Abstract
The p50 subunit of nuclear factor-kappa B (NF-κB) is generated from processing of the p105 precursor. We identified KIP1 ubiquitination-promoting complex 1 (KPC1) as the ubiquitin (Ub) ligase mediating this process. Overexpression of KPC1 results in tumor suppression, probably due to the generation of p50–p50 homodimers. It appears that high levels of KPC1 and nuclear p50 are important for maintaining the non-malignant state.
Keywords: 26S proteasome, KPC1, NF-κB, p105, tumor growth regulation, ubiquitin
The nuclear factor-kappa B (NF-κB) family of transcriptional regulators is essential for transcription of a variety of genes involved in the immune response, cell survival, proliferation, and differentiation. It is well established that NF-κB plays an important role in linking chronic inflammation and malignant transforma- tion.1
Ubiquitination is an enzymatic reaction in which the C-terminal glycine of the 76-amino acid residue protein ubiquitin (Ub) is covalently conjugated to the target substrate, in most cases through the ε-amino group of an internal lysine residue(s). Ub itself has 7 internal lysine residues (K6, K11, K27, K29, K33, K48, and K63) that together with its N-terminal methionine, can participate in the assembly of different Ub chains linked to the substrate. The resulting diversity essentially generates a “code” that enables a single molecule to regulate many different cellular processes, for example proteasomal degradation (mainly K48-based Ub chains) or protein–protein interactions (mainly linear, K63- and K11-based Ub chains).
Ubiquitination plays a pivotal role in different steps of the NF-κB activation pathway. A variety of proinflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin-1 β (IL-1β), and lipopolysaccharide (LPS) bind to their specific membrane receptors, recruiting a cascade of adaptor proteins that are modified by different Ub chains. In general, there are 2 types of NF-κB activation: ‘canonical’ activation resulting in activation of IκB kinase (IKKα/β) and NF-κB essential modulator (NEMO), degradation of IκBs, and translocation of p50–p65 dimers into the nucleus; and ‘alternative’ activation resulting in activation of IKKα, processing of p100, and translocation of p52–RelB dimers into the nucleus. Here we briefly summarize the involvement of Ub modification in the TNFα-induced ‘canonical’ NF-κB activation pathway (Figure 1).
TNFα binds the tumor necrosis factor receptor 1 (TNFR1), leading to recruitment of TNF receptor-associated protein with death domain (TRADD). The complex with TRADD also includes receptor-interacting protein-1 (RIP1), which is ubiquitinated through K63-based Ub chains by TNF receptor-associated factor (TRAF) and cellular inhibitor of apoptosis protein (c-IAP) following TNFα stimulation. This Ub scaffold recruits NEMO and the TAK1-binding protein (TAB)–TAK1–complex to activate IKK. Alternatively, RIP1 can be modified by K48-based Ub chains, a process mediated by the tumor necrosis factor α-induced protein 3 (A20), and subsequently degraded by the proteasome, thus attenuating the TNFα signal. In addition, c-IAPs catalyze the assembly of K11-based Ub chains on RIP1 that are recognized by NEMO.2 Finally, RIP1 and NEMO are ubiquitinated by “head-to-tail” linear Ub chains in which the C-terminus of Ub is conjugated via a peptide bond to the α-amino group of the first methionine of the previous Ub. These linear Ub chains are synthesized by the linear Ub chain assembly complex (LUBAC) Ub ligase, which is composed of the heme-oxidized IRP2 ligase-1 (HOIL-1L), HOIL-1L–interacting protein (HOIP), and SHANK-associated RH domain interacting protein (SHARPIN). Linear Ub chains have a distinct role in IKK activation.3
IκΒ inhibitory proteins (IκB, p105, and p100) prevent the entry of active NF-κB dimers into the nucleus. Following activation of IKK, phosphorylation and proteasomal degradation of IκB proteins occurs, releasing the dimers. Furthermore, the precursor protein NF-κB1 (p105) can be activated through limited processing; after ubiquitination the C-terminal portion is degraded by the proteasome, yielding p50.4,5 This subunit can further homodimerize or heterodimerize with members of the REL family of proteins, generating the active transcription factor. Proteasomal processing of p105 occurs primarily under basal conditions, requiring prior ubiquitination by what was until recently an unidentified ligase. After stimulation, the protein is phosphorylated by IKKβ on serine residues 927 and 932.6 This modification recruits the SCFβTRCP Ub ligase,7 resulting in complete degradation of p105.8 It appears that the action of another currently unidentified Ub ligase leads to increased processing of the precursor after cell stimulation.
Collectively, these studies indicate that different modes of Ub chains regulate numerous steps of NF-κB activation. Our recent study9 reveals a previously missing link in the NF-κB activation pathway – the ligase that catalyzes processing of the p105 precursor under both basal and stimulated conditions.
We show that the KIP1 ubiquitination-promoting complex (KPC), a heterodimer made of KPC1 (RNF123) and KPC2 (UBAC1), interacts with the precursor protein p105 in an ankyrin repeat-dependent manner. The RING finger domain ligase KPC1 ubiquitinates p105 in vivo and in vitro and promotes both basal and signal-induced proteasomal processing of the precursor.
