Abstract
Accumulation of type I collagen fibrils in tumors is associated with an increased risk of metastasis. We recently demonstrated that the collagen sensor discoidin domain receptor 1 (DDR1) interacts with type I collagen fibrils to allow proteolysis-based cancer cell invasion through the formation of a new class of invadosomes, termed linear invadosomes.
Keywords: invadosomes, invadopodia, DDR1, type I collagen
Type I collagen is one of the most long-studied proteins, initially because of its abundance; type I collagen is the major matrix component of skin, tendon, and bone. In physiology, type I collagen fibrils play a crucial structural role in maintaining tissue and the overall body. The biochemistry of collagen has been widely studied, and its fibrillogenesis, organization, homeostasis, and cross-linking are highly controlled by cells. Type I collagen in tissues is always organized into fibrils, which promote various processes such as cell adhesion.
In pathophysiological conditions, accumulation of type I collagen may lead to several types of damage such as tissue fibrosis, osteopetrosis, and artherosclerosis. In cancer, the situation is more complex. In some cases, type I collagen fibrils can be considered as a simple physical and structural barrier inhibiting tumor cell growth and dissemination. Indeed, for tumors that are encapsulated, such as primary liver tumors, a capsule composed of type I collagen fibrils encompasses the tumor and inhibits its expansion.1 In these conditions, the presence of the capsule is considered a good prognostic factor for disease evolution. In contrast, type I collagen is overexpressed in a large number of cancers, for example breast and lung cancers, and paradoxically this high expression is correlated with an increased risk of metastasis.2 Collagen overexpression is not the only parameter involved in cancer progression; indeed, the size, diameter, morphology, and cross-linking of type I collagen fibrils all have an impact on tumor cell proliferation and metastatic growth.3,4 Type I collagen degradation is known to be mediated by a limited number of enzymes, including members of the matrix metalloproteinase (MMP) family. In addition, type I collagen fibrils are able to activate MMPs, and especially the coupled membrane-type 1 matrix metalloproteinase (MT1-MMP)/MMP2.5
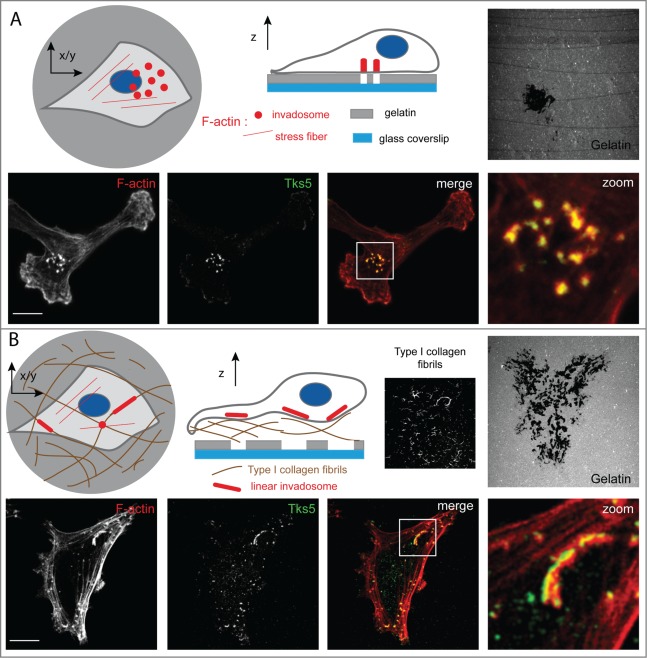
MMPs are key proteins involved in the proteolysis-based invasion that is mediated by invadosomes in cancer cells. Invadosomes are F-actin–based structures that are able to adhere and degrade the extracellular matrix (ECM).6 We analyzed the effect of type I collagen fibrils on invadosome formation. Interestingly, we demonstrated that seeding cancer cells on type I collagen fibrils induced invadosome linearization (Fig. 1). As a result of their organization, which is aligned along the collagen fibrils, we termed these structures linear invadosomes. Type I collagen fibrils promoted linear invadosome formation in all cancer cell lines tested so far, even in cancer cells that were unable to constitutively form invadosomes. The formation of linear invadosomes was associated with an increase in the capacity to degrade ECM, including type I collagen fibrils themselves.7 These data highlighted that type I collagen fibrils are a major inducer of invadosomes in cancer cells.
Figure 1.
Type I collagen induces linear invadosomes. (A) Schematic representation and confocal images of cancer cells seeded on fluorescent gelatin exhibiting classical invadosomes. Invadosomes are organized in individual dots and degrade the underlying extracellular matrix. Shown are representative confocal images of cells stained for tyrosine kinase substrate 5 (Tks5, also known as Fish; green) and filamentous actin (F-actin; red). Gelatin was stained with fluorescein (gray). Panels on the right show a 4× zoom of the white squares. Scale bar: 10 μm. (B) Schematic representation and confocal images of cancer cells seeded on type I collagen fibrils and fluorescent gelatin presenting as linear invadosomes. Linear invadosomes are aligned along fibrils and degrade gelatin and the collagen fibrils themselves. Shown are representative confocal images of cancer cells stained for Tks5 (green) and F-actin (red). Collagen I fibrils were labeled with 546-succinimidyl-ester (gray) and gelatin was stained with fluorescein (gray). Panels on the right show 4x zoom of the white squares. Scale bar: 10 μm.
