Abstract
Taspase 1 (TASP1) cleaves the mixed-lineage leukemia (MLL) and transcription factor (TF) IIA families of nuclear proteins to orchestrate various biological processes. TASP1 is not a classical oncogene, but assists in cell proliferation and permits oncogenic initiation through cleavage of MLL and TFIIA. TASP1 is thus better classified as a “non-oncogene addiction” protease, and targeting TASP1 offers a novel and attractive anticancer therapeutic strategy.
Keywords: Taspase 1, MLL, TFIIA, cyclin, CDK inhibitor
Taspase 1 Cleaves MLL and TFIIA Families of Nuclear Proteins to Orchestrate Various Genetic Programs
Site-specific proteolysis regulates critical aspects of biology. Unlike reversible post-translational protein modifications such as phosphorylation, acetylation, and methylation, proteolysis by degradation or site-specific cleavage renders permanent structural changes, thereby potentially resulting in long-lasting functional consequences. Taspase 1 (threonine aspartase 1; TASP1) was originally identified as a protease responsible for cleaving mixed-lineage leukemia 1 (MLL, also known as KMT2A) protein for proper regulation of HOX gene expression.1,2 TASP1 encodes a highly conserved 50-kDa α−β proenzyme that undergoes intramolecular autoproteolysis, generating a mature α28/β22 heterodimeric enzyme with an overall α/β/β/α structure (Fig. 1).1 TASP1-mediated proteolytic cleavage occurs after distinct aspartate residues of the conserved IXQL(V)D/G motif,3 suggesting that TASP1 evolved from hydrolyzing asparagine and glycosylasparagine to cleaving polypeptides after aspartates. Bona fide TASP1 substrates include MLL1, MLL2 (KMT2B), TFIIAα−β (GTF2A1), ALFα−β (TFIIA-like factor, GTF2A1L), and Drosophila host cell factor 1 (HCF1).1,4-6 Of note, all TASP1 substrates are translated as α−β precursors that undergo proteolysis to form mature α/β heterodimers, like TASP1 itself. Interestingly, all of these substrates are broad-acting nuclear factors that play important roles in transcription regulation, suggesting a critical role for TASP1 in the orchestration of various genetic programs through the cleavage of its substrates.
Figure 1.
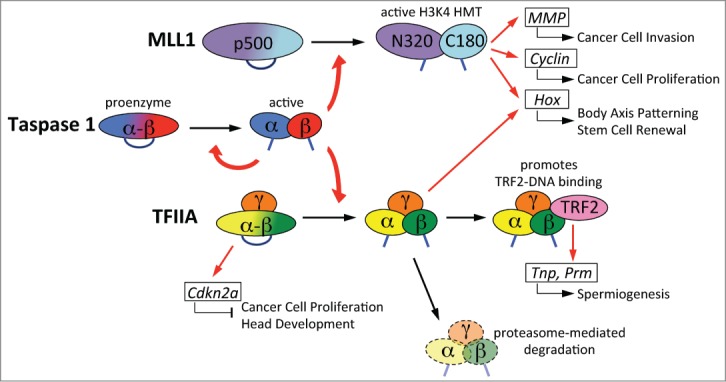
Taspase 1 processes transcription factors that are required for organogenesis, oncogenesis, and tumor progression. The proenzyme Tasp1α–β is activated by intramolecular autoproteolysis, and mature Tasp1 cleaves MLL1 p500 and TFIIAα−β. The mature MLL1 N320/C180 heterodimer has full histone H3 lysine 4 (H3K4) histone methyltransferase (HMT) activity and induces the expression of various genes. Both uncleaved TFIIAα−β and cleaved TFIIAα/β bind to TFIIAγ, encoded by another gene. In contrast to TFIIAα−β/γ, which is a stable protein, mature TFIIAα/β/γ is susceptible to proteasome-mediated degradation. TFIIAα/β/γ is specifically required for the induction of a subset of genes, whereas both TFIIAα−β/γ and TFIIAα/β/γ are active in bulk transcription. MMP, matrix metalloproteinase; Tnp, transition protein; Prm, protamine.
