Abstract
The Usp28 deubiquitinase antagonizes Fbw7-mediated turnover of multiple oncoproteins, including Myc, Jun, and Notch, and promotes tumorigenesis in the intestine. Our recent study reveals that Usp28 also counteracts autocatalytic ubiquitination of Fbw7, suggesting a complex role for Usp28 in the regulation of Fbw7 activity and tumor development.
Keywords: Fbw7, oncogene, SCF, tumor suppressor, ubiquitin, Usp28
Abbreviations
- Fbw7
F-box and WD40 domain-containing protein 7
- Usp28
Ubiquitin-specific protease 28
- SCF
Skp1–Cullin1–F-box protein
Fbw7, the substrate recognition subunit of the SCF (Skp1–Cullin1–F-box protein) ubiquitin ligase, promotes ubiquitination and turnover of multiple oncoproteins. Mutations in Fbw7 frequently occur in human cancers (most notably in colorectal carcinomas and T-cell leukemias) and compromise substrate ubiquitination.1 Accumulation of Fbw7 substrates, such as Myc, Mcl1, and cyclin E, fuels tumorigenesis by driving cell proliferation, survival, and genomic instability. Fbw7 also promotes its own degradation via autocatalytic ubiquitination, either in response to signaling or when substrate levels become low.
Fbw7-dependent substrate ubiquitination is countered by the Usp28 deubiquitinating enzyme. In human tumor cell lines, shRNA-mediated depletion of Usp28 downregulates Myc levels and attenuates proliferation.2 As a cysteine protease that stabilizes Myc, a key driver of cancer progression, Usp28 is a candidate oncoprotein and a potential target for cancer therapy.
Interestingly, however, recent studies have revealed a range of genetic alterations in Usp28 in human tumors, including amplifications, deletions, and point mutations. For example, Usp28 is overexpressed in colorectal and ovarian carcinomas, whereas prostate carcinomas and melanomas harbor recurrent focal deletions at the Usp28 locus.3, 4 Furthermore, Usp28 protein expression is downregulated in several cancer types relative to normal tissue, suggesting that loss of Usp28 may also be beneficial during tumorigenesis.5
Analysis of Usp28 knockout mice may help reconcile these controversial observations. As expected from in vitro studies, ablation of one allele of Usp28 uniformly downregulates Fbw7 substrates in murine tissues.6 In contrast, complete loss of Usp28 produces tissue-specific effects—in the intestine Fbw7 substrates are further downregulated whereas in other tissues, including lung, liver, and pancreas, they match or exceed the wildtype levels. Interestingly, Fbw7 is regulated reciprocally; it remains stable upon loss of Usp28 in the intestine but is downregulated in most other tissues and in cultured mouse embryonic fibroblasts (MEFs), suggesting that Usp28 controls Fbw7 stability in a tissue-specific manner.
Indeed, experiments in MEFs and human tumor cells demonstrate that Usp28 directly deubiquitinates and stabilizes Fbw7.6 Importantly, deubiquitination of Fbw7 requires significantly lower levels of Usp28 than needed for Fbw7 substrates, explaining the differential response to mono- and biallelic deletion of Usp28. Apparently, half of the normal dose of Usp28 maintains stable Fbw7, but is not sufficient for Fbw7 substrate stabilization. In contrast, complete knockout of Usp28 triggers autocatalytic ubiquitination and degradation of Fbw7, sparing Fbw7 substrates.
Autocatalytic ubiquitin transfer by Fbw7 is regulated by phosphorylation and Pin1-dependent isomerization.7, 8 Consistent with these findings, inhibition of Pin1 restores Fbw7 levels and drives Fbw7 substrate degradation in Usp28-null MEFs. In tissues of Usp28 knockout mice, Pin1 expression inversely correlates with Fbw7 levels, suggesting that the abundance or activity of Pin1 may determine the outcome of Usp28 loss for Fbw7 substrates.6
Usp28-dependent control of Fbw7 and its substrates closely resembles regulation of Mdm2 and p53 by the Hausp deubiquitinase. Hausp stabilizes both Mdm2 and p53, but its higher affinity for Mdm2 results in a biphasic response to the loss of Hausp.9 Other deubiquitinase–ligase pairs will likely exhibit a similar relationship, revealing a common regulatory paradigm in ubiquitin networks. But what are the advantages of such bifunctional regulation?
We propose that dual control of Fbw7 activity by Usp28 maintains physiological levels of Fbw7 substrates during homeostasis (Fig. 1). Activation of Usp28 will stabilize both Fbw7 substrates and Fbw7, suppressing prolonged accumulation of proto-oncoproteins. A similar principle is observed in the regulation of several oncogenic transcription factors; for example, phosphorylation events that activate Myc and Jun simultaneously prime them for degradation, creating a time-restricted window of activity. Potentially, such mechanisms evolved as safeguards against unscheduled activation of proto-oncoproteins and the emergence of cancer-initiating cells.
