ABSTRACT
Interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) signaling is considered to have important oncogenic functions in prostate cancer (PCa). However, a recent study highlighted the central role of IL-6/STAT3 signaling in regulation of the ARF–MDM2–p53 senescence axis. This reversal of the postulated oncogenic properties of IL-6/STAT3 signaling in PCa has important therapeutic implications.
KEYWORDS: ARF, CDK4/CDK6 inhibitors, IL-6, Metastasis, Prostate cancer, Senescence, STAT3
Interleukin 6 (IL-6) is a pleiotropic cytokine that is involved in the regulation of many cellular functions. In particular, there are multiple examples of its influence on apoptosis, cell cycle, migration, and angiogenesis. Since IL-6 levels are increased in the tissues and sera of patients with prostate cancer (PCa),4 targeting of this cytokine should be discussed on the basis of scientific results. It is important to know that the signaling pathways of signal transducer and activator of transcription (STAT), mitogen-activated protein kinases (MAPK), and phosphotidylinositol 3-kinase (PI3-K) are upregulated during IL-6 treatment. Whereas there is no doubt that MAPK and PI3-K pathways are oncogenic, the role of STAT3, which is essential for IL-6 downstream action in normal and malignant prostate tissue, is heavily debated. Moreover, STAT3 is crucial for the regulation of cellular events in PCa and there have been many attempts to evaluate its role in this disease. Experimental approaches with antisense oligonucleotides, CpG-STAT3-specific siRNA,5 and anti-IL-610 or anti-IL-6 receptor antibodies9 have been developed, however there is no clear strategy for their application in PCa therapy. Anti–IL-6 therapy did not show any therapeutic or survival benefit in patients with advanced or metastatic PCa. Moreover, anti–IL-6 agents (e.g., siltuximab) or JAK/STAT3 inhibitors were not only ineffective in metastatic PCa, but also enhanced expression of proliferation markers in PCa patients6 or in vivo xenografts.8
LNCaP cells/xenografts are most frequently used in PCa research and contrasting results were reported in vitro and in vivo after IL-6 treatment.4,7 For this reason, we took advantage of a prostate epithelial-specific loss of Pten transgenic mouse model and in addition deleted Stat3 or IL-6. With such a specific in vivo approach, Pencik and associates showed for the first time that ARF is transcriptionally regulated by STAT3. Loss of IL-6 or Stat3 in this PCa model leads to massive metastasis formation and early lethality caused by complete loss of cellular senescence. These data determine the antioncogenic role of the IL-6–Stat3 pathway. Similarly, the loss of STAT3 and/or ARF in a large collection of human PCa patient samples correlated with bad prognosis with high significance. Loss of ARF was twice as sensitive as the Gleason score in predicting PCa patients with bad prognosis. In summary, IL-6–STAT3 signaling is required for regulation of the controlled senescence and cancer progression pathway controlled by ARF–MDM2–p53 (Fig. 1). Thus, this model contradicts the dogma that IL-6–STAT3 signaling has an oncogenic function during PCa development. The study by Pencik and associates therefore calls for in-depth understanding and evaluation of conventional blockade of the IL-6–STAT3 pathway in the treatment of autoimmune disease, underscoring the risk of PCa development. The results should be also considered for re-evaluation of long-term treatments of PCa, rheumatoid arthritis, Castleman’s disease, and Crohn’s disease that rely on blockage of the IL-6 pathway. One should keep in mind that the cells initially inhibited by IL-6 may become predisposed to loss of tumor protein (TP53, best known as p53), accompanied by metastatic progression.8
Figure 1.
