Abstract
The role of p53, the original “guardian of the genome”, in skin has remained elusive. We have explored p53 function in human epidermal cells and demonstrated the importance of a mitosis-differentiation checkpoint to suppress potentially precancerous cells. This model places epidermal endoreplication as an antioncogenic mechanism in the face of irreparable genetic alterations.
Keywords: DNA damage, DNA repair, replication stress, MDC, mitotic slippage, OID
Abbreviations
- KO
knockout
- MDC
mitosis-differentiation checkpoint
- OID
oncogene-induced differentiation
- UV
ultraviolet
TP53 (tumor protein p53) is the paradigm of tumor suppressors and the most frequently mutated gene in cancer (present in about 50% of cancers).1 Alteration of the p53 gene is often associated with environmental mutagens such as ultraviolet (UV) light or smoking. As a result of sun exposure, the incidence of p53 mutations is strikingly high in skin squamous carcinomas (80–90%).2 In spite of these numbers, the role of p53 in human tissue renewal, including epidermal homeostasis, remains intriguing. Since experimenting in humans in vivo is not possible for obvious reasons, mouse models have become a strong reference for skin research. However, as observed for other key genes, p53 knockout (KO) mice develop normally.3 In addition, mouse and human skin displays different physiological features.
To address the role of p53 in human skin using new approaches, we have adapted the shRNA technology for the inactivation of endogenous proteins in human primary keratinocytes. We describe the consequences of silencing p53 in these epidermal cells in a recent issue of Cell Reports.4 We can now achieve efficiencies of about 90% for cell transduction with lentiviral vectors, which is the closest we can get to human KO epidermis. In steady-state healthy epidermis, p53 is thought to play a role in cell differentiation. However, contrary to our expectations, we found that knockdown of p53 in human keratinocytes favored squamous differentiation. Ironically, these results were highly consistent with our previous observations.
We have proposed a novel model of the keratinocyte epidermal cell cycle in which differentiating cells do not undergo quiescence but instead continue post-mitotic DNA replication in the absence of cell division (endoreplication).5 Endoreplication is a consequence of a process that some authors have called ‘mitotic slippage’.6 This term is somewhat controversial but in our opinion it expresses well the following sequence of events that occur during endoreplication: (1) In the case of difficulty or danger, mitotic checkpoints block cell division; (2) these cells are unable to properly stay in mitosis (G2/M); (3) as a result, they ‘slip’ through the G2/M checkpoints and restart DNA replication; (4) however, as the cells cannot divide, they become polyploid.
Endoreplication is controversial within the field of skin biology because it is still a poorly understood concept despite some very good reviews that highlight its biological importance.7 Defining endoreplication and explaining its potential functions is often a challenge. Nonetheless, endoreplication might be more widespread in human tissues than we think. Moreover, it might have an important role in the maintenance of homeostasis. We have shown that in keratinocytes mitotic slippage occurs in response to cell cycle deregulation that causes DNA replication errors (‘replication stress’) and triggers mitotic checkpoints.4,5
Our current paper in Cell Reports we believe that we answer 2 questions regarding epidermal homeostasis: (1) the function of p53 in normal keratinocytes; and (2) the importance of endoreplication against precancerous mutations. Why?
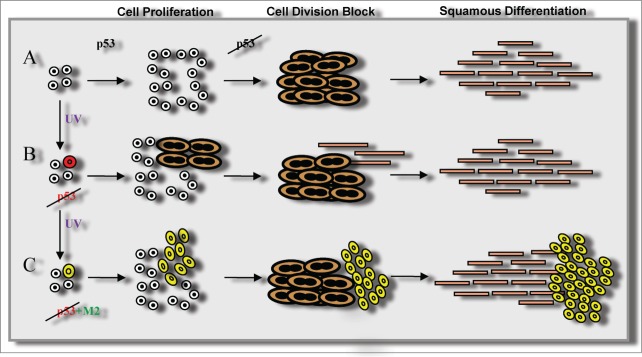
In human skin, p53 is expressed in the proliferative layers of the epidermis.4 p53 is upregulated by UV irradiation and is thought to trigger apoptosis of sunburn cells but, as mentioned above, its function in steady state epidermis is unclear. After knocking down p53, we were able to unravel its role in keratinocytes by subtraction. We also overexpressed a temperature-sensitive form of p53 that behaves as an inactive mutation at 39°C or as the active wild-type protein at 32°C.8 Similar to our observations for the knockdown, overexpression of the inactive conformation triggered terminal differentiation. p53 is a critical keeper of cell cycle pace, thus its absence caused cell cycle deregulation leading to replication stress, mitotic slippage, and endoreplication. This in turn triggered squamous differentiation, therefore forcing mutant cells to detach (Fig. 1). Interestingly, at 32°C the wild-type conformation attenuated differentiation, indicating that p53 protects the proliferative compartment and putatively the stem cells.4
Figure 1.
