Abstract
CD44 was recently identified as a positive feedback regulator of Wnt/β-catenin signaling. This regulation occurs at the level of low-density lipoprotein receptor-related protein 6 phosphorylation and membrane targeting. These findings broaden our understanding of the Wnt pathway activation process and open new perspectives for anti-CD44 therapies in diseases associated with Wnt induction, including colorectal cancer.
Abbreviations
- ERM
ezrin-radixin-moesin
- Fz
Frizzled
- LEF
lymphoid enhancer factor
- LRP6
low-density lipoprotein receptor-related proteins
- TCF
T-cell factor
Wnt/β-catenin signaling is activated upon binding of Wnt ligands to both Frizzled (Fz) receptors and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors (reviewed in 1). This activation results in the inhibition of β-catenin degradation and its cytosolic accumulation. Subsequent β-catenin translocation to the nucleus leads to T-cell factor/lymphoid enhancer factor (TCF/LEF)-driven transcription. Among the Wnt target genes are regulators of cell proliferation, growth, differentiation, and migration. Hence, Wnt/β-catenin signaling plays key roles in development (reviewed in 1). This pathway also regulates homeostasis of bones, hematopoiesis, and renewal of tissues such as the intestine or the skin, and therefore remains essential throughout life. Consequently, misregulation of Wnt/β-catenin signaling leads to developmental defects, malformations, degenerative and metabolic diseases, and cancer (reviewed in 1). A tight regulation of Wnt signaling involves feedback control mechanisms in which the expression of several Wnt-signaling components is regulated by Wnt/β-catenin signaling itself (reviewed in 1).
The cell adhesion molecule CD44 was identified as a canonical Wnt target in the intestine, where it is highly expressed in intestinal stem and proliferative progenitor cells. Loss of CD44 in ApcMin/+ mice significantly reduced the tumor number in the small intestine, thus indicating an involvement of CD44 in Wnt-induced tumorigenesis.2 Furthermore, increased CD44 expression correlates with late stages and poor prognosis of colorectal cancer.3 However, considering CD44 solely as a Wnt target gene might only be part of the story. In fact, our recent paper4 shows that CD44 also acts as a positive Wnt feedback regulator. In several cell lines, silencing of all CD44 isoforms suppressed Wnt-induced activation and nuclear translocation of β-catenin, as well as TCF/LEF-driven transcription. Conversely, overexpression of CD44 isoforms enhanced Wnt signaling regardless of the isoform, suggesting the involvement of a function common to all CD44 isoforms in the regulation of this pathway. A CD44 isoform with deletion of the cytoplasmic domain had no effect on Wnt pathway activation, indicating that the CD44 cytoplasmic domain is essential for the involvement of CD44 in Wnt signaling. Ezrin, a member of the Ezrin-Radixin-Moesin (ERM) protein family, links CD44 to the cytoskeleton (reviewed in 5). Interference with the binding of ezrin to CD44 resulted in loss of CD44 regulatory function. The association of CD44 to ERM proteins and to the cytoskeleton seems to be essential for Wnt signaling.
Wnt pathway activation at the level of Wnt3a, LRP6, Dishevelled, or β-catenin revealed regulation by CD44 at the level of membrane receptors. Co-immunoprecipitation experiments identified a Wnt-inducible LRP6–CD44 complex. Downregulation of CD44 demonstrated a role of CD44 both in LRP6 phosphorylation, an event that is Wnt-dependent, and in LRP6 membrane targeting, a step that is Wnt independent. These findings suggest a dual mechanism of action of CD44 in Wnt signaling (Fig. 1). Both of these functions depend on the cytoplasmic domain of CD44 and its binding to F-actin via Ezrin. Finally, experiments in Xenopus laevis demonstrated an in vivo requirement of CD44 for Wnt/β-catenin signaling in CNS development, as indicated by reduced expression of the Wnt-target genes tcf-4 and engrailed-2 in CD44 morphants.
Figure 1.
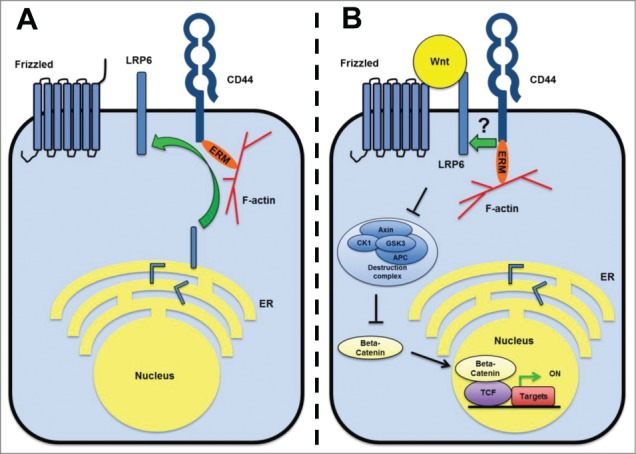
Dual impact of CD44 on Wnt/β-catenin signaling. (A) In the absence of Wnt ligands, CD44 bound to F-actin via ERMs regulates membrane targeting and cell surface expression of LRP6. (B) In the presence of Wnts, CD44 forms a complex with LRP6 and participates in LRP6 activation. This step is also highly dependent on the association of CD44 with the actin cytoskeleton via ERMs. Abbreviations: APC, adenomatous polyposis coli; CK1, casein kinase 1; ER, endoplasmic reticulum; ERM, ezrin-radixin-moesin; GSK3, glycogen synthase kinase; LRP6, low-density lipoprotein receptor-related proteins; TCF, T-cell factor.
