ABSTRACT
DNA replication must be tightly regulated to ensure accurate duplication of the genome and its transfer to the daughter cells. When these regulatory mechanisms fail, replicative stress and DNA damage ensue, eventually leading to cell cycle inhibition or cell death. We have recently uncovered that MYCN-dependent expansion of neuroprogenitor cells is accompanied by replication stress, which is restrained by the MRN complex, a direct transcriptional target of the MYCN proto-oncogene.
KEYWORDS: DNA damage response, DNA replication, microcephaly, MRN complex, Neuroprogenitor cells, N-myc, proto-oncogene, Replication stress
DNA replication is a fundamental process that ensures accurate duplication of the genetic information during each cell cycle. Various perturbations originating from endogenous or exogenous sources can interfere with proper progression and completion of the replication process. Coordinated activity of the biochemical components of replication and the DNA damage response (DDR) is essential to sense replication stress (RS), activate the replication checkpoint, and ensure the stability of the forks until replication resumes.
The MRE11/RAD50/NBS1 (MRN) complex is best known as a crucial player in sensing, processing, and repairing DNA double-strand breaks (DSBs),1 but findings describing its role in DNA replication, via stabilization and restart of stalled and collapsed forks, are rapidly accumulating (reviewed in ref. 2).2 Hypomorphic mutations of the MRE11, NBS1, and RAD50 genes are responsible for human disorders collectively classified among the DDR-defective syndromes.1 Common features of these syndromes include developmental and/or degenerative disorders of the nervous system, indicating a pivotal role of the DDR proteins during neuroprogenitor expansion and differentiation and/or for their survival.3 The reason for such frequent involvement of the nervous system in DDR-defective syndromes is currently not understood. An increased sensitivity of developing or differentiated neurons to DNA damage (especially DSBs) has been postulated, but the source of such damage is still a matter of debate.
Recently, we have been struck by the similarities in the phenotypes of two apparently unrelated genetic syndromes that are characterized by moderate to severe microcephaly: Nijmegen Breakage Syndrome, caused by NBN gene mutations, and Feingold syndrome, caused by MYCN haploinsufficiency.4,5 Both syndromes have been reproduced in animal models via central nervous system (CNS)-restricted gene knockouts, which confirmed the essential role of the two proteins in defining brain size and controlling pre- and postnatal cerebellar development.6,7 Indeed, both Mycn and Nbn gene KOs in the CNS are characterized by dramatically impaired proliferation of granule cell progenitors (GCPs) with lack of foliation and disorganized Purkinje cell distribution (Fig. 1). On the basis of this existing groundwork, we speculated that MYCN and the MRN complex (to which NBS1 belongs) might be connected through a unique pathway that is essential for the safe expansion of neuroprogenitor cells, such as GCPs, during development.
Figure 1.
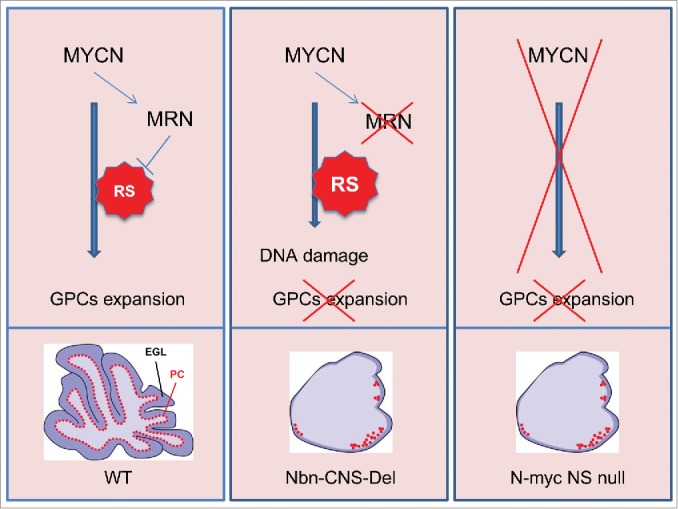
MYCN regulates expression of the MRE11/RAD50/NBS1 (MRN) complex to control the replication stress associated with granule cell progenitor (GCP) expansion during cerebellar development. MYC proteins promote DNA replication by activating the cell cycle machinery, by stimulating gene expression related to metabolic pathways, and by directly regulating replication origin firing. DNA replication under high MYC expression, how- 115 ever, generates replication stress. This has been attributed to (1) a higher density of replicating origins; (2) increased replication fork stalling or collapse; (3) collisions between replication and transcriptional apparatuses; and (4) CDC45 recruitment on replication origins. During the expansion wave of cerebellar 120 GCPs, the high expression of MYCN induces RS and increased expression of the MRN complex, whose function is strictly required to prevent the deleterious effects of RS on DNA integrity. Indeed, MRN inhibition at this level leads to DNA damage accumulation and GCP cell death. According to this model, the 125 cerebellum of the Nbn-CNS-Del KO mouse is characterized by extremely reduced foliation, minimal expansion of GCPs, and reduction and mislocalization of the Purkinje cells (PC). These features are strikingly similar to those observed in the nervous system-restricted N-myc KO mouse.
