ABSTRACT
Sirtuin-1 (SIRT1) is associated with longevity and cell survival. Recently, we unveiled a new role of SIRT1 in hepatic ischemia/reperfusion (I/R) injury and identified a novel interaction between SIRT1 and mitochondrial outer membrane protein mitofusin-2 (MFN2), in which SIRT1-dependent deacetylation of MFN2 regulates mitochondria and autophagy in the liver.
KEYWORDS: Autophagy, ischemia/reperfusion, mitochondria, mitofusin 2, sirtuin 1
Abbreviations
- HCC
hepatocellular carcinoma
- I/R
ischemia/reperfusion
- MFN
mitofusin
- MPT
mitochondrial permeability transition
- SIRT1
sirtuin-1
Limited blood flow or tissue ischemia can occur as a result of various pathologic conditions or surgical procedures. Although prolonged ischemia inevitably harms the affected organ, ischemic tissues and cells experience more severe damage upon restoration of blood flow, a paradoxical phenomenon termed ischemia/reperfusion (I/R) injury. A pivotal event in I/R-induced cell death is the onset of the mitochondrial permeability transition (MPT) whereby the inner membrane conductance pores of the mitochondria open, leading to loss of integrity of the permeability barrier.1 MPT onset is associated with uncoupling of oxidative phosphorylation, ATP depletion, and ultimately cell death.1
Autophagy is a major degradative pathway for cytosol and organelle turnover, and is critical for sequestering and degrading potentially cytotoxic proteins and damaged mitochondria that accumulate during I/R.2 Thus, enhancing autophagy is protective against I/R injury, whereas failure to execute autophagy causes greater cellular damage following I/R. The protective role of autophagy during liver I/R has been substantiated by multiple approaches, including starvation prior to ischemia, genetic overexpression of key autophagy proteins, or exposure to pharmacologic autophagy inducers, all of which stimulate autophagy and subsequently increase resiliency against I/R injury.2
Whereas underactivation of autophagy can lead to accumulation of abnormal constituents and consequent cell injury, overactivation of autophagy can also be detrimental. This dual role of autophagy has been further recognized in cancer, and details on the complex mechanisms by which autophagy modulates cell proliferation or responses to chemo/radiation therapy continue to emerge. Autophagy can prevent tumor growth by suppressing the accumulation of dysregulated proteins, or promote tumor survival by providing energy and raw materials to meet the increased metabolic demands related to rapid tumor growth.3
In our recent work, we investigated the role of sirtuin-1 (SIRT1), a deacetylase, in I/R injury.4 Liver biopsies from patients or mice subjected to hepatic inflow occlusion showed depletion of SIRT1 levels (Fig. 1A). The loss of SIRT1 in prolonged ischemia was also observed in isolated primary hepatocytes, and was partially attributable to activation of calpains. Importantly, overexpression of SIRT1 enhanced mitochondrial autophagy, thereby protecting cells against global MPT and necrosis, whereas hepatocytes from SIRT1-null mice showed increased vulnerability to I/R-induced cell death, indicating that SIRT1 depletion contributes to I/R injury.
Figure 1.
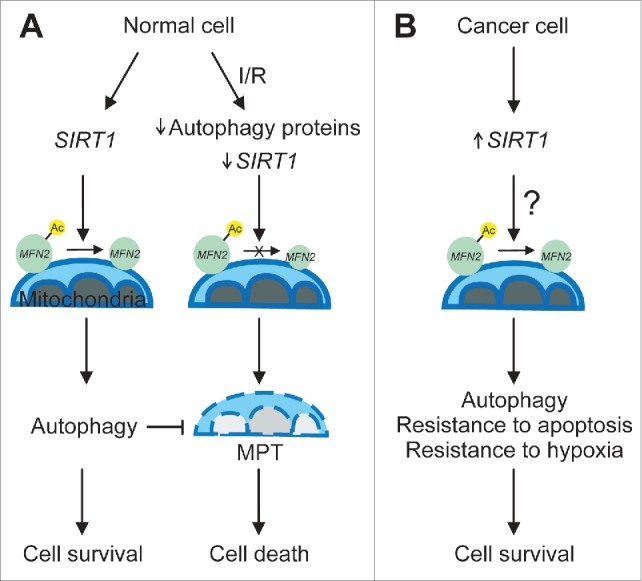
Mechanism of sirtuin 1 (SIRT1)-dependent mitofusin 2 (MFN2) deacetylation in normal and cancer cells. (A) In healthy normal cells, SIRT1 deacetylates MFN2, leading sequentially to enhancement of autophagy, suppression of the mitochondrial permeability transition (MPT), and cell survival. Following prolonged ischemia/reperfusion (I/R), levels of some autophagy proteins and SIRT1 are depleted, thereby blocking MFN2 deacetylation. As a result, SIRT1 loss induces widespread mitochondrial damage, which in turn causes MPT onset and ultimate cell death. (B) In tumor cells, SIRT1 expression is increased and MFN2 might exist predominantly as a deacetylated form. Increased deacetylation of MFN2 in cancer cells could serve as a contributing factor for enhanced autophagy and resistance to apoptotic stimuli and hypoxia, which supports cell survival and hyperproliferation. Ac-MFN2 and MFN2 represent acetylated and deacetylated MFN2, respectively.