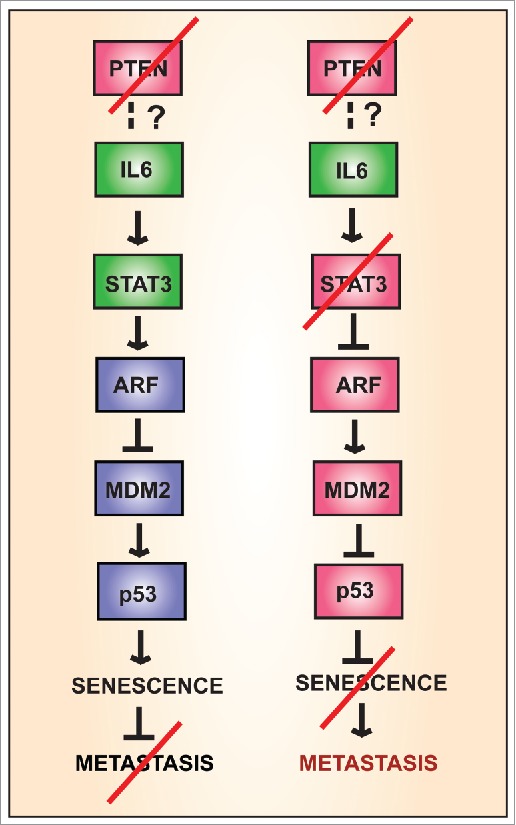
Activation of signaling pathways by interleukin 6 (IL-6) in the presence or absence of the tumor suppressor PTEN. Activation of signal transducer and activator of transcription 3 (STAT3) is associated with inhibition of metastasis.
The results of the study by Pencik and associates should motivate translational researchers to investigate expression of several downstream targets of STAT3-ARF in PCa. The studies should also be extended to tumor-initiating cells in which activation of STAT3 by IL-6 is clearly demonstrated. In addition, STAT3 is involved in cell cycle control and plays an important role during the G1 to S cell cycle transition. The key regulator of G1 phase progression is cyclin D1, a direct STAT3 target gene. Cyclin D1 overexpression is implicated in androgen-independent metastatic PCa.3 Cyclin D1, together with other D cyclins, binds and activates cyclin-dependent kinase 4 and 6 (CDK4 and CDK6) to promote cell cycle progression. The direct connection of cyclin D1 as a known STAT3 target gene with CDK4 and CDK6 favors future studies and possible therapeutic opportunities using selective CDK4/CDK6 inhibitors (Alao et al., 2007). In vitro and in vivo animal studies involving androgen receptor antagonists such as bicalutamide, flutamide, or nilutamide may show synergy with the antitumor effects of CDK4/CDK6 inhibitors such as Palbociclib (PD-0332991).2 Therefore, we aim to conduct studies to identify subgroups of patients with aggressive forms of PCa who might benefit from combined antiandrogens and CDK4/CDK6 inhibitors.
In summary, the key issues in individual PCa patients have to be considered, especially with regard to IL-6–STAT3 as a key tumor suppressor pathway that is indispensible for regulation of the ARF–MDM2–p53 axis and senescence. This paradigmatic change in the mechanistic view of prostate carcinogenesis demonstrates the urgent need for the development of appropriate novel combination therapies and understanding their impact on androgen-independent metastatic PCa.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007. 6:24; PMID:17407548; http://dx.doi.org/ 10.1186/1476-4598-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015. 14:130-46; PMID:25633797; http://dx.doi.org/ 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000. 6:1891-5; PMID:10815912 [PubMed] [Google Scholar]
- 4.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol 2001. 159:2159-65; PMID:11733366; http://dx.doi.org/ 10.1016/S0002-9440(10)63067-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S, Kortylewski M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin Cancer Res 2015. 21:3771-82; PMID:25967142; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S, Reddy M, Puchalski T, Safer K, Prabhakar U, et al. . The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate 2011. 71:1455-65; PMID:21321981; http://dx.doi.org/ 10.1002/pros.21362 [DOI] [PubMed] [Google Scholar]
- 7.Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res 2003. 9:370-6; PMID:12538490 [PubMed] [Google Scholar]
- 8.Pencik J, Schlederer M, Gruber W, Unger C, Walker SM, Chalaris A, Marie IJ, Hassler MR, Javaheri T, Aksoy O, et al. . STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun 2015. 6:7736; PMID:26198641; http://dx.doi.org/ 10.1038/ncomms8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res 2011. 3:177-89; PMID:21625400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH, Keller ET. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res 2006. 66:3087-95; PMID:16540658; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3447 [DOI] [PubMed] [Google Scholar]


