Abstract
Engulfment of apoptotic cells is predominantly executed by phagocytes via the recognition of “eat me” signals like phosphatidylserine (PS). Various PS-specific receptors exist on phagocytes, including Tyro3, Axl, and MerTK receptor tyrosine kinases (TAMs), T-cell immunoglobulin and mucin domain containing 1 and 4 (TIM1/4), and the newly identified CD300 family. The aim of the present auto-commentary is to highlight recent findings regarding the Cd300lf and Cd300lb receptors and their emerging roles in the development of autoimmune disease.
Keywords: CD300 receptors, apoptotic cells, efferocytosis, phosphatidylserine, professional phagocytes, autoimmune disease
Abbreviations
- ACs
apoptotic cells
- BAI1
brain-specific angiogenesis inhibitor 1
- DAP12
DNAX activation protein of 12 kDa
- IRI
ischemia/reperfusion injury
- ITIM
harbor immune-receptor tyrosine-based inhibitory motifs
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PS
phosphatidylserine
- TAMs
Tyro3, Axl and MerTK receptor tyrosine kinases
- TIM1/4
T-cell immunoglobulin and mucin domain containing 1 and 4
Large numbers of apoptotic cells (ACs) are constantly being generated that must be phagocytized by neighboring cells or “professional” phagocytes such as macrophages and dendritic cells.1 If they are not cleared ACs can undergo necrosis; at this stage they become proinflammatory and immunogenic, possibly leading to the development of autoimmune diseases like arthritis and systemic lupus erythematous. Thus, efficient clearance of ACs (efferocytosis) by professional phagocytes is essential for maintaining cellular homeostasis. Once guided to ACs by “find me” signals, phagocytes recognize ACs through their display of characteristic cell surface molecules, known as “eat me” signals.1 The most common “eat me” signal is phosphatidylserine (PS), which when exposed on the outer leaflet of the plasma membrane signals to phagocytes to engulf the ACs. Multiple receptors for PS exist on phagocytic cells, although not necessarily simultaneously, including brain-specific angiogenesis inhibitor 1 (BAI1), Tyro3, Axl, and MerTK receptor tyrosine kinases (TAMs), Stabilin1/2, and T-cell immunoglobulin and mucin domain containing 1 and 4 (TIM1/4) (Fig. 1).1 Most recently, several reports have highlighted the emerging role of the CD300 receptors as a protein family involved in efferocytosis and regulation of the immune response.2-6
Figure 1.
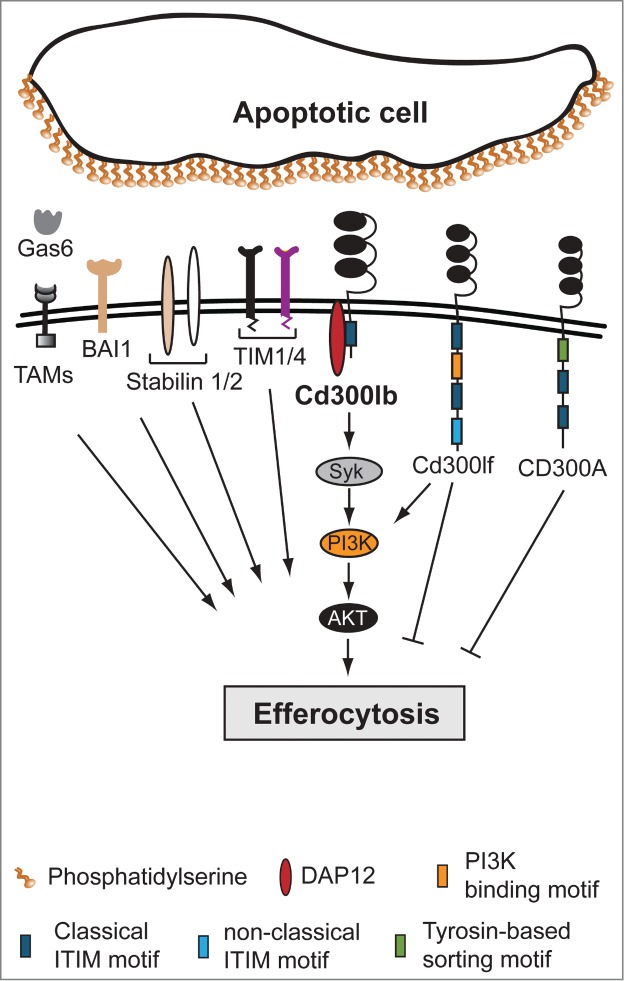
Simplified model for phosphatidylserine receptor-mediated efferocytosis. Apoptotic cells display various ‘eat me’ signals like phosphatidylserine (PS) on the outer leaflet of the plasma membrane that are recognized directly or indirectly (via bridging molecules such as Gas6) by different phagocytic PS receptors, including BAI1, TAMs, Stabilin1/2, TIM1/4, and CD300 family members, such as human CD300A or mouse Cd300lb and Cd300lf. The CD300 receptor family consists of both activating and inhibitory receptors. Inhibitory receptors, like CD300A, harbor immune-receptor tyrosine-based inhibitory motifs (ITIMs) within their intracellular tail to regulate signaling events. Cd300lf functions as an activating receptor via the PI3K pathway or as an inhibitory receptor, depending on cell type. The activating receptor Cd300lb requires binding to an adaptor protein, such as DAP12, to gain signaling capacity and regulates efferocytosis via the DAP12-Syk-PI3K-AKT pathway. BAI1, brain-specific angiogenesis inhibitor 1; DAP12, DNAX activation protein of 12 kDa; Gas6, growth arrest-specific 6; TAM, Tyro3, Axl, and MerTK receptor tyrosine kinase; TIM1/4, T-cell immunoglobulin and mucin domain containing 1 and 4.