NF-κB is overexpressed in many tumors and is known to be antiapoptotic and to stimulate the expression of cell cycle promoters and growth factors. Nevertheless, the role of NF-κB in tumorigenesis is still somewhat controversial. In some models, NF-κB possesses strong proinflammatory and antiapoptotic activities, whereas under certain conditions its activation demonstrates a clear tumor suppressor phenotype.10 In our study we demonstrate that overexpression of KPC1 in glioblastoma and breast xenograft models leads to enhanced generation of p50 and consequently to significant suppression of tumor growth. The most probable explanation for this phenomenon comes from analysis of RNA purified from tumors that overexpress either KPC1, the catalytic enzyme, or p50, the product of the processing reaction. This analysis revealed a significant increase in the expression of tumor suppressors, possibly as a result of p50 subunit bias in the cell and generation of p50–p50 homodimers rather than the ‘canonical’ tumorigenic p50–p65 heterodimers. In human head and neck tumors and glioblastoma, we found a significant decrease in KPC1 staining intensity and p50 presence in the nucleus, compared to normal tissue. We hypothesize that KPC1 and p50 are important for maintaining the “normal” state of the cell and preventing its malignant transformation.
Figure 1.
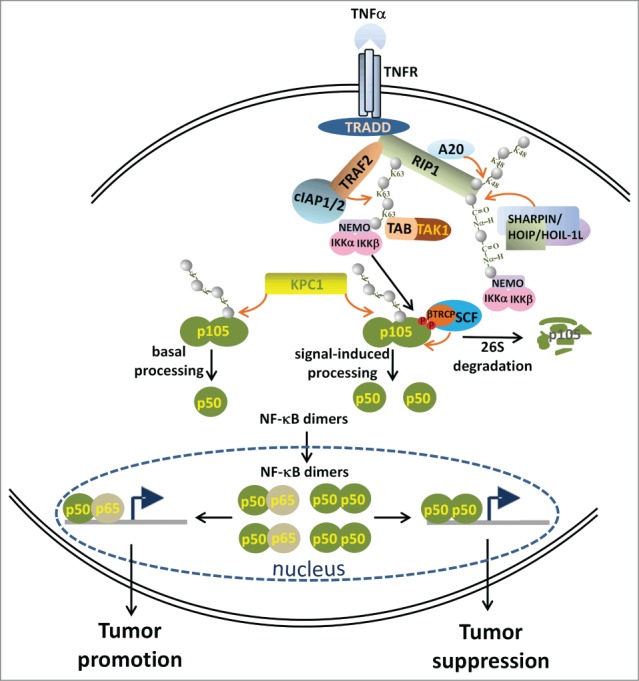
TNFα-induced ‘canonical’ nuclear factor-kappa B (NF-κB) activation pathway. Schematic representation of the involvement of components of the ubiquitin (Ub) system in different steps of NF-κB activation. Please refer to the text for details. The orange arrow denotes ubiquitination. The gray sphere denotes Ub.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Research in the laboratory of A.C. is supported by grants from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF), the Israel Science Foundation (ISF), the I-CORE Program of the Planning and Budgeting Committee and the ISF (Grant1775/12), the EU Treat PolyQ Network, the Nobel Laureates Invitation Program of Seoul National University, the Deutsch-Israelische Projektkooperation (DIP), and the Program for Targeting Cancer by Modulating Protein Dynamics supported by Albert Sweet, Malibu, CA, USA. A.C. is an Israel Cancer Research Fund (ICRF) USA Professor.
References
- 1. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev 2012; 246(1):379-400. [DOI] [PubMed] [Google Scholar]
- 2. Iwai K. Diverse roles of the ubiquitin system in NF-kappaB activation. Biochim Biophys Acta 2014; 1843(1):129-36. [DOI] [PubMed] [Google Scholar]
- 3. Iwai K, Fujita H, Sasaki Y. Linear ubiquitin chains: NF-kappaB signalling, cell death and beyond. Nat Rev Mol Cell Biol 2014; 15(8):503-8. [DOI] [PubMed] [Google Scholar]
- 4. Fan CM, Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature 1991; 354(6352):395-8. [DOI] [PubMed] [Google Scholar]
- 5. Orian A, et al. . Ubiquitin-mediated processing of NF-kappa B transcriptional activator precursor p105. Reconstitution of a cell-free system and identification of the ubiquitin-carrier protein, E2, and a novel ubiquitin-protein ligase, E3, involved in conjugation. J Biol Chem 1995; 270(37):21707-14. [DOI] [PubMed] [Google Scholar]
- 6. Salmeron A, et al. . Direct phosphorylation of NF-kappaB1 p105 by the IkappaB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J Biol Chem 2001; 276(25):22215-22. [DOI] [PubMed] [Google Scholar]
- 7. Orian A, et al. . SCF(beta)(-TrCP) ubiquitin ligase-mediated processing of NF-kappaB p105 requires phosphorylation of its C-terminus by IkappaB kinase. EMBO J 2000; 19(11):2580-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heissmeyer V, et al. . Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol Cell Biol 2001; 21(4):1024-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kravtsova-Ivantsiv Y, et al. . KPC1-Mediated Ubiquitination and Proteasomal Processing of NF-kappaB1 p105 to p50 Restricts Tumor Growth. Cell 2015; 161(2):333-47. [DOI] [PubMed] [Google Scholar]
- 10. Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer 2012; 12(2):121-32. [DOI] [PubMed] [Google Scholar]


