Abstract
P2X7 (also known as P2RX7) is a plasma membrane receptor for extracellular ATP that is expressed at a high level by immune and tumor cells. Previous data showed that increased P2rx7 expression by tumor cells accelerates tumor progression. We have now looked at the other side of the relationship by investigating the effect of a lack of host P2rx7 expression on tumor growth. Our novel observations highlight a surprising role of host P2rx7 in restraining tumor progression.
Keywords: extracellular ATP, immunity, purinergic receptors, tumors
Extracellular ATP has a crucial role in a multiplicity of pathophysiological conditions, including inflammation and cancer.1 Responses to extracellular ATP are mediated by P2Y and P2X receptors, which participate in different ways in the promotion (or inhibition) of chemotaxis, phagocytosis, cytokine release, cell death, and proliferation. One of the most intriguing P2 receptors is P2X7 (gene symbol P2RX7, protein P2RX7), a peculiar cation-selective plasma membrane channel that, when over-activated, undergoes a channel-to-pore transition responsible for a reversible permeabilization of the plasma membrane to low MW aqueous solutes.2 In the past, P2RX7 has been mostly associated with cytotoxicity and the release of proinflammatory cytokines, primarily interleukin-1β (IL-1β). However, more recently it has become clear that under conditions of low-intensity tonic stimulation this receptor has a potent growth-promoting activity, and in fact has been implicated in tumor progression.3 Evidence supporting a major contribution of P2RX7 to neoplastic growth comes from in vivo experiments showing that silencing or pharmacological blockade of P2rx7 slows down tumor progression and prevents metastatic spread.4,5 Vice versa, forced P2rx7 expression accelerates tumor progression. A correlative finding supporting a role of P2RX7 in tumor growth is the increased expression of P2RX7 by many malignant human cancers.5 The tumor promoting activity of P2RX7 is due to a multiplicity of direct and indirect actions: stimulation of tumor cell growth, activation of cytokine release from tumor-infiltrating inflammatory cells, stimulation of release of immunosuppressive factors from myeloid-derived suppressor cells (MDSCs), stimulation of VEGF release, and enhancement of tumor neovascularization.5,6 The few studies carried out so far have concentrated on investigating the effect of changes in expression or pharmacological blockade of tumor P2rx7 on tumor growth. On the contrary, in a recent study we focused on the role of host P2rx7.7
We were prompted to start this investigation by recent reports underscoring the role of this receptor in the host immune response against allotransplants and in graft-versus-host reactions.8 Our hypothesis was that, given its pivotal role in the immune reaction against allogeneic cells, P2RX7 should also be heavily involved in the immune response against tumors. We anticipated that lack of host P2RX7 would strongly impair antitumor immunity. To verify this hypothesis we analyzed the progression of B16 melanomas and CT26 colon carcinomas in 2 syngeneic mice strains (C57Cl/6 and BALB/c) genetically deleted of P2rx7 (C57Bl/6-p2rx7-KO and BALB/c-P2rx7-KO, respectively) and in the corresponding wild-type (wt) strains.7 Tumor growth was accelerated several fold in the P2rx7-KO strains compared to P2rx7 wt hosts.
Such unrestricted growth was due to an absolute lack of antitumor immune reaction, as documented by the near total absence of intratumor inflammatory cell infiltrate, whether neutrophils, lymphocytes or macrophages. Lack of inflammatory cell infiltration was paralleled by a drastic reduction in plasma and intratumor IL-1β and VEGF levels in P2rx7-KO compared to wt hosts. Bone marrow transplantation experiments showed that unrestricted tumor progression specifically depended on lack of P2rx7 expression in the host immune cells since tumors inoculated into P2rx7-KO mice reconstituted with P2rx7-wt bone marrow showed a growth rate similar to those inoculated into P2rx7-wt host. Accordingly, tumors inoculated into P2rx7-wt mice reconstituted with P2rx7-KO bone marrow showed a growth rate similar to those inoculated into P2rx7-KO host. Deletion of P2rx7 hindered host immune cell responses in several ways, some of which were very surprising. For example, tumor cells were unable to activate dendritic cells (DCs) from the P2rx7-deleted host in an in vitro cytokine release assay and, more surprisingly, immune cells lacking P2rx7 were incapable of chemotaxis in a classical in vitro wound healing experiment. Therefore, it appears that lack of P2rx7 causes a basic inability to mount an immune response against allogeneic cells, cancer cells included.
These results highlight a novel role of P2RX7 in cancer. This receptor has long been associated with apoptosis or necrosis following the pioneering observations published by our laboratory several years ago.9 Subsequently, it has been firmly linked to activation of the NLRP3 inflammasome and to IL-1β release. More recently, we have revealed its growth-promoting activity. It is clear that full understanding of the role of P2RX7 in the host/tumor interaction requires a thorough characterization of the multiple P2RX7-dependent responses on both the host and the tumor sides. As is now clear, P2RX7 has different and opposite effects if expressed on the tumor or the host cells, since tumor P2rx7 potentiates tumor growth whereas host P2rx7 restrains it. Tumor growth inhibition appears to be due mainly, if not exclusively, to facilitation (or even permission) of DC/cancer cell interaction, stimulation of cytokine release, promotion of chemotaxis, and tumor infiltration by inflammatory cells. The different roles of P2RX7 in the promotion of inflammation (on the host side) and the stimulation of proliferation (on the tumor side) might explain why in different experimental models P2rx7 blockade may promote rather than slow down tumor progression.10 Figure 1 provides a schematic rendition of the different and contrasting responses to P2RX7 activation.
