ABSTRACT
Pluripotent stem cells must be endowed with efficient genome surveillance. Here we describe the multiple mechanisms that ensure their genome integrity, including high susceptibility to apoptosis and efficient prevention of DNA lesions. In induced pluripotent stem cells, apoptosis hypersensitivity is mediated by increased expression of proapoptotic BCL-2 protein, whereas DNA damage is prevented by the upregulation of several antioxidant enzymes. Antioxidants might be therefore employed for safer stem cell therapies.
KEYWORDS: Antioxidant, apoptosis, Bcl-2, DNA damage, genome surveillance, glutathione, LORD-Q, oxidative stress, pluripotent stem cell
Pluripotent stem cells have the capacity to self-renew and to differentiate into all cell types of the organism. Therefore, maintenance of their genomic stability must be stringently controlled, as any genetic alteration could impair their functionality and tissue renewal. Mutations can also favor uncontrolled proliferation or predispose cells to further mutations associated with cancer development. In fact, there is evidence that embryonic and induced pluripotent stem cells (iPSCs) have enhanced tumorigenic potential and share several features with cancer cells.1 Because they originate from differentiated somatic cells, potential genomic instability and tumorigenicity are relevant concerns for iPSCs. A better understanding of genomic surveillance mechanisms is therefore crucial with regard to future therapeutic applications of iPSCs.
To date, the mechanisms that maintain genomic integrity in human iPSCs have been largely elusive. We and others recently found that genome surveillance in pluripotent stem cells is basically achieved by 2 mechanisms, namely a very low accumulation of DNA lesions and a hypersensitivity to apoptosis, which enables rapid removal of cells once DNA damage has occurred.2,3 Compared to differentiated cells such as fibroblasts, iPSCs were found to be exceptionally sensitive to apoptosis and readily died even after exposure to low-damage doses of genotoxic agents. Unlike human embryonic stem cells,4 in iPSCs this apoptosis hypersensitivity is not restricted to genotoxic insults, but is also observed after treatment with agents causing Golgi or ER stress. Interestingly, as a result of the low expression level of death receptors iPSCs are largely resistant to stimuli activating the extrinsic apoptosis pathway. Thus, iPSCs display a selective sensitivity to the mitochondrial death pathway.
To explore the mechanisms underlying this apoptosis-prone state of iPSCs, we investigated the expression of various apoptosis regulators. Transcript levels of members of the inhibitor-of-apoptosis protein family, including XIAP, BIRC2, and particularly BIRC3, were considerably reduced in iPSCs compared to fibroblasts. Human embryonic stem cells have been previously reported to be primed for apoptosis by the presence of constitutively active BAX at the Golgi that can rapidly translocate to mitochondria upon genotoxic insults.4 In human iPSCs, however, BAX is evenly distributed in the cytosol but not localized at the Golgi.2 Nevertheless, in line with constitutive expression of p53 (TP53), mRNA expression of proapoptotic p53 target genes of the BCL-2 family including BAK1, BIM (BCL2L11), and NOXA (PMAIP1) is strongly upregulated, whereas the levels of several antiapoptotic regulators including BCL2, BCLX (BCL2L1), BCLW (BCL2L2), and BCL2A1 are reduced. Thus, iPSCs reveal increased mitochondrial priming and a strong p53 response, resulting in a shift of the balance of from anti- to proapoptotic BCL-2 proteins.
Even the reprogramming of somatic cells to iPSCs is thought to cause DNA damage, which might explain why the absence of p53 improves reprogramming efficiency. Other sources of DNA damage include replication stress or reactive oxygen species (ROS) generated during mitochondrial respiration.5 To assess DNA damage in iPSCs we employed the highly sensitive LORD-Q (long-run real-time PCR-based DNA-damage quantification) method, which detects gene locus-specific DNA lesions of both the mitochondrial and nuclear genome.6 Interestingly, upon exposure to various genotoxic conditions, the accumulation of nuclear and mitochondrial (mt) DNA lesions was significantly lower in iPSCs than in fibroblasts.2 Moreover, comparison with a large panel of tumor cell lines revealed less frequent DNA damage in iPSCs than in transformed cells. Remarkably, when undifferentiated and differentiated iPSCs were exposed to genotoxins, differentiated iPSCs clearly displayed enhanced DNA damage compared to their undifferentiated counterparts, suggesting that protection of pluripotent stem cells against DNA damage is rapidly lost upon differentiation.2
The reduced DNA damage in iPSCs could be mediated by increased expression of DNA repair genes. However, since iPSCs exhibit protection against both nuclear and mtDNA damage, another explanation could be that there is less oxidative damage occurring, possibly due to higher levels of antioxidants. Indeed, measurement of the levels of glutathione (GSH), the most important cellular antioxidant, revealed 3- to 4-fold elevated levels of GSH in iPSCs compared to fibroblasts. Notably, the increase in ROS levels in fibroblasts was up to 10-fold higher, suggesting that oxidative stress is efficiently prevented in iPSCs.
In addition to GSH, iPSCs upregulate the expression of several antioxidant enzymes. For instance, we found that several glutathione S-transferases (GSTs), which act as antioxidant and detoxifying enzymes, were upregulated in iPSCs compared to their somatic precursor cells.2 Most prominent was GSTA2, transcript levels of which were more than 80,000-fold higher in iPSCs than in primary fibroblasts. In addition, iPSCs revealed more than 10,000-fold higher mRNA expression of glutathione peroxidase 2 (GPX2). Furthermore, expression of several peroxiredoxins, which scavenge ROS and organic hydroperoxides, and of glutathione reductase was considerably elevated, further supporting the potent antioxidant status of iPSCs.
