Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide yet has limited therapeutic options. We recently demonstrated that inhibition of de novo nicotinamide adenine dinucleotide (NAD+) synthesis is responsible for DNA damage, thereby initiating hepatocarcinogenesis. We propose that boosting NAD+ levels might be used as a prophylactic or therapeutic approach in HCC.
Keywords: Aryl hydrocarbon receptor (AhR), DNA damage, Estrogen receptor (ER), GEMMs, HCC, NAD+, nicotinamide riboside, prevention, pancreatic tumor, therapy, URI
Abbreviations
- AhR
aryl hydrocarbon receptor
- Dox
doxycycline
- ER
estrogen receptor
- HCC
hepatocellular carcinoma
- HSP90
heat shock protein 90
- NAD+
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NR
nicotinamide riboside
- PP1γ
protein phosphatase 1γ
- URI
unconventional prefoldin RPB5 interactor
Liver cancer is the second leading cause of cancer mortality worldwide (GLOBOCAN 2012), causing approximately 800,000 deaths each year. Hepatocellular carcinoma (HCC) is the most frequent primary liver neoplasm and has a very poor prognosis. Multiple risk factors, such as chronic hepatitis B and C (HBV and HCV) viral infections, dietary aflatoxin B1 poisoning, chronic alcohol abuse, or, most commonly, excessive nutrient-dependent obesity leading to non‐alcoholic steatohepatitis (NASH), play major roles in HCC development.1 Although there are several therapeutic approaches to the treatment of advanced HCC, they have limited clinical impact. For instance, the kinase inhibitor sorafenib improves patient survival but with life extension limited to a maximum of 2–3 months.1 The increasing prevalence of HCC has created an alarming situation.
We recently investigated the molecular mechanisms of HCC using genetically engineered mouse models (GEMMs) for the loss- and gain-of-function of unconventional prefoldin RPB5 interactor (URI).2 URI is member of the R2TP/URI-prefoldin like complex, which contains not only prefoldin subunits, but also RNA polymerase binding subunit 5 (RPB5), ATPases/helicases RuvB-like protein 2 (RUVBL2, also known as 48-kDa TATA box-binding protein-interacting [TIP48] or reptin) and RuvB-like protein 1 (RUVBL1, also known as 49-kDa TATA box-binding protein-interacting [TIP49] or pontin) and co-chaperones such as heat shock protein 90 (HSP90).3,4 URI mammalian function was first identified in vitro and URI was proposed to be a downstream target of the growth factor- and nutrient-regulated mTOR/S6K1 pathway, inhibiting activity of protein phosphatase (PP)1γ to sustain S6K1 survival signaling.5 URI was also described as an “addicting” oncogene that is amplified and overexpressed in various human cancers, including ovarian carcinomas, in which the cancer cells take advantage of upregulated URI to dampen PP1γ activity and provide survival signaling.6 Additionally, xenograft studies further demonstrated the role of URI in HCC development.7
To better understand URI function(s) in vivo, we recently generated conditional URI genetically engineered mouse models (GEMMs).2 Doxycycline (Dox)-switchable whole body gain-of-function URI mutant mice displayed spontaneous tumors in different organs, but prominently in the liver. This first observation in our laboratory suggested that URI may have oncogenic properties in vivo, in particular in liver cancer. Together with the above mentioned studies, and based on the fact that HCC occurs in association with mitochondrial dysfunction-mediated hepatocyte death,8 we used hepatocyte-specific loss-of-function and Dox-switchable hepatocyte gain-of-function (designated hURI-tetOFFhep) models to demonstrate that URI is oncogenic in liver and, induces DNA damage, a key initiating event in HCC development (Fig. 1).2
Figure 1.
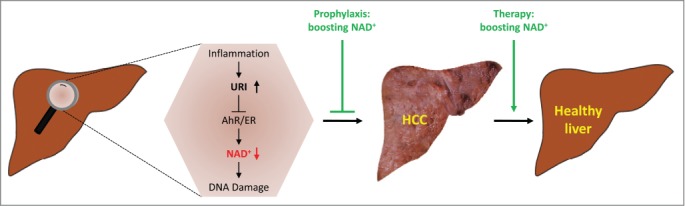
Boosting NAD+ as a strategy to prevent and cure hepatocellular carcinoma. Increased expression of unconventional prefoldin RPB5 interactor (URI) in response to inflammatory cues inhibits aryl hydrocarbon receptor (AhR)- and estrogen receptor (ER)-mediated transcription of enzymes implicated in de novo nicotinamide adenine dinucleotide (NAD+) synthesis, thereby causing DNA damage at early stages of tumorigenesis and hepatocellular carcinoma (HCC) development. Boosting NAD+ pools prevents DNA damage and tumor formation and cures early HCC.
Although in vitro and in vivo studies identified numerous pathways and their components in HCC,9 to date no single animal model described in the literature has encompassed the full spectrum of human HCC progression. The hURI-tetOFFhep mouse represents a unique model to temporally study HCC development. In fact, not only do hURI-tetOFFhep mice recapitulate human HCC during a multistep process but, importantly, these mice also display a human HCC signature associated with hepatitis viral infection.2 hURI-tetOFFhep mice represent the first genetic tool mimicking several features of human HCC, and therefore might be useful to better study human liver dysfunctions.
