ABSTRACT
Use of synthetic quantitative array technology led to the identification of positive and negative modulators of rapamycin-induced mitophagy in yeast. The Ubp3-Bre5 deubiquitination complex was shown to inhibit mitophagy but promote other types of autophagy, including ribophagy. We propose an ubiquitin-dependent regulatory switch between different types of autophagy.
KEYWORDS: Autophagy, deubiquitination, mitophagy, ubiquitination, Ubp3
In addition to their well-known metabolic functions and providing sufficient ATP by oxidative phosphorylation, mitochondria play other important cellular roles in processes such as iron-sulfur biogenesis, generation of reactive oxygen species (ROS), and regulation of apoptosis. Not surprisingly, mitochondrial dysfunction is linked to numerous human disorders including neurodegeneration, cancer, and diabetes.1 Pathways that counteract mitochondrial dysfunction are thus of major importance to gain a better understanding of the pathogenesis of these diseases. Selective degradation of dysfunctional mitochondria by mitophagy is one such important pathway.2 This process has attracted tremendous attention in recent years as PINK1 (PTEN induced putative kinase 1), encoding a mitochondrial kinase, and PARK2 (PARKIN RBR E3 ubiquitin protein ligase, best known as PARKIN), which are both known to be mutated in familial forms of Parkinson's disease, were shown to be required for the removal of dysfunctional mitochondria in mammalian cells.3 Several studies have established the critical role of ubiquitination and deubiquitination in the regulation of mitophagy in mammals. The model organism Saccharomyces cerevisiae was successfully applied to identify regulators of mitophagy and 2 genome-wide screens have been performed.4,5 Both studies revealed several genes required for mitophagy, yet only 2 genes overlapped between these studies. One of these was the gene encoding Atg32, a specific receptor located at the outer mitochondrial membrane that is essential for mitophagy. A role of deubiquitination/ubiquitination as known to exist in mammals has not been revealed in yeast until now. One reason for this could be the specific conditions applied for induction of mitophagy using cell growth into the post-log phase.
Rapamycin inhibits the TOR kinase and thereby induces mitophagy in an Atg32-dependent manner6 in addition to the well-known induction of bulk autophagy. We decided to perform a quantitative genome-wide screen for modulators of mitophagy using rapamycin.7 For this, we developed a new method termed synthetic quantitative array technology (SQA). The novel aspect of this technology is the combination of established “synthetic genetic array (SGA)” methodology with a biochemical high-throughput assay, allowing reliable quantification of impaired as well as enhanced mitophagy (Fig. 1). To measure mitophagy we used a modified version of the previously described alkaline phosphatase (ALP)-based assay.6 This assay relies on expression of an inactive proenzyme of alkaline phosphatase targeted to the mitochondrial matrix (mtALP) that can only become activated by the vacuolar proteinase A. Application of SQA technology followed by 2 consecutive rounds of validation led to the identification of 86 positive and 10 negative regulators of mitophagy.7 In addition, by SQA-based analysis of non-selective autophagy we found that 63 of these regulators are specific for mitophagy and 33 regulate autophagy in general. Parallel quantitative analysis of mitophagy and autophagy revealed an interesting subset of candidate genes that inversely affect mitophagy and bulk autophagy.
Figure 1.
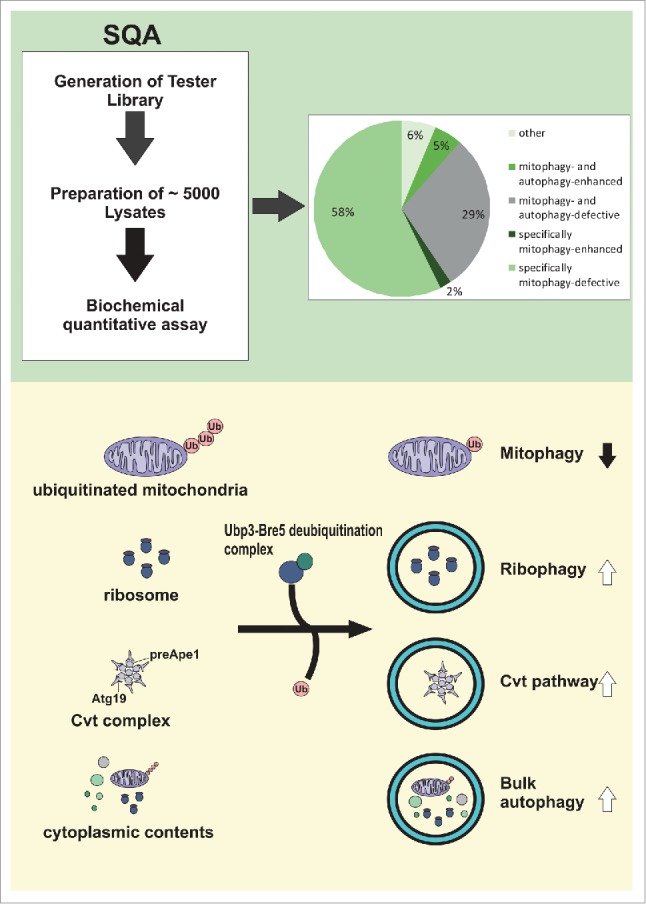
Genome-wide screen for modulators of rapamycin-induced mitophagy by synthetic array technology (SQA). Principle of SQA technology (top, left) and results of the quantitative genome-wide screen are shown (top, right). The Ubp3-Bre5 deubiquitination complex was identified as a negative modulator of mitophagy but a positive modulator of ribophagy, the cytoplasm-to-vacuole targeting (Cvt) pathway, and non-selective bulk autophagy (bottom).