Model for dual consequences of p53 inactivation in the skin. p53 surveys for correct execution of the cell cycle in epidermal homeostasis (A). Loss of p53 function as a single mutation causes mitotic slippage, squamous differentiation, and cell shedding, thus maintaining homeostasis (B), or contributes to malignancy when additional mutations (M2) affect cell division control and allow p53-deficient cells to divide (C).
Mitotic slippage, replication stress, and endoreplication are all fairly new concepts that we will probably read increasingly more about in the near future as they most likely have a role in cancer. We propose that these events represent a mitosis-differentiation checkpoint (MDC), a tool used by normal skin to remove precancerous cells bearing irreparable damage. Even though skin carcinomas are the most common malignancies, their incidence seems low considering that cells in the epidermis are continuously dividing and are chronically exposed to the mutagenic power of UV light. Therefore, the skin must have powerful protective mechanisms in play when the level of pigment (melanin) is not sufficient and the DNA repair machinery cannot repair the damage produced.
Mutations of p53 are highly frequent in skin carcinomas yet we propose the existence of a protective mechanism in normal skin. How can both concepts be reconciled? Inactivation of p53 in the whole mouse did not provoke early skin carcinomas although the animals died by 4 months of age from other types of cancer. However, skin-specific p53 KO mice did develop squamous cell carcinomas from 4 months onwards.9 Similarly, skin carcinomas in humans are usually associated with old age. It therefore seems likely that additional mutations are required for the loss of p53 to be tumorigenic. Nonetheless, progression of chemically-induced tumors was accelerated in p53 KO mice.10 Our model proposes a second “guardian of the genome” through the cell division block imposed by the MDC. If this checkpoint is mutated, the additional absence of p53, the original guardian of the genome, makes those cells more malignant (Fig. 1).
Our data point to a homeostatic maintenance role of endoreplication that might be common to other endoreplicating tissues. The list of these tissues in the human body has increased to include megakaryocytes, hepathocytes, endometrium, keratinocytes, and cardiomyocytes5 and may expand further in the future. Finally, we interpret our data to mean that skin carcinoma cells are malignant not simply because their cell cycle is deregulated but because, in addition, they are able to divide.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize that we could not cite every relevant publication because of length limitations; many missing articles are cited within the papers we do list. We thank all members of the laboratory and the associated clinicians.
Funding
We thank the Instituto de Salud Carlos III for their support, grant FIS PI11/02070.
References
- 1.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 2007; 26:2157-65; PMID:17401424; http://dx.doi.org/ 10.1038/sj.onc.1210302 [DOI] [PubMed] [Google Scholar]
- 2.Brash DE. Roles of the transcription factor p53 in keratinocyte carcinomas. Br J dermatol 2006; 154 Suppl 1:8-10; PMID:16712710; http://dx.doi.org/ 10.1111/j.1365-2133.2006.07230.x [DOI] [PubMed] [Google Scholar]
- 3.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr., Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356:215-21; PMID:1552940; http://dx.doi.org/ 10.1038/356215a0 [DOI] [PubMed] [Google Scholar]
- 4.Freije A, Molinuevo R, Ceballos L, Cagigas M, Alonso-Lecue P, Rodriguez R, Menendez P, Aberdam D, De Diego E, Gandarillas A. Inactivation of p53 in human keratinocytes leads to squamous differentiation and shedding via replication stress and mitotic slippage. Cell Rep 2014; 9:1349-60; PMID:25453755; http://dx.doi.org/ 10.1016/j.celrep.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 5.Gandarillas A, Freije A. Cycling up the epidermis: reconciling 100 years of debate. Exp Dermatol 2014; 23:87-91; PMID:24261570; http://dx.doi.org/ 10.1111/exd.12287 [DOI] [PubMed] [Google Scholar]
- 6.Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J Cell Biol 1994; 127:789-802; PMID:7962060; http://dx.doi.org/ 10.1083/jcb.127.3.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox DT, Duronio RJ. Endoreplication and polyploidy: insights into development and disease. Development 2013; 140:3-12; PMID:23222436; http://dx.doi.org/ 10.1242/dev.080531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalovitz D, Halevy O, Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell 1990; 62:671-80; PMID:2143698; http://dx.doi.org/ 10.1016/0092-8674(90)90113-S [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Cruz AB, Santos M, Lara MF, Segrelles C, Ruiz S, Moral M, Lorz C, Garcia-Escudero R, Paramio JM. Spontaneous squamous cell carcinoma induced by the somatic inactivation of retinoblastoma and Trp53 tumor suppressors. Cancer Res 2008; 68:683-92; PMID:18245467; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-3049 [DOI] [PubMed] [Google Scholar]
- 10.Kemp CJ, Donehower LA, Bradley A, Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell 1993; 74:813-22; PMID:8374952; http://dx.doi.org/ 10.1016/0092-8674(93)90461-X [DOI] [PubMed] [Google Scholar]