Mechanistically, we demonstrated that the potential inhibitory effect of URI on PP1γ5,6 does not play a major role in our model. However, URI inhibits aryl hydrocarbon (AhR)- and estrogen receptor (ER)- mediated transcription of enzymes implicated in L-tryptophan/kynurenine/NAD+ metabolism, thereby causing DNA damage to critically initiate hepatocarcinogenesis (Fig. 1).2 URI, in a complex with HSP90, abolishes the trafficking of AhR and ER, but the exact mechanism by which URI/HSP90 modulates AhR and ER complex inhibition and whether other components of the URI prefoldin complex participate in this function remains to be understood. Although crystallographic studies should better define the precise function and role of the R2TP/URI-prefoldin-like complex, we speculate that the URI prefoldin complex, acting through the chaperone function, may either stabilize and suppress AhR and ER nuclear translocation or induce an allosteric conformational change in the AhR and ER active site, preventing ligand binding and thus nuclear targeting of AhR and ER. We do not exclude the possibility that URI might also sterically hinder the AhR and ER ligand binding sites and abolish nuclear translocation and transcriptional activity. Inhibitors against HSP90 are currently being used in clinical trials for cancer treatment.10 Designing therapeutic strategies that inhibit HSP90 chaperone, scaffolding, or cotranscriptional activities should be meticulously considered.
To better prevent HCC and treat patients with liver cancer, we propose boosting NAD+ pools, for instance by nutritional supplementation with the vitamin B3 derivative nicotinamide riboside (NR) (Fig. 1).2 This notion suggests that people subjected to chronic dietary restriction or malnutrition affecting NAD+ concentrations might be predisposed to several diseases, including cancer. We extended our study to pancreatic tumors and demonstrated a conceptual role of oncogene-induced NAD+ depletion in DNA damage.2 Therefore, NR can be used to generally limit tumor growth in mouse models displaying high levels of genotoxic stress. We are currently testing the response to NR of several resected pancreatic human tumors with high levels of genotoxic stress using avatar mouse models. Responses to NR will be taken into consideration for progression into clinical trials and personalization of human cancer therapy.
Interestingly, inhibitors against nicotinamide phosphoribosyltransferase (NAMPT), the enzyme implicated in boosting NAD+ pools via the salvage pathway, are currently being used in several phase II clinical trials as anticancer therapy. Based on our data, this kind of therapy should be considered with caution because reducing NAD+ levels might be harmful through exposing healthy cells to genotoxic stress. To revolutionize cancer treatment, we instead propose that NAD+ boosters in combination with chemotherapeutic drugs might be useful to protect healthy cells against DNA damage and transformation into cancerous cells.
A defect in cell metabolism is therefore a fundamental characteristic for acquiring genomic instability and certainly other hallmarks of cancer. Thus, therapeutic intervention against metabolic alterations prior to development of genomic instability warrants further attention in the prevention and treatment of tumorigenesis. Facilitating the development of more efficient and stable NAD+ “boosters” would offer innovative new avenues and mechanism-based therapeutics to maintain human health and prevent and cure cancer and various associated metabolic dysfunctions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank Jean-Philippe Theurillat (IOR, Bellinzona, Switzerland) for sharing previous observations on URI expression in human HCC samples. I am also thankful to E. Wagner (CNIO, Madrid) and M. Bellahcene (WTCHG, Oxford) for critical reading of this manuscript. N.D is a recipient of the Spanish Ramón y Cajal fellowship.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (SAF2010-18518), the Association for International Cancer Research AICR-UK (11-0242), CNIO (BC1104-08) and the European Foundation for the Study of Diabetes (EFSD).
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118-27; PMID:21992124; http://dx.doi.org/ 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 2.Tummala KS, Gomes AL, Yilmaz M, Grana O, Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, et al.. Inhibition of de novo NAD synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 2014; 26: 826-39; PMID:25453901; http://dx.doi.org/ 10.1016/j.ccell.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, Azzag K, Robert MC, Ahmad Y, Neel H, et al.. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell 2010; 39:912-24; PMID:20864038; http://dx.doi.org/ 10.1016/j.molcel.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gstaiger M, Luke B, Hess D, Oakeley EJ, Wirbelauer C, Blondel M, Vigneron M, Peter M, Krek W. Control of nutrient-sensitive transcription programs by the unconventional prefoldin URI. Science 2003; 302:1208-12; PMID:14615539 [http://dx.doi.org/ 10.1126/science.1088401 [DOI] [PubMed] [Google Scholar]
- 5.Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R, Hess D, Krek W. S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell 2007; 28:28-40; PMID:17936702; http://dx.doi.org/ 10.1016/j.molcel.2007.08.010 [DOI] [PubMed] [Google Scholar]
- 6.Theurillat JP, Metzler SC, Henzi N, Djouder N, Helbling M, Zimmermann AK, Jacob F, Soltermann A, Caduff R, Heinzelmann-Schwarz V, et al.. URI is an oncogene amplified in ovarian cancer cells and is required for their survival. Cancer Cell 2011; 19:317-32; PMID:21397856; http://dx.doi.org/ 10.1016/j.ccr.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Gu J, Zheng Q, Li M, Lian X, Miao J, Jiang J, Wei W. RPB5-mediating protein is required for the proliferation of hepatocellular carcinoma cells. J Biol Chem; 286:11865-74; PMID:21310960; http://dx.doi.org/ 10.1074/jbc.M110.136929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014; 147:765-83; PMID:25046161; http://dx.doi.org/ 10.1053/j.gastro.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol 2013; 7:206-23; PMID:23428636; http://dx.doi.org/ 10.1016/j.molonc.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J; 280:1381-96; PMID:23356585; http://dx.doi.org/ 10.1111/febs.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]