Taspase 1 Plays Critical Roles in the Development of Various Organs
Our initial characterization of Tasp1−/− mice demonstrated that Tasp1 activates the histone methyl transferase activity of MLL1 and MLL2, and that this regulation is important for the subsequent induction of certain Hox and cyclin genes that are crucial for cell fate determination and cell cycle progression, respectively.1,4 Specifically, in the absence of Tasp1 the cell cycle is slowed down with decreased expression levels of cyclins E, A, and B, and increased expression of cyclin-dependent kinase (CDK) inhibitors including p16, p21 and p27, resulting in the smaller body size of Tasp1−/− mice.4 Subsequent mouse genetic studies were performed to clarify which substrate is important for the various phenotypes of Tasp1−/− mice by generating Mll1nc/nc, Mll2nc/nc, and TFIIAα−βnc/nc knockin mouse lines in which the endogenous D/G cleavage residues are replaced with non-cleavable amino acids. These studies revealed that proteolytic cleavage of TFIIAα−β, but not MLL1 or MLL2, is indispensable for male spermiogenesis,7 craniofacial development (Takeda et al., unpublished data), and maintenance of fetal liver hematopoietic stem cells (Niizuma et al., unpublished data) (Fig. 1). These findings highlight the new paradigm that a protease (TASP1) performs a critical function to initiate highly specific genetic programs through the cleavage of TFIIAα−β, a ubiquitously expressed general transcription factor.
Taspase 1 is a Non-Oncogene Addiction Protease in a Variety of Cancers
Since Tasp1 drives cell cycle progression through upregulation of cyclins and downregulation of CDK inhibitors in mice and mouse embryonic fibroblasts (MEFs),4 one might suppose that TASP1 is a potential oncogene. Accordingly, we investigated the importance of TASP1 in tumorigenesis. First, we found that TASP1 is generally overexpressed, but not mutated, in various human cancer cell lines and primary human cancer tissues.8 A deficiency of TASP1 in cancer cells, including glioblastoma and melanoma, enhances apoptosis and disrupts proliferation through decreased levels of antiapoptotic MCL-1 and increased levels of the CDK inhibitors p16, p21, and p27.8 Remarkably, deficiency of TASP1 synergizes with ABT-737, a BCL-2/BCL-XL inhibitor, and other chemotherapeutic agents to kill cancer cells.8 Interestingly, however, TASP1 fails to transform NIH/3T3 cells or primary MEFs, even in cooperation with established oncogenes such as MYC, RAS, and E1A.8 Likewise, Tasp1−/− MEFs demonstrate resistance to oncogenic transformation by classic oncogenic pairs.4 Additionally, in hepatocellular carcinoma cell lines, the full histone methyltransferase activity of MLL1 rendered by cleavage by TASP1 is required for the induction of matrix metalloproteinases (MMPs), which are key effectors of tumor invasiveness driven by the HGF-MET signaling pathway (Fig. 1).9 In summary, TASP1 does not fit the criteria for a conventional oncogene. Nevertheless, it enables full cancer characteristics, suggesting a heavy reliance of cancer cells on TASP1 for tumor maintenance. This kind of molecule is better classified as a “non-oncogene addiction” factor, offering a new class of attractive therapeutic targets for anticancer treatments. Successful application of this strategy in cancer therapy relies on the identification and characterization of tumor dependent, non-oncogenic factors that cancer cells heavily rely on but normal cells do not. Of note, despite the critical roles of Tasp1 in embryonic development, acute deletion of Tasp1 in adult mice does not incur obvious organismal distress and changes in hematopoiesis,3 supporting a potential application of TASP1 inhibitors in the treatment of human cancers. Cancer frequently hijacks key developmental pathways during tumorigenesis and thus frequently exhibits unique properties such as stem cell-like and dedifferentiated states that may underlie the preferential therapeutic benefit conferred by targeting TASP1 to treat cancers.
Targeting TASP1 in vivo
Accordingly, we sought to identify small-molecule TASP1 inhibitors (TASPINs). Using cell-based dual-fluorescent proteolytic screening, an arsenic acid NSC48300 was identified as the most potent and specific TASPIN.3 The sensitivity to NSC48300-mediated growth inhibition is in general agreement with the protein levels of TASP1 in many human cancer cell lines, among which an especially strong correlation is detected in breast and brain cancer cells.3 NSC48300 displays effective tumor growth inhibition in vivo in both the MMTV-neu mouse model of HER2/neu-driven breast cancer and the U251 xenograft model of glioblastoma.3 Moreover, conditional deletion of Tasp1 from mouse mammary glands through the generation of MMTV-neu;MMTV-cre;Tasp1F/− mice completely prevents HER2/neu-driven breast tumorigenesis.10 In accordance with the fact that Tasp1 controls expression of cyclins E, A, and B through the cleavage of MLL1 (Fig. 1),4 MMTV-neu;Mll1nc/nc mice are similarly protected from breast cancer.10 These data suggest that MLL1 is the primary Tasp1 substrate whose cleavage is required for HER2/neu-driven breast tumorigenesis, and that Tasp1 ablation suppresses tumor initiation. Interestingly, however, MMTV-wnt–driven breast cancer is not blocked by Tasp1 deletion, highlighting the various mechanisms underlying individual tumorigenesis.