We demonstrated that the Ubp3-Bre5 deubiquitination complex inhibits mitophagy but conversely promotes autophagy.7 We further showed that the same complex is a positive regulator of other types of selective autophagy such as the cytoplasm-to-vacuole targeting (Cvt) pathway and ribophagy. The latter finding is consistent with previously published results for ribophagy under starvation conditions.8 The role of deubiquitination in specifically modulating distinct autophagy pathways is remarkable in several respects. First, Ubp3/Bre5 was identified as the first negative regulator of mitophagy in S. cerevisiae. Second, a reciprocal regulation between mitophagy and other types of autophagy, including selective types thereof, has not been observed before and strongly implies the existence of a regulated switch that determines whether mitophagy or other types of autophagy are preferred. Third, it opens the possibility of using S. cerevisiae as a model to study the regulation of mitophagy by ubiquitination and deubiquitination. This notion is supported by a recent study using heterologous expression of human PARKIN in S. cerevisiae.9 We further showed that Ubp3/Bre5 dynamically translocates to mitochondria upon addition of rapamycin. The physiological importance of this mechanism could relate to the fact that mitochondria are essential organelles that cannot be generated de novo. It is thus entirely possible that deubiquitination of mitochondria evolved to prevent the complete or excessive removal of these organelles. This is of particular importance for post-mitotic tissues that are often affected in diseases linked to mitochondrial dysfunction.1 The antagonistic effects of Ubp3/Bre5 on mitophagy versus other types of autophagy (e.g., ribophagy) also make physiologic sense. Under conditions of mitochondrial dysfunction cells would preferentially focus on the removal of damaged mitochondria rather than degrade ribosomes or other cargo at the same time. Conversely, the removal of any existing stalled or defective ribosomes is certainly a high priority function that needs to be performed even at the expense of impaired mitophagy. Future studies are certainly needed to decipher the complex regulation of molecular switches for distinct autophagy pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005; 39:359-407; PMID:16285865; http://dx.doi.org/ 10.1146/annurev.genet.39.110304.095751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 2008; 27:306-14; PMID:18216873; http://dx.doi.org/ 10.1038/sj.emboj.7601972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12:9-14; PMID:21179058; http://dx.doi.org/ 10.1038/nrm3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell 2009; 17:87-97; PMID:19619494; http://dx.doi.org/ 10.1016/j.devcel.2009.06.013 [DOI] [PubMed] [Google Scholar]
- 5.Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell 2009; 17:98-109; PMID:19619495; http://dx.doi.org/ 10.1016/j.devcel.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendl N, Occhipinti A, Müller M, Wild P, Dikic I, Reichert AS. Mitophagy in yeast is independent of mitochondrial fission and requires the stress response gene WHI2. J Cell Sci 2011; 124:1339-50; PMID:21429936; http://dx.doi.org/ 10.1242/jcs.076406 [DOI] [PubMed] [Google Scholar]
- 7.Müller M, Kotter P, Behrendt C, Walter E, Scheckhuber CQ, Entian KD, Reichert AS. Synthetic quantitative array technology identifies the ubp3-bre5 deubiquitinase complex as a negative regulator of mitophagy. Cell Rep 2015; 10:1215-25; PMID:25704822; http://dx.doi.org/ 10.1016/j.celrep.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 8.Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol 2008; 10:602-10; PMID:18391941; http://dx.doi.org/ 10.1038/ncb1723 [DOI] [PubMed] [Google Scholar]
- 9.Pereira C, Costa V, Martins LM, Saraiva L. A yeast model of the Parkinsons disease-associated protein Parkin. Exp Cell Res 2015; 333:73-9; PMID:25728007; http://dx.doi.org/ 10.1016/j.yexcr.2015.02.018 [DOI] [PubMed] [Google Scholar]