We also found profound differences in the acetylation status in the mitochondria after SIRT1 overexpression. Immunoprecipitation analysis revealed that SIRT1 interacts with the mitochondrial outer membrane proteins mitofusins (MFNs), but not with voltage-dependent anion channels. Furthermore, overexpression of SIRT1 increased its association with MFN2, leading to deacetylation of this isoform, whereas no changes were observed in MFN1 acetylation. Since knockdown and deletion mutants of MFN2 abrogated SIRT1-mediated autophagy and cytoprotection against I/R injury, we concluded that MFN2 is a target for SIRT1 and that the acetylation status of this mitochondrial outer membrane protein may play an integral role in I/R injury in the liver. In addition, our study provides evidence that SIRT1, which localizes in the cytosol and nucleus, directly regulates mitochondrial function and adaptive responses to ischemic stress, suggesting an intimate connection between these compartments. Of note, the mitochondria themselves possess 3 different sirtuin isoforms, SIRT3–5.
The importance of SIRT1 in hepatocellular carcinoma (HCC) has been reported.5 Human hepatic tumor tissue and various HCC cell lines have higher levels of SIRT1 compared to non-diseased counterparts. Moreover, silencing of SIRT1 with a small hairpin RNA suppresses the proliferation of HCC cells, implying a positive role of SIRT1 in tumor advancement. Overexpression of SIRT1 has also been reported in other tumors, including colorectal and lung cancer.6,7 Therefore, similar to its role in normal cells, SIRT1 appears to promote cancer cell survival.
Interestingly, some cancer cells appear to overexpress both SIRT1 and MFN2. A recent study in human lung adenocarcinoma demonstrates that levels of MFN2 are markedly higher in cancer tissues than in neighboring normal tissues.8 Furthermore, knockdown of MFN2 in a lung adenocarcinoma cell line delayed cell proliferation and invasion, suggesting a role of MGN2 in tumor promotion. In contrast, studies in other cancer tissues have shown lower levels of MFN2 expression, suggesting a tumor suppressor role. However, as shown in our study, the acetylation/deacetylation status of MFN2, rather than levels of MFN2 itself, may directly govern mitochondrial and cellular integrity. As tumor cells often have smaller and fewer mitochondria with some ultrastructural and membrane potential alterations, it has long been thought that cancer cells predominantly rely on aerobic glycolysis rather than mitochondrial oxidative phosphorylation. However, growing evidence indicates that mitochondria play an essential role in energy production, survival, and proliferation of cancer cells.9,10 It has been well documented that cancer cells are innately resistant to intrinsic or mitochondrial apoptosis. The mechanisms underlying this resistance are multifactorial, including activation of antiapoptotic signaling, inhibition of mitochondrial outer membrane permeabilization, and blockade of the MPT. Since SIRT1-mediated deacetylation of MFN2 prevents onset of the MPT and cell death in ischemic hepatocytes, it is tempting to speculate that high levels of SIRT1 in cancer cells under the microenvironment of hypoxia might be an additional mechanism conferring cytoprotection against apoptotic insults (Fig. 1B).
Overall, our work characterizes MFN2 as a novel substrate of SIRT1 to specifically regulate autophagy in the liver and promote mitochondrial integrity and cell survival after I/R injury. Future work is necessary to elucidate the details of the SIRT1-MFN2 interaction and how these proteins contribute to mitochondrial adaptations to stresses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment of Funding
This work was supported in part by US National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK079879 and DK090115 (to J-S Kim) and National Institute on Aging AG028740 (to J-S Kim).
References
- 1.Kim J-S, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Comm 2003; 304: 463-70; PMID:12729580; http://dx.doi.org/ 10.1016/S0006-291X(03)00618-1 [DOI] [PubMed] [Google Scholar]
- 2.Kim J-S, Nitta T, Mohuczy D, O'Malley KA, Moldawer LL, Dunn WA, Behrns KE. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology 2008; 47:1725-36; PMID:18311843; http://dx.doi.org/ 10.1002/hep.22187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12:401-10; PMID:22534666; http://dx.doi.org/ 10.1038/nrc3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biel TG, Lee S, Flores-Toro JA, Dean JW, Go KL, Lee MH, Law BK, Law ME, Dunn WA Jr, Zendejas I, Behrns KE, Kim JS. Surtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ 2015; PMID:26184910; http://dx. doi.org/ 10.1038/cdd.2015.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, Yu J, Ng HK, Ling MT, Huang AL, Cai XF, Ko BC. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res 2011; 71:4138-49; PMID:21527554; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4274 [DOI] [PubMed] [Google Scholar]
- 6.Lv L, Shen Z, Zhang J, Zhang H, Dong J, Yan Y, Liu F, Jiang K, Ye Y, Wang S. Clinicopathological significance of SIRT1 expression in colorectal adenocarcinoma. Med Oncol 2014; 31:965; PMID:24816737; http://dx.doi.org/ 10.1007/s12032-014-0965-9 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Wang L, Zheng L, Zhan X, Xu B, Jiang J, Wu C. SIRT1 expression is associated with poor prognosis of lung adenocarcinoma. Onco Targets Ther 2015; 30:977-84; PMID:25995644; http://dx.doi.org/ 10.2147/OTT.S82378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou Y, Li R, Liu J, Zhang Y, Zhang X, Jin B, Liu Y, Wang Z, Zhong H, Wen S, Han B. Mitofusin-2 over-expresses and leads to dysregulation of cell cycle and cell invasion in lung adenocarcinoma. Med Oncol 2015; 32:132. PMID:25796500; http://dx.doi.org/ 10.1007/s12032-015-0515-0 [DOI] [PubMed] [Google Scholar]
- 9.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci 2010; 107:8788-93. PMID:20421486; http://dx.doi.org/ 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol 2014; 9:712; PMID:24301801; http://dx.doi.org/ 10.1038/msb.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]