Future Directions
There are several lines of evidence suggesting that targeting TASP1 could represent a novel and attractive strategy for cancer therapeutics. Current efforts are focused on developing next-generation TASPINs with safer and more specific pharmacological profiles and selecting responsive cancers that might benefit from treatment with anti-TASP1 strategies.10 It is predicted that inhibition of TASP1 will be generally tolerable in adults for the treatment of cancers. Caution should be paid, however, to potential developmental sequelae when treating pregnant women and children because embryonic loss of Tasp1 in mice results in severe perinatal lethality.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hsieh JJD, Cheng EHY, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell 2003; 115:293-303; PMID:14636557; http://dx.doi.org/ 10.1016/S0092-8674(03)00816-X [DOI] [PubMed] [Google Scholar]
- 2.Hsieh JJD, Ernst P, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Proteolytic cleavage of MLL generates a complex of N- and C-terminal fragments that confers protein stability and subnuclear localization. Mol Cell Biol 2003; 23:186-94; PMID:12482972; http://dx.doi.org/ 10.1128/MCB.23.1.186-194.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DY, Lee Y, Van Tine BA, Searleman AC, Westergard TD, Liu H, Tu H-C, Takeda S, Dong Y, Piwnica-Worms DR, et al.. A pharmacologic inhibitor of the protease Taspase1 effectively inhibits breast and brain tumor growth. Cancer Res 2012; 72:736-46; PMID:22166309; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda S, Chen DY, Westergard TD, Fisher JK, Rubens JA, Sasagawa S, Kan JT, Korsmeyer SJ, Cheng EHY, Hsieh JJD. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev 2006; 20:2397-409; PMID:16951254; http://dx.doi.org/ 10.1101/gad.1449406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capotosti F, Hsieh JJD, Herr W. Species selectivity of mixed-lineage leukemia/trithorax and HCF proteolytic maturation pathways. Mol Cell Biol 2007; 27:7063-72; PMID:17698583; http://dx.doi.org/ 10.1128/MCB.00769-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, Spicuglia S, Hsieh JJD, Mitsiou DJ, Høiby T, Veenstra GJC, Korsmeyer SJ, Stunnenberg HG. Uncleaved TFIIA is a substrate for taspase 1 and active in transcription. Mol Cell Biol 2006; 26:2728-35; PMID:16537915; http://dx.doi.org/ 10.1128/MCB.26.7.2728-2735.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyama T, Sasagawa S, Takeda S, Hess RA, Lieberman PM, Cheng EH, Hsieh JJ. Cleavage of TFIIA by Taspase1 Activates TRF2-Specified Mammalian Male Germ Cell Programs. Dev Cell 2013; 27:188-200; PMID:24176642; http://dx.doi.org/ 10.1016/j.devcel.2013.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DY, Liu H, Takeda S, Tu H-C, Sasagawa S, Van Tine BA, Lu D, Cheng EHY, Hsieh JJD. Taspase1 functions as a non-oncogene addiction protease that coordinates cancer cell proliferation and apoptosis. Cancer Res 2010; 70:5358-67; PMID:20516119; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda S, Liu H, Sasagawa S, Dong Y, Trainor PA, Cheng EH, Hsieh JJ. HGF-MET signals via the MLL-ETS2 complex in hepatocellular carcinoma. J Clin Invest 2013; 123:3154-65; PMID:23934123; http://dx.doi.org/ 10.1172/JCI65566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Van Tine BA, Oyama T, Wang PI, Cheng EH, Hsieh JJ. Taspase1 cleaves MLL1 to activate cyclin E for HER2/neu breast tumorigenesis. Cell Res 2014; 24:1354-66; PMID:25267403; http://dx.doi.org/ 10.1038/cr.2014.129 [DOI] [PMC free article] [PubMed] [Google Scholar]


