ABSTRACT
Delta-like protein 3 (DLL3) is a novel and tractable tumor-initiating cell-associated target for the antibody-drug conjugate SC16LD6.5 in high-grade pulmonary neuroendocrine tumors. Elevated expression of DLL3, an inhibitor of Notch pathway activation, marks the second recent observation that impairment of Notch receptor signaling may play a critical role in neuroendocrine tumorigenesis.
KEYWORDS: DLL3, Notch, SCLC, TIC, Tumor-initiating cell
High-grade pulmonary neuroendocrine tumors, such as small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), represent a minority of lung cancer cases yet remain among the largest unmet needs, with fewer than 10% of patients surviving 5 years.1 LCNEC has no standard of care, and is increasingly being treated like SCLC. Platinating agents such as cisplatin or carboplatin, together with etoposide, remain the standard-of-care frontline therapy as they been have for more than 30 years.2 Although most patients respond, these responses are short-lived. Topotecan is approved in the second line, but response rates are dismal (˜17%) and physicians dislike the drug because the risk/benefit profile is slim.
Most lung tumors have mutations in cellular tumor antigen p53 (TP53, p53) and are comprised of cells with either epithelial (e.g., non-small cell lung adenocarcinoma) or neuroendocrine (SCLC and LCNEC) features (Fig. 1). Cases have been reported in which epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma transforms into neuroendocrine small cell tumors; both tumor types originating in the same patient have identical p53 mutations but differ in the oncogene driver mutations commonly associated with each lung cancer subtype, which are mutually exclusive.3 Given the known association between activating EGFR mutations in lung adenocarcinoma and retinoblastoma gene (RB1) mutations in SCLC, it stands to reason that a precancerous stem cell harboring p53 mutations may be the cell of origin for both subtypes.
Figure 1.
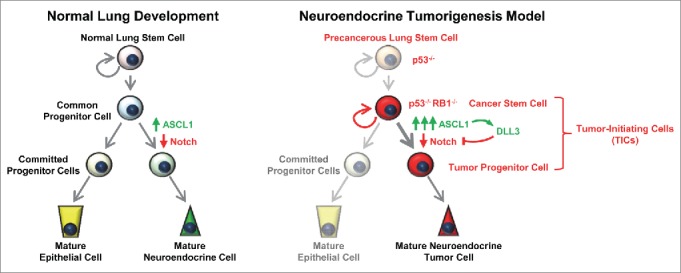
Delta-like protein 3 (DLL3) may mediate achaete-scute complex homolog 1 (ASCL1)-driven cell fate decisions in normal lung development and high-grade pulmonary neuroendocrine tumors. Activation of ASCL1 expression and inhibition of the Notch pathway drive neuroendocrine cell fates in the developing lung (left panel). DLL3 appears to be downstream of ASCL1 and its expression is substantially increased in neuroendocrine tumor-initiating cells, where ASCL1 expression is also increased (right panel). Inhibition of the Notch pathway by upregulated DLL3 may contribute to neuroendocrine tumorigenesis.
In high-grade pulmonary neuroendocrine tumors in which RB1 is mutated, expression of the transcription factor achaete-scute complex homolog 1 (ASCL1) is usually elevated (Fig. 1). ASCL1 plays an important role in dictating neuroendocrine cell fates and correlates with tumor-initiating cell (TIC) capacity.4,5 Inhibition of the Notch pathway similarly regulates neuroendocrine differentiation in the lung.6 Using TICs isolated from SCLC and LCNEC patient-derived xenografts (PDXs), we performed whole transcriptome sequencing and identified DLL3 as significantly overexpressed compared with normal tissues, which was further validated in primary biopsies obtained directly from patients.7 DLL3 is a member of the Notch receptor ligand family that is normally expressed during development and differs from other family members in that it is typically localized to the Golgi. DLL3 interacts with Notch1 and DLL1 in the Golgi (the latter indirectly through the lunatic fringe), retaining and/or redirecting them to endosomes for destruction and thereby preventing them from reaching the cell surface where they can activate Notch signaling in trans.8,9 DLL3 thus appears to be a dominant negative inhibitor of the Notch receptor pathway. DLL3 is transcriptionally regulated downstream of the ASCL1 oncogenic driver in SCLC tumor cells,5 and expression of the 2 genes is significantly correlated in SCLC and LCNEC PDX models.7 DLL3 may thus mediate Notch pathway inhibition downstream of ASCL1 to facilitate neuroendocrine tumorigenesis, - unifying previous observations.
