Abstract
Hypoxia-inducible factor 1 (HIF-1) promotes glycolysis in cancer cells, hence sustaining survival. We recently reported that HIF-1 suppresses fatty acid β-oxidation in malignant cells through medium- and long-chain acyl-CoA dehydrogenases. This promotes tumor progression by controlling the level of reactive oxygen species and via crosstalk between metabolism and PTEN signaling.
Keywords: HIF-1, fatty acid catabolism, PTEN
Louis Pasteur discovered in 1857 that aerating yeast broth caused yeast cell growth to increase, while conversely decreasing the fermentation rate. This phenomenon, now known as the Pasteur effect, involves the transition from fermentation (anaerobic glycolysis) to oxidative respiration (oxidative phosphorylation) in the presence of O2. However, when studying the metabolism of cancer cells, Otto Warburg observed that most cancer cells predominantly produced energy by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, rather than by oxidation of pyruvate in mitochondria as in most normal cells. Warburg reported that malignant tumor cells typically had a glycolytic rate up to 200 times higher than that of their normal tissues of origin, and that this occurred even if oxygen was plentiful. Warburg called this process aerobic glycolysis, and it is now universally known as the Warburg effect.1
Regarding the underlying mechanisms, the Warburg effect was originally assumed to be a consequence of damage to the mitochondria in cancer cells. As most cancer cells proliferate under low-oxygen environments (hypoxia) within tumors it is now believed that, as an adaptation to hypoxic stress, alteration of cancer genes enhances glycolysis while shutting down mitochondrial respiration because mitochondria are involved in the cellular apoptosis process that would otherwise kill cancer cells. Early evidence demonstrated that hypoxia-inducible factor 1 (HIF-1) is behind this adaptation. HIF-1 regulates glucose transporters 1 and 3 (GLUT1, GLUT3) and other glycolytic enzymes such as hexokinases (HK1 and HK2) and phosphoglycerate kinase 1 (PGK1) to enhance the flux from glucose to pyruvate. HIF-1 modulates lactate dehydrogenase A (LDHA) to promote lactate production and generation of the electron acceptor NAD+ at the last step of glycolysis. Recent studies by Kim et al. and Papandreou et al. showed that HIF-1 activates pyruvate dehydrogenase kinase isozyme 1 (PDK1), which blocks the conversion of pyruvate to acetyl-CoA,2,3 thus attenuating mitochondrial respiration. We and others also found that the HIF-1 pathway regulates mitochondrial biogenesis via suppression of PGC-1β through the oncogene C-MYC and the induction of mitophagy through activation of BNIP3.4,5 Those results shed light on the mechanism underlying the Warburg effect by providing evidence that oncogenic HIF-1 shunts pyruvate entry into the TCA cycle via acetyl-CoA, thus avoiding cell death under hypoxic conditions.
During cancer progression, cancer cells usually adopt a comprehensive metabolic reprogramming that goes well beyond glycolysis.6 In fact, changes in the metabolism of amino acids or lipids in cancer cells have been extensively described.7,8 Lipid catabolism is known to be linked to that of glucose and amino acids via the central metabolic hub of the TCA cycle. If it is necessary to block the entry of glucose into the TCA cycle to yield acetyl-CoA, we wondered what happens to the catabolism of fatty acids through fatty acid β-oxidation (FAO), which provides another major source of acetyl-CoA, under hypoxic stress.
