Abstract
Expression of AEG-1 (also known as MTDH or LYRIC) is elevated in many cancers including glioblastoma multiforme (GBM), in which it functions as an oncogene. AEG-1 activates AKT signaling and physically interacts with AKT2 in GBM. Disruption of this interaction reduces glioma cell survival and invasion, uncovering a novel potential target for development of an effective therapy against GBM.
Keywords: AEG-1, AKT2, glioblastoma, MTDH
Abbreviations
- AEG-1
astrocyte elevated gene 1
- AKT
protein kinase B
- GBM
glioblastoma multiforme
- RTK
receptor tyrosine kinase
- PI3K
phosphatidylinositol 3-kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
Glioblastoma multiforme (GBM), graded by the World Health Organization (WHO) as a grade IV malignant glioma, is the most lethal form of glioma. Most patients who are diagnosed with GBM survive only 12 to 15 months.1 Treatment options remain multimodal, including surgery, radiation, and chemotherapy, and impart only incremental changes in cognitive functions and survival.2 Perhaps the most challenging complications in GBM therapy are a consequence of the highly invasive nature and complex heterogeneous molecular makeup of this cancer, which contribute to the aggressiveness of this disease. Over the past decade, many multitargeted therapies have been evaluated with only limited success. As a result, defining novel targets and developing targeted therapies remains a priority for this invariably fatal neoplasm.
Astrocyte-elevated gene-1 (AEG-1) was identified using subtraction hybridization from primary human fetal astrocytes as an human immunodeficiency virus (HIV)-1- and tumor necrosis factor (TNF)-α−inducible gene that is upregulated in the majority of cancers analyzed to date.3, 4 Subsequent studies identified AEG-1 as metadherin (MTDH) in the mouse and lysine-rich CEACAM1 coisolated (LYRIC) in the rat.3 AEG-1 promotes tumor growth via multiple oncogenic signaling pathways and regulates a wide array of key cellular processes involved in cancer progression including migration, invasion, angiogenesis, and metastasis. As a multifunctional protein, AEG-1 interacts with a plethora of proteins in different cancer types and these defined interactions facilitate tumor progression.5
AKT, also known as protein kinase B, acts as a nexus-signaling molecule in the receptor tyrosine kinase/phosphatidylinositol 3-kinase (RTK/PI3K) pathway and functions as an essential regulator of cell proliferation, survival, migration, and invasion.6 The AKT gene family consists of 3 homologous isoforms, AKT1, AKT2, and AKT3. A recent study comprehensively analyzed the expression levels of all 3 AKT isoforms in malignant glioma.7 In high-grade gliomas both AKT2 mRNA and protein levels were increased, whereas AKT3 mRNA and protein levels were reduced. AKT2 knockdown in glioma cell lines reduced colony formation and induced apoptosis; however, knockdown of AKT1 did not alter cell growth or promote apoptosis, suggesting that AKT2 and AKT3 may be the key regulators of GBM cell growth.7
Our previous studies demonstrated that AEG-1 functions as a downstream target of Ha-Ras, and that induction of AEG-1 was attenuated by treatment with the PI3K inhibitor LY294002 or overexpression of phosphatase and tensin homolog (PTEN).8 Promoter mapping suggested that Ha-Ras enhanced the binding of c-Myc to the E-box elements in the promoter region of AEG-1 via the PI3K/AKT/c-Myc pathway, thereby regulating AEG-1 transcription.8 In another study, AEG-1 overexpression inhibited serum starvation-induced apoptosis by activating the Ras and PI3K-AKT signaling pathways.9 Subsequently, AEG-1 regulation of AKT signaling pathways was shown to be critical in cell proliferation, invasion, apoptosis resistance, chemoresistance, and angiogenesis in multiple cancer contexts by several groups.5 However, the mechanism by which AEG-1 regulates AKT remained unclear.
In our recent study,10 we demonstrated a novel specific interaction between AEG-1 and AKT2 in glioma. We showed that AEG-1 specifically interacts with AKT2, but not with AKT1 or AKT3 isoforms, in GBM. Immunohistochemical analysis revealed a high correlation between the expression of AEG-1 and AKT2 in clinical glioma samples. Using TCGA data analysis we also confirmed the clinical significance of these 2 key molecules for survival of glioma patients—higher expression was associated with poorer survival. Results from our functional assays indicated that disruption of the AEG-1–AKT2 interaction reduced AEG-1–mediated invasion. This observation was consistent with a previous report that AKT2 promotes breast cancer cell migration and invasion. Interestingly, we found that AEG-1–AKT2 interaction prolonged stabilization of AKT2 phosphorylation at S474. To explore the upstream regulators of AEG-1–AKT signaling, we examined AKT signaling upon insulin stimulation when AEG-1 expression was knocked down with siRNA and found that AEG-1 knockdown effectively blocked insulin-induced activation of AKT2-S474. In contrast, AEG-1 overexpression maintained S474 phosphorylation when activation of PI3K-AKT was inhibited by PTEN and LY294002, and this was associated with parallel changes in the AKT downstream molecules p-glycogen synthase kinase 3 (p-GSK3β) and cyclin D1. We also showed that phosphorylation of the proapoptotic protein Bcl-2-associated death promoter (BAD) was regulated by the AEG-1–AKT2 interaction, suggesting that the AEG-1–AKT2 complex protects glioma cells from the classic intrinsic mitochondrial apoptosis pathway. Together, these data highlight the importance of AEG-1–AKT2 interaction in glioma proliferation, survival, and invasion (Fig. 1).