The human CD300 and mouse Cd300 family are diverse groups of molecules including both activating and inhibitory receptors that are expressed mainly by myeloid cells.2 Activating receptors, such as CD300C and Cd300lb, require association with adaptor proteins to gain signaling capacity (Fig. 1). Inhibitory receptors, such as CD300A, harbor immune-receptor tyrosine-based inhibitory motifs (ITIMs) within their intracellular tail that regulate the signaling events elicited after ligand recognition. A unique family member, Cd300lf, contains both activating and inhibitory motifs (Fig. 1).
Recent studies provide important insights into the mechanisms by which CD300 molecules recognize their cellular ligands and carry out their immune response-specific functions. For example, CD300A-dependent recognition of PS and phosphatidylethanolamine (PE) results in the inhibition of efferocytosis.7 Cd300lf was shown to bind PS, phosphatidylglycerol (PG), and ceramide,3,4,8 with PS being the predominant physiological ligand.4,8 Importantly, Cd300lf can initiate positive or negative signaling events to regulate efferocytosis, and lack of Cd300lf expression has been associated with the exacerbation of allergic responses and the development of autoimmune diseases.3,4 Intriguingly, whether Cd300lf functions as an activating or inhibitory receptor probably depends on the signaling capability within a cell type; Cd300lf can promote efferocytosis by Cd300lf-overexpressing L929-fibroblasts whereas transfection of Cd300lf into J774 macrophages inhibits their phagocytic activity.4
Cd300lb has been demonstrated to be an activating receptor that gains activation potential by association with the adaptor protein DAP12.9,10 Antibody cross-linking of CD300LB and Cd300lb initiates the production and release of proinflammatory cytokines from mast cells, and promotes cell adhesion to the extracellular matrix.9 However, the precise role of Cd300lb in regulating myeloid cell functions remains elusive, in part due to the unknown nature of its cellular ligand.
Yamanishi et al.5 have identified the PS binding receptors TIM1 and TIM4 as endogenous ligands for Cd300lb, but not PS itself; however, the significance and specificity of these interactions were not clear. The most recent report by Murakami et al.10 provides important insights into Cd300lb ligand identity and its downstream signaling. Using a variety of methods the authors demonstrate that Cd300lb directly recognizes PS, but not other phospholipids, and further show that Cd300lb-mediated efferocytosis is dependent on PS. In agreement with the previous report,5 Cd300lb was shown to bind TIM1- or TIM4-expressing cells. However, in contrast to the previous study, Murakami et al.10 demonstrated that the binding of Cd300lb to TIM1- or TIM4-expressing cells is dependent on PS, as binding was increased in the presence of PS liposomes and Cd300lb failed to associate with either TIM1 or TIM4 in the absence of PS. These findings indicate that Cd300lb does not recognize TIM1 or TIM4 directly but interacts with these proteins indirectly, most likely through bound PS-expressing cell fragments.