Although the majority of DLL3 protein is intracellular some escapes to the cell surface when overexpressed in tumors, where it is detectable by flow cytometry and immunohistochemistry, and is readily accessible to antibodies. Large tissue microarrays representing 187 SCLC and 57 LCNEC patients showed that roughly 73% of patients have substantial DLL3 expression on the cell surface. Because DLL3 is both available on the surface of tumor cells and not detected in normal tissues, it can be leveraged as a Trojan horse for potent cytotoxin delivery. The antibody-drug conjugate (ADC) SC16LD6.5 (rovalpituzumab tesirine, Rova-T™) is a DLL3-targeted ADC that utilizes a DLL3-specific monoclonal antibody conjugated to a cell-cycle independent pyrrolobenzodiazepine dimer toxin to target and kill DLL3+ tumor cells. In mice bearing SCLC and LCNEC PDX tumors, a single course of therapy over 7 days was able to debulk tumors and prevent recurrence in 7 of 12 models assessed. This antitumor activity was dependent on the ADC, as 30-fold excess naked antibody and the free toxin equivalent had no impact on PDX tumor growth. Moreover, SC16LD6.5 efficacy correlated significantly with DLL3 expression, as determined using an FFPE-compatible anti-DLL3 immunohistochemistry antibody. Although cisplatin/etoposide (C/E) regimens similarly impacted tumor growth over the first few weeks following exposure, tumor recurrence was widespread and rapid—an unfortunate hallmark of high-grade pulmonary neuroendocrine tumors in the clinic, and perhaps not surprising given that C/E treatment was unable to significantly impact TIC frequency. The durable and complete responses observed in mice following SC16LD6.5 treatment suggested that TICs, and more specifically cancer stem cells (CSCs; the self-renewing proportion of TICs), are eliminated by SC16LD6.5, which should translate to more durable responses in the clinic.
The identification of DLL3 overexpression in approximately three-quarters of SCLC patients complements the recent observation that approximately 25% of SCLC tumors have non-synonymous Notch receptor mutations.10 Whether ASCL1-driven DLL3 overexpression is mutually exclusive among patients with these inhibitory Notch receptor mutations is of substantial interest and, if true, would support the argument that Notch pathway inhibition is fundamental to neuroendocrine tumor etiology. Therapeutic intervention with a small molecule targeting inhibitory Notch receptor mutations in an effort to reactivate Notch signaling may not be practical; however, effective targeting of surface DLL3 with an ADC is not only tractable, but already being pursued clinically with SC16LD6.5/Rova-T.
To date, early clinical results from CSC-targeted naked antibody and small molecule therapeutics have failed to impress and/or are difficult to interpret given their co-administration with standard-of-care chemotherapeutic regimens. Targeting CSC with a single-agent ADC designed to seek out and actively destroy the cells underlying tumor growth and recurrence, without the need for combination therapies to debulk or sensitize tumors, should soon provide evidence as to whether this approach will translate into significant and durable responses in cancer patients.
Disclosure of potential conflicts of interest
S.J.D. is a shareholder in Stemcentrx Inc., a privately held and financed company.
References
- 1.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010; 134:1628-38; PMID:21043816 [DOI] [PubMed] [Google Scholar]
- 2.William WN Jr., Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol 2011; 8:611-9; PMID:21691321; http://dx.doi.org/ 10.1038/nrclinonc.2011.90 [DOI] [PubMed] [Google Scholar]
- 3.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al.. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015; 6:6377; PMID:25758528; http://dx.doi.org/ 10.1038/ncomms7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nat 1997; 386:852-5; PMID:9126746; http://dx.doi.org/19176379 10.1038/386852a0 [DOI] [PubMed] [Google Scholar]
- 5.Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, Nelkin BD, Ball DW. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res 2009; 69:845-54; PMID:19176379; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Dev 2012; 139:4365-73; PMID:23132245; http://dx.doi.org/26311731 10.1242/dev.083840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A, et al.. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015; 7:302ra136; PMID:26311731; http://dx.doi.org/ 10.1126/scitranslmed.aac9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet 2011; 20:905-16; PMID:21147753; http://dx.doi.org/ 10.1093/hmg/ddq529 [DOI] [PubMed] [Google Scholar]
- 9.Serth K, Schuster-Gossler K, Kremmer E, Hansen B, Marohn-Kohn B, Gossler A. O-Fucosylation of DLL3 Is Required for Its Function during Somitogenesis. PloS one 2015; 10:e0123776; PMID:25856312; http://dx.doi.org/ 10.1371/journal.pone.0123776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L, Bosco G, et al.. Comprehensive genomic profiles of small cell lung cancer. Nature 2015; 524:47-53; PMID:26168399; http://dx.doi.org/ 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]