To address this issue we first investigated the expression of MYCN and the MRN complex components. Indeed, we found that MYCN transcriptionally controls expression of the three components of the MRN complex by directly binding to their promoters at canonical E-boxes.8
Interestingly, overexpression of the MYC oncogenes is known to induce replication stress (RS), DDR activation, and eventually genomic instability via several mechanisms. Indeed, MYC accelerates S-phase execution, increases the number of firing replication origins, alters their spatio/temporal activity, frequently induces collision between replication and transcriptional apparatuses, and induces fork stalling and collapse. Moreover, it directly binds replication origins and also increases CDC45 recruitment on origins (reviewed in ref. 9).9 Although the relevance of MYC-induced RS has been largely investigated in cancer cells, whether it also occurs under more physiologic conditions has not been reported to date. In our recent report, we demonstrated that MYCN induces RS and RS-associated DDR during the physiologic replication of GCPs promoted by activation of the Hedgehog pathway. Moreover, MRN inactivation via NBS1 RNA interference or pharmacologic inhibition of MRE11 exonuclease by mirin resulted in impaired MYCN-dependent cell proliferation. Indeed, MRN inhibition by mirin also resulted in accumulation of DDR markers and replication stress-associated DNA lesions in a MYCN- and S-phase-dependent manner. This occurred in an overexpression model but also during the more physiologic proliferation of primary GCPs stimulated by an Hh agonist. Overall, our findings indicate that: (1) neuronal progenitor cell expansion promoted by the Hh pathway in the postnatal cerebellum is characterized by a relatively high rate of RS imposed by MYCN; and (2) induction of the MRN complex by the same proto-oncogene is essential to restrain the deleterious effects of RS on DNA integrity and thus on cell proliferation/survival (Fig. 1).
Knockout of the NBN gene in mouse embryo fibroblasts causes accumulation of toxic replication intermediates, leading to CHK1 activation and p53-dependent slowdown of cell cycle progression, suggesting the activation of a replication checkpoint.10 Although these findings also strongly support the role of the MRN complex in controlling replication stress, they differ from ours with respect to the outcome of MRN inhibition. Indeed, the functional inactivation of the MRN complex via mirin in MYCN-expressing neural cells failed to cause CHK1 phosphorylation and S phase arrest, but activated ATM, p53, and apoptosis.8 Whether these major differences reflect the different cell context or the different approach to MRN inhibition remains to be established, as does the exact contribution of the MRN complex to activation of the ATR-CHK1-dependent replication stress checkpoint.
In a broader view, our data allow the speculation that waves of neuroprogenitor expansion might be associated with an “intrinsic level” of replication stress requiring an higher activity of DDR proteins (including but not limited to the MRN complex) to avoid the accumulation of replication-induced DNA damage, thus providing a potential explanation for the recurrent neuronal phenotypes of DDR-defective syndromes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was partially supported by grants from the Italian Association for Cancer Research to GG (IG12116 and IG17734). M.P. is a recipient of a fellowship FIRC David Raffaelli.
References
- 1.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 2011; 12:90-103; PMID:21252998; http://dx.doi.org/ 10.1038/nrm3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafrance-Vanasse J, Williams GJ, Tainer JA. Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair. Prog Biophys Mol Biol 2015; 117:182-93; PMID:25576492; http://dx.doi.org/ 10.1016/j.pbiomolbio.\2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinnon PJ. Maintaining genome stability in the nervous system. Nat Neurosci 2013; 16:1523-9; PMID:24165679; http://dx.doi.org/ 10.1038/nn.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrzanowska KH, Gregorek H, Dembowska-Baginska B, Kalina MA, Digweed M. Nijmegen breakage syndrome (NBS). Orphanet J Rare Dis 2012; 7:13; PMID:22373003; http://dx.doi.org/ 10.1186/1750-1172-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcelis CL, Hol FA, Graham GE, Rieu PN, Kellermayer R, Meijer RP, Lugtenberg D, Scheffer H, van Bokhoven H, Brunner HG, et al.. Genotype-phenotype correlations in MYCN-related Feingold syndrome. Hum Mutat 2008; 29:1125-32; PMID:18470948; http://dx.doi.org/ 10.1002/humu.20750 [DOI] [PubMed] [Google Scholar]
- 6.Frappart PO, Tong WM, Demuth I, Radovanovic I, Herceg Z, Aguzzi A, Digweed M, Wang ZQ. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat Med 2005; 11:538-44; PMID:15821748; http://dx.doi.org/ 10.1038/nm1228 [DOI] [PubMed] [Google Scholar]
- 7.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev 2002; 16:2699-712; PMID:12381668; http://dx.doi.org/ 10.1101/gad.1021202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petroni M, Sardina F, Heil C, Sahun-Roncero M, Colicchia V, Veschi V, Albini S, Fruci D, Ricci B, Soriani A, et al.. The MRN complex is transcriptionally regulated by MYCN during neural cell proliferation to control replication stress. Cell Death Differ 2015; PMID:26068589; http://dx.doi.org/ 10.1038/cdd.2015.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohban S, Campaner S. Myc induced replicative stress response: How to cope with it and exploit it. Biochimica Et Biophysica Acta 2015; 1849:517-24; PMID:24735945; http://dx.doi.org/ 10.1016/j.bbagrm.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Bruhn C, Zhou ZW, Ai H, Wang ZQ. The essential function of the MRN complex in the resolution of endogenous replication intermediates. Cell Rep 2014; 6:182-95; PMID:24388752; http://dx.doi.org/ 10.1016/j.celrep.2013.12.018 [DOI] [PubMed] [Google Scholar]


