Abstract
SESTRINs, proteins encoded by the SESN1–3 genes in mammals, are well-established suppressors of the mechanistic target of rapamycin complex 1 (mTORC1) kinase. Recently, we found that SESTRINs bind the GATOR2 protein complex, which is a regulator of RRAGA/B guanosine triphosphatase. Three independent studies support the RRAGA/B-dependence of mTORC1 regulation by SESTRINs; however, the role of GATOR2 in this process requires clarification.
Keywords: GATOR, GID, mTORC1, RRAG-A/B, SESTRINs
SESTRINs, a protein family composed of SESTRIN1, SESTRIN2, and SESTRIN3 in mammals, are potent suppressors of the mechanistic target of rapamycin complex 1 (mTORC1) kinase.1 mTORC1 integrates numerous signals from nutrients, growth factors, and stress to control metabolism, autophagy, and cell growth. mTORC1 activity inversely correlates with the lifespan of most eukaryotes and mTORC1 contributes to several different pathologies including cancer, diabetes, and neurodegenerative diseases.2 mTORC1 is activated by 2 groups of small guanine triphosphatases (GTPases): Ras homolog enriched in brain (RHEB) and members of the Ras-related GTP-binding protein (RRAG) family, working as RRAG-A/B and RRAG-C/D heterodimers. RHEB activity is inhibited by the tuberoses sclerosis protein complex (TSC). TSC in turn is controlled by several stress insults via activation of 5´-AMP-activated protein kinase (AMPK), and we have shown previously that SESTRINs inhibit mTORC1 via the AMPK-TSC axis.3 Amino acids and glucose control mTORC1 via RRAGs, which stimulate translocation of mTORC1 to the lysosomes where it can be activated by RHEB. RRAG-A/B is activated by its GTPase exchange factor (GEF) called ragulator and is inhibited by the protein complex GATOR1 (GTPase activating protein [GAP] activity toward RRAG complex 1) working as a GAP. GATOR1 in turn is suppressed by the GATOR2 protein complex within the GATOR supercomplex.4
Three groups recently reported a novel mechanism of mTORC1 inhibition by SESTRINs via suppression of RRAG-dependent mTORC1 lysosomal translocation.5-7 SESTRINs are critical for mTORC1 inhibition by amino acid withdrawal in mammalian cells. Surprisingly, SESTRINs do not affect RRAG-A/B GDP/GTP loading, suggesting that they are not regulators of RRAG-A/B GAP or GEF. Although all 3 groups agreed on the importance of RRAGs for mTORC1 suppression by SESTRINs, they proposed different mechanisms for this regulatory activity.
Using mass spectrometry and immunoprecipitation analyses of SESTRIN2 complexes, our group identified GATOR2 as a strong SESTRIN2 interactor.7 At the same time, Sabatini's group isolated SESTRIN1–3 as GATOR2 interactors,5 showing strong similarity to our data. Both groups demonstrated that SESTRINs regulate mTORC1 in a GATOR- and RRAG-dependent manner. However, SESTRINs do not affect GATOR1–GATOR2 interactions, suggesting that they do not work by simply titrating GATOR2 away from GATOR1.
Li's group proposed a different mechanism of mTORC1 regulation by SESTRINs.6 According to the study of Peng et al., SESTRINs directly bind RRAGs and work as RRAG-A/B guanine dissociation inhibitors (GDIs).6 A portion of the SESTRIN protein shares homology with a GDI motif of GDI1 protein that controls activity of the RAB GTPase family members. Mutations in the GDI motif significantly impair the ability of SESTRIN2 to inhibit mTORC1 and delivery of a peptide GDI motif is sufficient to inhibit mTORC1.6
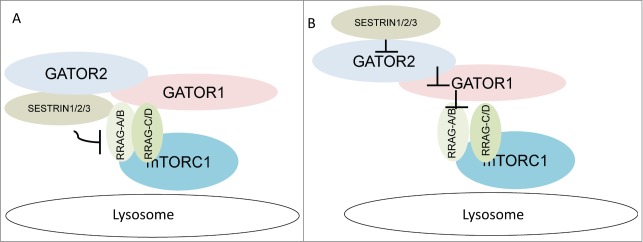
There are several major concerns that remain unexplained. First, do SESTRINs directly interact with RRAGs and what is the role of the GATOR complex in the regulation of RRAG-A/B and mTORC1 activities by SESTRINs? Despite the high abundance of GATOR2 peptides among our mass spectrometry data, we did not observe any RRAG peptides, indicating that this interaction is very weak and might be indirect. Second, according to Li's model, SESTRIN2 must have some regulatory role over RRAGs even in the absence of GATOR1 as a result of intrinsic RRAG-A/B GTPase activity,6 although data from our group and Sabatini's group indicate a strong requirement for the GATOR complex in the regulation of mTORC1 by SESTRINs.5,7 Finally, in contrast to Peng et al., we were not able to detect any colocalization of SESTRIN2 with lysosomes, indicating that SESTRINs might play a major role in the regulation of GATOR and RRAGs in the cytoplasm.6,7 To reconcile these studies, we propose that GATOR is required for the interaction between SESTRIN2 and RRAGs, potentially providing a bridge between them and somehow bringing the potential GDI domain of SESTRINs into close proximity of the RRAG-A/B protein. Thus, GATOR might control interactions between SESTRINs and RRAGs and assist in mTORC1 inhibition by SESTRINs (Fig. 1A). Alternatively, SESTRINs might interact solely with GATOR2, and the GDI activity of SESTRIN toward RRAGA/B might be mediated by GATOR (Fig. 1B). We demonstrated that the interaction between SESTRIN2 and GATOR2 is mediated by WD-repeat domain 24 (WDR24) and SEH1-like (SEH1L) proteins, thus it would be important to examine whether the GDI domain of SESTRIN is required for the interaction between SESTRINs and GATOR2.