In GSH-depleted cells knockdown of GPX2, but not of GSTA2, rendered the cells significantly more vulnerable to DNA damage following hydrogen peroxide exposure.2 Vice versa, ectopic overexpression of GPX2 in fibroblasts was sufficient to confer DNA protection. Interestingly, the expression of GPX2 has been implicated in the proliferation and self-renewal capacity of gastrointestinal crypt stem cells and malignant epithelial cells.7 GPX2 expression is largely controlled by NRF2, an antioxidant transcription factor, which can considerably improve the self-renewal capacity of stem cells.7,8 It is thus conceivable that the strong antioxidant defense is not only involved in genome surveillance, but also required for self-renewal and delayed differentiation of iPSCs.
Several lines of evidence suggest that the occurrence of mtDNA lesions in particular must be prevented for the maintenance of pluripotency. This is for exemplified in mice with defective proof-reading by DNA polymerase γ; these mice exhibit not only an accumulation of mtDNA mutations, but also demonstrate stem cell exhaustion and premature aging.9 Moreover, iPSCs reveal reduced mitochondrial biogenesis and appear to rely more on anaerobic, rather than aerobic, mitochondrial respiration.10 Thus, stem cells maintain low ROS levels not only by their high antioxidant activity, but also by reduced oxygen consumption.
In conclusion, studies by us and others suggest that pluripotent stem cells are able to defend their genomic integrity through their exceptional hypersensitivity to apoptosis as well as by maintaining low ROS levels to prevent DNA damage (Fig. 1). Antioxidant supplementation might therefore improve the safety of iPSCs for future therapeutic applications.
Figure 1.
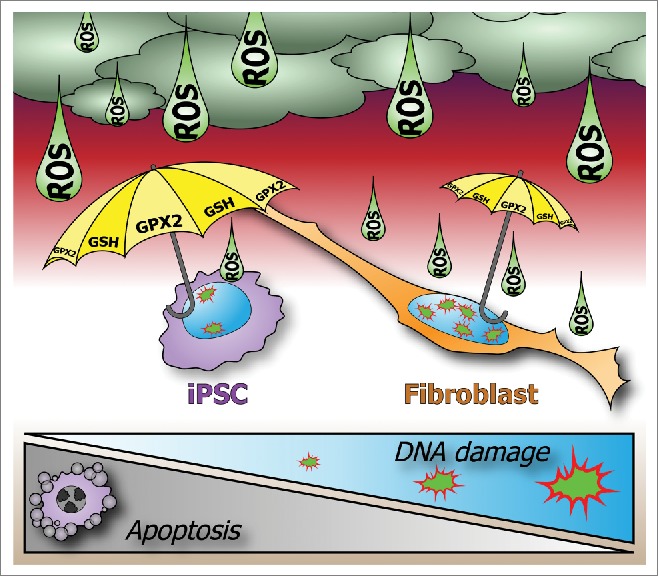
Maintenance of genome integrity in pluripotent stem cells. Pluripotency requires strict genome surveillance to prevent transmission of mutations. Induced pluripotent stem cells (iPSCs) maintain genomic integrity through hypersensitivity to apoptosis and strong protection from DNA damage. Unlike fibroblasts, iPSCs upregulate glutathione peroxidase-2 (GPX2) and glutathione (GSH), which scavenge reactive oxygen species (ROS) and prevent DNA damage.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (SFB 685, GRK 1302) and the Innovation Grant of the Excellence Initiative of the University of Tübingen.
References
- 1.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 2011; 11:268-77; PMID:21390058; http://dx.doi.org/ 10.1038/nrc3034 [DOI] [PubMed] [Google Scholar]
- 2.Dannenmann B, Lehle S, Hildebrand DG, Kübler A, Grondona P, Schmid V, Holzer K, Fröschl M, Essmann F, Rothfuss O, et al. . High glutathione and glutathione peroxidase-2 levels mediate cell-type-specific DNA damage protection in human induced pluripotent stem cells. Stem Cell Reports 2015; 4:886-98; PMID:25937369; http://dx.doi.org/ 10.1016/j.stemcr.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 2010; 28:661-73; PMID:20073085; http://dx.doi.org/ 10.1002/stem.307 [DOI] [PubMed] [Google Scholar]
- 4.Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active bax at the golgi and are primed to undergo rapid apoptosis. Mol Cell 2012; 46:573-83; PMID:22560721; http://dx.doi.org/ 10.1016/j.molcel.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol 2014; 24:268-74; PMID:24721782; http://dx.doi.org/ 10.1016/j.tcb.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehle S, Hildebrand DG, Merz B, Malak PN, Becker MS, Schmezer P, Essmann F, Schulze-Osthoff K, Rothfuss O. LORD-Q: a long-run real-time PCR-based DNA-damage quantification method for nuclear and mitochondrial genome analysis. Nucleic Acids Res 2014; 42:e41; PMID:24371283; http://dx.doi.org/ 10.1093/nar/gkt1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R, Kipp AP. Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. Ann NY Acad Sci 2012; 1259:19-25; PMID:22758632; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06574.x [DOI] [PubMed] [Google Scholar]
- 8.Jang J, Wang Y, Kim HS, Lalli MA, Kosik KS. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells 2014; 32:2616-25; PMID:24895273; http://dx.doi.org/ 10.1002/stem.1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines HL, Turnbull DM, Greaves LC. Human stem cell aging: do mitochondrial DNA mutations have a causal role? Aging Cell 2014; 13:201-5; PMID:24382254; http://dx.doi.org/ 10.1111/acel.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 2010; 28:721-33; PMID:20201066; http://dx.doi.org/ 10.1002/stem.404 [DOI] [PubMed] [Google Scholar]