Importantly, Murakami et al.10 showed that Cd300lb promotes efferocytosis via its association with DAP12, thereby initiating positive signals that activate the PI3K pathway. Notably, Cd300lb is able to mediate efferocytosis without any other PS receptor present; however, Cd300lb-mediated efferocytosis can be further enhanced through cooperation with TIM1 or TIM4, indicating that Cd300lb likely synergizes with other PS receptors. Unlike most other PS receptors (e.g., BAI1, MerTK), Cd300lb expression is restricted to myeloid cells, suggesting that Cd300lb regulates immune cell responses rather than functioning as a general PS binding receptor. Interestingly, although TIM4 expression is also limited to immune cells, TIM4 does not have any signaling capacity and requires β1 integrins as co-receptors, whereas Cd300lb acts as a signaling molecule that, upon recognition of PS, is able to promote efferocytosis via the DAP12-Syk-PI3K-AKT pathway (Fig. 1).
The role of Cd300lb in the development of autoimmune diseases and/or resolution of inflammation remains unclear. Although the most recent results clearly showed that Cd300lb positively regulates efferocytosis, its exact function remains to be elucidated. Cd300lb-deficient mice are protected from ischemia/reperfusion injury (IRI).5 Since blockade of PS exposure on the cell surface by Annexin V is able to improve kidney and liver IRI, it is tempting to speculate that Cd300lb controls IRI via PS recognition. In addition, Cd300lb-deficient mice are resistant to sepsis-induced lethality.6 The excessive inflammatory responses induced by microbes promote apoptosis of lymphocytes and epithelial cells, suggesting that Cd300lb mediates the pathology of sepsis via the recognition of ACs.
Thus, although the exact function of each of the CD300 family members requires further elucidation, a growing body of evidence suggests that CD300 receptors are emerging as an important new family of proteins that positively or negatively regulate immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author Contributions
The content of this manuscript was discussed with all authors. The manuscript was written by O.H.V., J.E.C. and K.K. and reviewed by all authors.
References
- 1. Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol 2012; 7:61-98; PMID:21910624; http://dx.doi.org/ 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]
- 2. Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood 2013; 121:1951-60; PMID:23293083; http://dx.doi.org/ 10.1182/blood-2012-09-435057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Izawa K, Yamanishi Y, Maehara A, Takahashi M, Isobe M, Ito S, Kaitani A, Matsukawa T, Matsuoka T, Nakahara F, et al. . The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity 2012; 37:827-39; PMID:23123064; http://dx.doi.org/ 10.1016/j.immuni.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 4. Tian L, Choi SC, Murakami Y, Allen J, Morse HC, 3rd, Qi CF, Krzewski K, Coligan JE. p85alpha recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat commun 2014; 5:3146; PMID:24477292; http://dx.doi.org/ 10.1038/ncomms4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamanishi Y, Kitaura J, Izawa K, Kaitani A, Komeno Y, Nakamura M, Yamazaki S, Enomoto Y, Oki T, Akiba H, et al. . TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med 2010; 207:1501-11; PMID:20566714; http://dx.doi.org/ 10.1084/jem.20090581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamanishi Y, Takahashi M, Izawa K, Isobe M, Ito S, Tsuchiya A, Maehara A, Kaitani A, Uchida T, Togami K, et al. . A soluble form of LMIR5/CD300b amplifies lipopolysaccharide-induced lethal inflammation in sepsis. J Immunol 2012; 189:1773-9; PMID:22772446; http://dx.doi.org/ 10.4049/jimmunol.1201139 [DOI] [PubMed] [Google Scholar]
- 7. Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 2012; 119:2799-809; PMID:22302738; http://dx.doi.org/ 10.1182/blood-2011-08-372425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SC, Simhadri VR, Tian L, Gil-Krzewska A, Krzewski K, Borrego F, Coligan JE. Cutting edge: mouse CD300f (CMRF-35-like molecule-1) recognizes outer membrane-exposed phosphatidylserine and can promote phagocytosis. J Immunol 2011; 187:3483-7; PMID:21865548; http://dx.doi.org/ 10.4049/jimmunol.1101549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamanishi Y, Kitaura J, Izawa K, Matsuoka T, Oki T, Lu Y, Shibata F, Yamazaki S, Kumagai H, Nakajima H, et al. . Analysis of mouse LMIR5/CLM-7 as an activating receptor: differential regulation of LMIR5/CLM-7 in mouse versus human cells. Blood 2008; 111:688-98; PMID:17928527; http://dx.doi.org/ 10.1182/blood-2007-04-085787 [DOI] [PubMed] [Google Scholar]
- 10. Murakami Y, Tian L, Voss OH, Margulies DH, Krzewski K, Coligan JE. CD300b regulates the phagocytosis of apoptotic cells via phosphatidylserine recognition. Cell Death Differ 2014; 21:1746-57; PMID:25034781; http://dx.doi.org/ 10.1038/cdd.2014.86 [DOI] [PMC free article] [PubMed] [Google Scholar]