Figure 1.
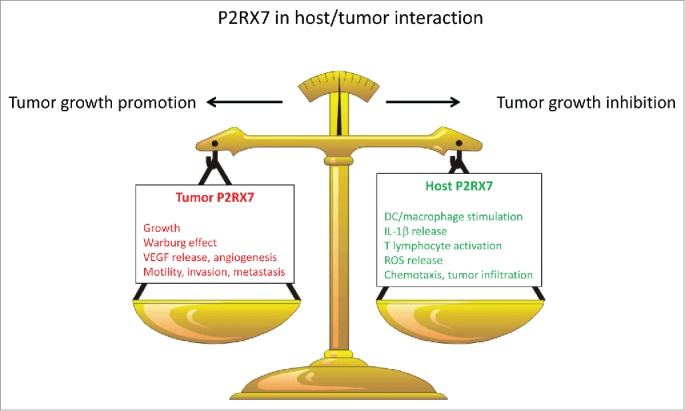
Contrasting roles of the P2X7 receptor (P2RX7) in the host–tumor interaction. Whether tumor (red) or host (green) P2RX7-dependent actions prevail will dictate whether a tumor will progress and metastasize or be successfully restrained by the host immune response. VEGF, vascular endothelial growth factor; DC, dendritic cell; IL-1β, interleukin-1β; ROS, reactive oxygen species.
Given this dual (and contrasting) P2RX7 function in host/tumor interactions, what is the future of P2RX7-targeted anticancer therapy? We believe that despite this complexity P2RX7 is an exceedingly interesting novel target for anticancer therapy. All of our in vivo experiments demonstrate that systemic administration of pharmacologic P2rx7 blockers in P2rx7-wt tumor-bearing hosts has a strong anticancer effect.5,7 In other words, the growth-inhibiting activity due to blockade of tumor P2rx7 prevails over the mild immunosuppressive effect due to inhibition of host P2rx7. This should be no surprise as it is known that mild immunosuppression is the price that must be paid to successfully fight cancer. In conclusion, P2RX7 has a complex and apparently contradictory personality, but there is an intrinsic rationale to its behavior that can be exploited for our benefit.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by grants from the AIRC (n. IG 13025), Telethon (n. GGP06070), ERA-NET Neuron “Nanostroke,” EU COST Program n. BM1406, the Ministry of Health of Italy (n. RF-2011–02348435), the Italian Ministry of Education, University and Research (n. RBAP11FXBC_001) and funds from the University of Ferrara, and was also supported by the 7th Framework Program HEALTH-F2–2007–202231 “ATPBone.”
References
- 1.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol 2014; 99:16–34; PMID:24078669; http://dx.doi.org/ 10.1113/expphysiol.2013.071951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Virgilio F. Dr. Jekyll/Mr. hyde: the dual role of extracellular ATP. J Auton Nerv Syst 2000; 81:59–63; PMID:10869701; http://dx.doi.org/ 10.1016/S0165-1838(00)00114-4 [DOI] [PubMed] [Google Scholar]
- 3.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res 2012; 72:5441–7; PMID:23090120; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1600 [DOI] [PubMed] [Google Scholar]
- 4.Jelassi B, Chantome A, Alcaraz-Perez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 2011; 30:2108–22; PMID:21242969; http://dx.doi.org/ 10.1038/onc.2010.593 [DOI] [PubMed] [Google Scholar]
- 5.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 2012; 72:2957–69; PMID:22505653; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1947 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi G, Vuerich M, Pellegatti P, Marimpietri D, Emionite L, Marigo I, Bronte V, Di Virgilio F, Pistoia V, Raffaghello L. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis 2014; 5:e1135; PMID:24651438; http://dx.doi.org/ 10.1038/cddis.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adinolfi E, Capece M, Franceschini A, Falzoni S, Giuliani A, Rotondo A, Sarti A, Bonora M, Syberg S, Corigliano D., et al.. Accelerated tumor progression in mice lacking the P2X7 receptor. Cancer Res 2015; 75:635-644 PMID:25542861 [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E., et al.. Graft-vs.-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 2010; 16:1434–8; PMID:21102458; http://dx.doi.org/ 10.1038/nm.2242 [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Bronte V, Collavo D, Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5'-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol 1989; 143:1955–60; PMID:2789252 [PubMed] [Google Scholar]
- 10.Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Juhel T, Hebuterne X, Gilson E, Schmid-Allilana A, Boyer O, Adriouch S., et al.. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res 2015; 75:835-845; PMID:25564520 [DOI] [PubMed] [Google Scholar]