Figure 1.
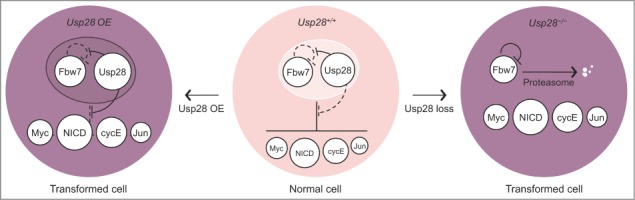
Dual regulation of Fbw7 function by Usp28 maintains cellular homeostasis. Usp28 limits Fbw7-mediated ubiquitination of proto-oncogenic substrates, but primarily deubiquitinates and stabilizes Fbw7. During homeostasis, the Fbw7–Usp28 complex maintains low levels of Fbw7 substrates. Loss of Usp28 triggers autocatalytic ubiquitination and turnover of Fbw7, leading to the accumulation of Fbw7 substrates and promoting oncogenic transformation. Overexpression (OE) of Usp28 blocks both autocatalytic and substrate-directed ubiquitination by Fbw7 and equivalently enhances cell transformation.
Physiological levels of Usp28 are required to maintain Fbw7 substrates within homeostatic boundaries. In a cell culture model of oncogenic transformation, loss of Usp28 strongly upregulates Fbw7 substrates and promotes anchorage-independent growth (Fig. 1). Intriguingly, ectopic expression of Usp28 in this system equivalently stabilizes Fbw7 substrates and promotes transformation, despite highly increased levels of Fbw7. Thus, opposite extremes of Usp28 abundance produce equivalent phenotypes through opposite mechanisms: complete loss destabilizes Fbw7, whereas overexpression stabilizes both Fbw7 and its substrates.
A prediction derived from our analysis is that Usp28 may function as a tumor promoter or suppressor in different tissues, depending on whether the signaling context favors autocatalytic ubiquitination of Fbw7. In the intestine, Usp28 deletion does not promote Fbw7 turnover and consequently induces Fbw7 substrate degradation. Accordingly, in a mouse model of Apcmin-driven driven intestinal cancer, ablation of Usp28 attenuates tumorigenesis and prolongs survival.10
In tissues where the signaling favors autocatalytic turnover of Fbw7, deletion of Usp28 may functionally mimic Fbw7 loss-of-function and promote tumorigenesis. Our observations in MEFs predict that under these conditions, loss and overexpression of Usp28 may equivalently upregulate Fbw7 substrate levels and help establish or maintain the malignant state. We imagine that bifunctional regulatory motifs, similar to the Usp28–Fbw7–Fbw7 substrate circuit, are widespread in signaling networks and could explain the occurrence of opposite genetic alterations of a single gene in cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in the Popov laboratory is supported by the German Research Foundation (DFG Research Grant PO1458–3/1 and PO1458–4/1) and by an institutional grant from the Deutsche Krebshilfe.
References
- 1.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008l; 8, 83–93; PMID:18094723 [DOI] [PubMed] [Google Scholar]
- 2.Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R., Moll R., Elledge S.J., Eilers M.. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol 2007; 9, 765–74; PMID:17558397; http://dx.doi.org/ 10.1038/ncb1601 [DOI] [PubMed] [Google Scholar]
- 3.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G.. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 12, R41; PMID:21527027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucl Acids Res 2015; 43:D805-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 2010; 28, 1248–50; PMID:21139605; http://dx.doi.org/ 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- 6.Schülein-Völk C, Wolf E, Zhu J, Xu W, Taranets L, Hellmann A, Jänicke LA, Diefenbacher ME, Behrens A, Eilers M, et al. Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep 2014; 9, 1099–109; PMID:25437563 [DOI] [PubMed] [Google Scholar]
- 7.Schülein C, Eilers M, Popov N. PI3K-dependent phosphorylation of Fbw7 modulates substrate degradation and activity. FEBS Lett 2011; 585, 2151–7; PMID:21620836; http://dx.doi.org/ 10.1016/j.febslet.2011.05.036 [DOI] [PubMed] [Google Scholar]
- 8.Min S-H, Lau AW, Lee TH, Inuzuka H, Wei S, Huang P, Shaik S, Lee DY, Finn G, Balastik M, et al. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol. Cell 2012; 46, 771–83; PMID:22608923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell 2004; 13, 879–86; PMID:15053880 [DOI] [PubMed] [Google Scholar]
- 10.Diefenbacher ME, Popov N, Blake SM, Schülein-Völk C, Nye E, Spencer-Dene B, Jaenicke LA, Eilers M, Behrens A. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J Clin Invest 2014; 124, 3407–18; PMID:24960159 [DOI] [PMC free article] [PubMed] [Google Scholar]