Molecular analysis of linear invadosomes revealed specific features, such as the absence of the focal adhesion markers vinculin, talin, and integrins usually present in classic invadosomes. However, they retained important invadosome markers such as cortactin, tyrosine kinase substrate 5 (Tks5, also known as Fish), and MMPs. Using several approaches, we determined that β1 integrin was not involved in linear invadosome formation and function,7 raising the question of which type I collagen fibril receptor is implicated. Our recent work established discoidin domain receptor 1 (DDR1) as the collagen sensor involved in linear invadosome formation.8 DDR1 belongs to a family of receptors known to interact with collagens, in particular fibrillar collagens I-III. DDR1 only binds collagens in their native physiological triple-helical conformation and does not recognize denatured collagens such as gelatin. The DDR receptor family is part of the large group of receptor tyrosine kinases (RTKs) and is composed of 2 members, DDR1 and DDR2. Ligand interaction with DDRs promotes tyrosine autophosphorylation as with classic RTKs, although with very slow and persistent kinetics. The DDRs are considered collagen sensors and act not only on tissue homeostasis, but also on many other cellular processes including cell proliferation, differentiation, adhesion, migration, and invasion. Accordingly, a number of recent studies showed that the DDRs are often upregulated in cancers. Notably, DDR1 was found to be overexpressed in lung and breast cancers, where a high expression level was correlated with a poor prognosis and metastasis formation.9
Our recent data demonstrated that DDR1 co-localized with linear invadosomes in tumor cells and was required for their formation and matrix degradation ability.8 Unexpectedly, DDR1 kinase activity was not required for invadosome formation or activity. Indeed, blocking DDR1 kinase activity using nilotinib or blocking antibodies did not inhibit linear invadosome formation and activity. Interestingly, we demonstrated that Src tyrosine kinase inhibition did not affect the formation of linear invadosomes. We further found that the RhoGTPase Cdc42 was activated on collagen in a DDR1-dependent manner. Cdc42 and its specific guanine nucleotide exchange factor (GEF) Tuba co-localized to linear invadosomes, and both are required for linear invadosome formation. Cdc42 involvement is in agreement with the affiliation of these structures with the invadosome family.10 Thus, we determined that upon contact with type I collagen fibrils, cancer cells are able to form linear invadosomes in a DDR1/Tuba-Cdc42–dependent manner to degrade and invade the ECM. Moreover, our results confirmed that DDR1 depletion blocked cell invasion in a collagen-rich environment.
Overall, our work established that type I collagen is an inducer of invadosomes through DDR1 activation via a Cdc42-Tuba pathway. Thus, DDR1 is the sensor used by tumor cells to interact with fibrillar collagen I, leading to the organization of invadosomes that concentrate the proteolytic machinery of the cells, including MT1-MMP to degrade the extracellular matrix, and facilitate invasiveness. Based on their capacity to stimulate cell invasion and the overexpression of DDRs in different cancers, DDR1-dependent linear invadosomes should be a good target for the prevention of metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the members of the Cytoskeleton and Cancer group and our collaborators, who collectively participated to the original manuscripts.
Funding
This work was supported by grants from ANR-13-JJC-JSV1–0005, La Ligue Nationale contre le Cancer and Fondation ARC pour la Recherche sur le Cancer. V.M. is supported by funding from “Equipe Labellisée Ligue Nationale contre le Cancer 2011.”
References
- 1.Wu TH, Yu MC, Chen TC, Lee CF, Chan KM, Wu TJ, Chou HS, Lee WC, Chen MF. Encapsulation is a significant prognostic factor for better outcome in large hepatocellular carcinoma. J Surg Oncol 2012; 105:85–90; PMID:22161900; http://dx.doi.org/ 10.1002/jso.22060 [DOI] [PubMed] [Google Scholar]
- 2.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet 2003; 33:49–54; PMID:12469122; http://dx.doi.org/ 10.1038/ng1060 [DOI] [PubMed] [Google Scholar]
- 3.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al.. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139:891–906; PMID:19931152; http://dx.doi.org/ 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 2013; 73:1721–32; PMID:23345161; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzam HS, Thompson EW. Collagen-induced activation of the M(r) 72,000 type IV collagenase in normal and malignant human fibroblastoid cells. Cancer Res 1992; 52:4540–4; PMID:1322793 [PubMed] [Google Scholar]
- 6.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Ann Rev Cell Dev Biol 2011; 27:185–211; PMID:21801014; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154216 [DOI] [PubMed] [Google Scholar]
- 7.Juin A, Billottet C, Moreau V, Destaing O, Albiges-Rizo C, Rosenbaum J, Genot E, Saltel F. Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Mol Biol Cell 2012; 23:297–309; PMID:22114353; http://dx.doi.org/ 10.1091/mbc.E11-07-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juin A, Di Martino J, Leitinger B, Henriet E, Gary AS, Paysan L, Bomo J, Baffet G, Gauthier-Rouviere C, Rosenbaum J, et al.. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol 2014; 207:517–33; PMID:25422375; http://dx.doi.org/ 10.1083/jcb.201404079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012; 31:295–21; PMID:22366781; http://dx.doi.org/ 10.1007/s10555-012-9346-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Martino J, Paysan L, Gest C, Lagree V, Juin A, Saltel F, Moreau V. Cdc42 and Tks5: a minimal and universal molecular signature for functional invadosomes. Cell Adh Migr 2014; 8:280–92; PMID:24840388; http://dx.doi.org/ 10.4161/cam.28833 [DOI] [PMC free article] [PubMed] [Google Scholar]