The activity of the Wnt cascade is regulated at each step from the cell surface to the nucleus (reviewed in 1). What could be the additional contribution of CD44? One important characteristic of CD44 is its ability to bind to the cytoskeleton through ERM proteins. This network formed by the CD44–ERM–actin complex might provide a platform necessary for the tight association between LRP6 and kinases such as glycogen synthase kinase 3β and casein kinase 1γ. Additionally, trafficking of the LRP6-containing vesicles from the Golgi to the membrane might take place within the F-actin rich cortex. These F-actin tracks might be tethered to the plasma membrane through the CD44-ERM complex. Although these hypotheses are highly speculative one should note that CD44 can be found on coat protein complex 1 (COP1) vesicles involved in vesicle trafficking.6 Additionally, CD44 was shown to co-localize with soluble NSF attachment protein receptors (SNAREs) that play important roles in the fusion of vesicles with the plasma membrane.7
CD44 is not the only Wnt-target gene providing positive feedback regulation in Wnt/β-catenin signaling. Other positive regulators like LEF1 or Fz-receptors have been shown to be upregulated upon activation of canonical Wnt signaling. Positive feedback loops between CD44 and Wnt signaling might be required during development or for homeostasis of various organs, including the limbs or the skin. The intestinal epithelium, a highly dynamic tissue that is renewed every 4 to 5 days, is also a strong candidate for such regulation. Indeed, renewal of the intestinal epithelium requires permanently active Wnt signals in the intestinal stem and proliferative progenitor cells. Inactivation of canonical Wnt signaling in the intestine causes a complete loss of proliferation of intestinal progenitor cells at the bottom of the intestinal crypts. Severe effects on stem cell maintenance and proliferation were also observed.8 Interestingly, an isoform-specific function of CD44 in this intestinal stem and proliferative progenitor compartment was recently suggested.9
In conclusion, our findings reveal a completely new aspect of CD44, namely as a regulator of Wnt signaling at the level of LRP6, and add another regulatory function to the already described roles of CD44 in signaling. In colorectal cancer, CD44 might act as a Wnt target gene in concert with Met.10 Additionally, CD44 might influence the activity of the Wnt pathway in a positive feedback loop. Distinguishing the role of CD44 as a target gene or as a Wnt-regulator might be difficult. However, anti-CD44 therapies should be seriously considered, especially in colorectal cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20:781–810; PMID:15473860; http://dx.doi.org/ 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 2.Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res 2008; 68:3655–61; PMID:18483247; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2940 [DOI] [PubMed] [Google Scholar]
- 3.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 1993; 53:4754–6; PMID:7691404 [PubMed] [Google Scholar]
- 4.Schmitt M, Metzger M, Gradl D, Davidson G, Orian-Rousseau V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ 2014; PMID:25301071; http://dx.doi.org/ 10.1038/cdd.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer 2010; 46:1271–7; PMID:20303742; http://dx.doi.org/ 10.1016/j.ejca.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 6.Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, Oda K. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic 2010; 11:1552–66; PMID:20874812; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01123.x [DOI] [PubMed] [Google Scholar]
- 7.Chintagari NR, Jin N, Wang P, Narasaraju TA, Chen J, Liu L. Effect of cholesterol depletion on exocytosis of alveolar type II cells. Am J Respir Cell Mol Biol 2006; 34:677–87; PMID:16439800; http://dx.doi.org/ 10.1165/rcmb.2005-0418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003; 17:1709–13; PMID:12865297; http://dx.doi.org/ 10.1101/gad.267103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeilstra J, Joosten SP, Vermeulen L, Koster J, Medema JP, Versteeg R, Spaargaren M, Pals ST. CD44 expression in intestinal epithelium and colorectal cancer is independent of p53 status. PLoS One 2013; 8:e72849; PMID:24009708; http://dx.doi.org/ 10.1371/journal.pone.0072849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 2002; 62:5126–8; PMID:12234972 [PubMed] [Google Scholar]