Figure 1.
Two potential mechanisms of mTORC1 regulation by SESTRINs via GATOR and RRAG-A/(B) proteins. SESTRINs interact with GATOR and RRAGs and regulate mTORC1 activity via a RRAG-dependent mechanism. Two models can explain the regulation of RRAG-A/B activity by SESTRINs. In model (A), SESTRINs interact directly but weakly with RRAG-A/B. GATOR2, being a strong interactor for SESTRIN2, stabilizes interactions between SESTRINs and RRAGs, thus supporting the inhibitory effects of SESTRINs on RRAG-A/B. In model (B), interactions between SESTRINs and RRAGs are solely mediated by GATOR, which transmits an inhibitory signal from SESTRINs toward RRAG-A/B.
To reconcile the discrepancy in localization studies we propose that the interaction between SESTRINs and GATOR–RRAGs complexes is a dynamic process and under certain conditions SESTRINs can be associated with lysosomes, although most of the proteins associated with GATOR are still located in the cytoplasm in close proximity to lysosomes. Our findings are consistent with recently published data from Avruch's laboratory8 showing a predominantly cytoplasmic localization of RRAGs based on protein fractionation studies. In the future it would be important to analyze the localization of endogenous SESTRINs and RRAGs, and the role of SESTRINs in the control of RRAG localization in the cell under normal and stress conditions.
Interestingly, yeast mTORC1 is regulated by amino acids via a mechanism mediated by Gtr1 and Gtr2, orthologs of mammalian RRAG-A/B and RRAG-C/D, and the SEA complex (SEAC), an analog of mammalian GATOR. SEAC functions as a whole complex, suggesting that GATOR might also work as an entire complex to regulate RRAG-A/B activity.9,10 However, the fact that yeasts do not express sestrins indicates that they are not obligatory mediators of AA signaling. Surprisingly, sestrin-deficient C. elegans and D. melanogaster do not show any significant abnormalities beyond an accelerated aging phenotype, indicating that sestrins might provide adaptation to stress rather than functioning as bona fide mTORC1 controllers.1 Nevertheless, the redundancy of SESTRIN family members in mammals indicates that each member of the family might play distinctive roles under certain stress and metabolic conditions. Different SESTRINs might cooperate with each other to provide a robust firewall against uncontrolled mTORC1 activation under stress conditions, preventing accumulation of damage and switching anabolism to a stress mode.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH CA172660 to AB.
References
- 1.Budanov AV, Lee JH, Karin M. Stressin' Sestrins take an aging fight. EMBO Mol Med 2010; 2:388-400; PMID:20878915; http://dx.doi.org/ 10.1002/emmm.201000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/ 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008; 134:451-60; PMID:18692468; http://dx.doi.org/ 10.1016/j.cell.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014; 24(7):400-6; PMID:24698685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins Interact with GATOR2 to Negatively Regulate the Amino-Acid-Sensing Pathway Upstream of mTORC1. Cell Rep 2014; 9(1):1-8; PMID:25263562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng M, Yin N, Li MO. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell 2014; 159:122-33; PMID:25259925; http://dx.doi.org/ 10.1016/j.cell.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Rep 2014; 9:1281-91; PMID:25457612; http://dx.doi.org/ 10.1016/j.celrep.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshiro N, Rapley J, Avruch J. Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging. J Biol Chem 2014; 289:2658-74; PMID:24337580; http://dx.doi.org/ 10.1074/jbc.M113.528505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dokudovskaya S, Waharte F, Schlessinger A, Pieper U, Devos DP, Cristea IM, Williams R, Salamero J, Chait BT, Sali A, et al.. A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae. Mol Cell Proteomics 2011; 10:M110 006478; http://dx.doi.org/ 10.1074/mcp.M110.006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panchaud N, Peli-Gulli MP, De Virgilio C. SEACing the GAP that nEGOCiates TORC1 activation: evolutionary conservation of Rag GTPase regulation. Cell Cycle 2013; 12:2948-52; PMID:23974112; http://dx.doi.org/ 10.4161/cc.26000 [DOI] [PMC free article] [PubMed] [Google Scholar]