Figure 1.
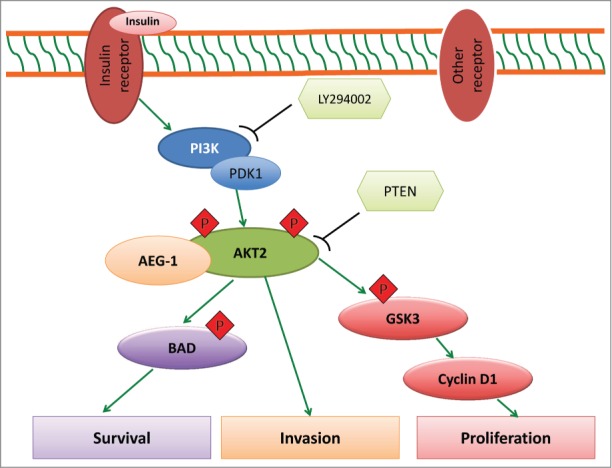
AEG-1–AKT2 signaling in glioma. Upon binding of insulin or other ligands to their respective receptors, PI3K, PDK1, or other kinases become activated and then phosphorylate AKT at T308 or S473/474. By forming a complex with AKT2, AEG-1 stabilizes the phosphorylation of AKT at S473/474. Phospho-AKT then activates downstream target genes and regulates a cascade of events implicated in tumor progression including unlimited cell proliferation, survival, and invasion. AEG-1, astrocyte elevated gene 1; BAD, Bcl-2-associated death promoter; GSK3, glycogen synthase kinase 3; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase.
AEG-1 was previously shown to crosstalk with several signaling pathways to regulate cancer cell proliferation and promote glioma survival.5 For that reason, we investigated the potential crosstalk between AEG-1–AKT2 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling using an I kappa B kinase (IKK) inhibitor, caffeic acid phenyl ester (CAPE). CAPE modestly affected AEG-1–AKT2 signaling, suggesting that NF-κB activation by AEG-1 is independent of AKT activation. Finally, we showed that conditional expression of the AKT2-PH domain combined with AEG-1 silencing significantly enhanced survival in an orthotopic mouse model of human GBM. These findings further confirm that AEG-1–AKT2 is a critical protein–protein signaling complex in glioma. We are now exploring this interaction in other cancer contexts in which both of these molecules are highly upregulated.
We confirmed that the AKT2-PH domain is responsible for interacting with AEG-1 in glioma and mapped the interacting regions in AEG-1. This allowed us to define a target site for the development of candidate small-molecule drugs capable of disrupting this interaction. We predict that targeting AEG-1 or AKT2 alone will not be sufficient to engender a cure for this aggressive cancer. Rather, small-molecule inhibitors designed to disrupt AEG-1–AKT2 interactions in combination with conventional treatment modalities might represent a viable strategy to develop novel therapeutics for GBM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- 1.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 2013; 15 Suppl 2:ii1-56; PMID:24137015; http://dx.doi.org/ 10.1093/neuonc/not151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008; 359:492-507; PMID:18669428; http://dx.doi.org/ 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- 3.Lee SG, Kang DC, DeSalle R, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC, the beginning: initial cloning, structure, expression profile, and regulation of expression. Adv Cancer Res 2013; 120:1-38; PMID:23889986; http://dx.doi.org/ 10.1016/B978-0-12-401676-7.00001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: clinical significance. Adv Cancer Res 2013; 120:39-74; PMID:23889987; http://dx.doi.org/ 10.1016/B978-0-12-401676-7.00002-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emdad L, Das SK, Dasgupta S, Hu B, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res 2013; 120:75-111; PMID:23889988; http://dx.doi.org/ 10.1016/B978-0-12-401676-7.00003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chautard E, Ouedraogo ZG, Biau J, Verrelle P. Role of Akt in human malignant glioma: from oncogenesis to tumor aggressiveness. J Neuro Oncol 2014; 117:205-15; PMID:24477623; http://dx.doi.org/ 10.1007/s11060-014-1382-9 [DOI] [PubMed] [Google Scholar]
- 7.Mure H, Matsuzaki K, Kitazato KT, Mizobuchi Y, Kuwayama K, Kageji T, Nagahiro S. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol 2010; 12:221-32; PMID:20167810; http://dx.doi.org/ 10.1093/neuonc/nop026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A 2006; 103:17390-5; PMID:17088530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 2008; 27:1114-21; PMID:17704808; http://dx.doi.org/ 10.1038/sj.onc.1210713 [DOI] [PubMed] [Google Scholar]
- 10.Hu B, Emdad L, Bacolod MD, Kegelman TP, Shen XN, Alzubi MA, Das SK, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) interacts with Akt isoform 2 to control glioma growth, survival and pathogenesis. Cancer Res 2014; 74:7321-32; PMID:25304263; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2978 [DOI] [PMC free article] [PubMed] [Google Scholar]