In a recent study by Huang et al., we started to investigate whether hypoxic stress regulates lipid metabolism in human hepatocellular carcinoma cells and found that hypoxic stress led to significant lipid accumulation in cancer cells that is dependent on HIFs. To address the mechanisms underlying hypoxia-induced lipid accumulation, we screened for genes related to lipid metabolism that are regulated by hypoxia or HIFs. This PCR array-based screening led to the discovery of downregulation of several lipid catabolism genes, especially medium-chain acyl-CoA dehydrogenases (MCAD) and long-chain acyl-CoA dehydrogenases (LCAD), under hypoxic conditions in Hep3B and HepG2 cells and also in PC3 prostate cancer cells.9 Further study revealed a role of the HIF-1/C-MYC/PGC-1β regulatory axis in this hypoxia-mediated regulation of MCAD and LCAD by which HIF-1 suppressed β-oxidation (FAO) in hypoxic cancer cells. As expected, we observed that suppression of MCAD and LCAD resulted in decreased levels of cellular reactive oxygen species (ROS) that partially contributed to enhanced cancer progression. However, a surprising discovery was that that suppression of LCAD, but not MCAD, led to more significant tumor growth in vivo that involved the phosphatase and tensin homolog (PTEN) signaling pathway.9
This study extends our understanding of how HIF-1 switches cancer metabolism under hypoxic conditions. Previous studies have documented that HIF-1 enhances glycolytic metabolism by upregulating glucose transporters and glycolytic enzymes to enhance the flux in glycolysis, inducing PDK1 to block the conversion of pyruvate to acetyl CoA, activating LDHA to convert pyruvate to lactate, and repressing mitochondrial biogenesis (Fig. 1). We further established that HIF-1 inhibits catabolism of fatty acids by repressing LCAD and MCAD; this leads to accumulation of fatty acids, which blunts PTEN expression and enhances cancer cell proliferation (Fig. 1). We now have a more comprehensive view of the role of HIF-1 in regulating the Warburg effect and overall cancer metabolic reprogramming. Further investigation is warranted to identify novel strategies for targeting these metabolic pathways and HIF-1 signaling for cancer therapies.
Figure 1.
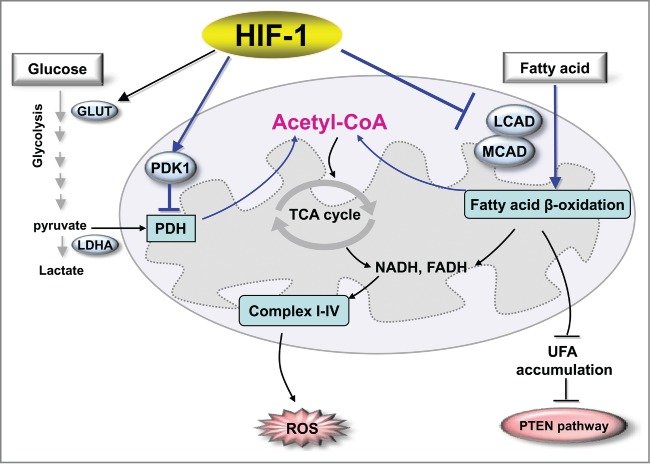
HIF-1 regulates the metabolic reprogramming of cancer cells. For glucose metabolism, HIF-1 enhances glycolytic metabolism by upregulating glucose transporters and glycolytic enzymes to enhance the flux of glycolysis, by inducing PDK1 to block the conversion of pyruvate to acetyl CoA, and by activating LDHA to convert pyruvate to lactate. In the regulation of lipid metabolism, HIF-1 inhibits catabolism of fatty acids by repressing LCAD and MCAD, leading to suppression of ROS levels and accumulation of fatty acid that blunts PTEN expression and enhances cancer cell proliferation. Abbreviations: HIF-1, hypoxia-inducible factor 1; GLUT, glucose transporter; LDHA, lactate dehydrogenase A; PDK1, pyruvate dehydrogenase kinase isozyme 1; PDH, pyruvate dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase, UFA, unsaturated fatty acid; PTEN, phosphatase and tensin homolog; TCA, tricarboxylic acid cycle; ROS, reactive oxygen species.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Our research work was supported in part by the National Basic Key Research Program of China (2014CB910604), Research Fund for the Doctoral Program of Higher Education of China (20133402110020), and the Fundamental Research Funds for the Central Universities in China.
References
- 1. Warburg O. On the origin of cancer cells. Science 1956, 123:309–14; PMID:13298683 [DOI] [PubMed] [Google Scholar]
- 2. Kim J, Tchernyshyov I, Semenza G L, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism 2006; 3:177-85; PMID:16517405; http://dx.doi.org/ 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 3. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell metabolism 2006; 3:187-97; PMID:16517406; http://dx.doi.org/ 10.1016/j.cmet.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 4. Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of c-Myc activity. Cancer Cell 2007; 11:407-20; PMID:17482131; http://dx.doi.org/ 10.1016/j.ccr.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 5. Zhang H, Marce MB, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 2008; 283: 10892-903; PMID:18281291; http://dx.doi.org/ 10.1074/jbc.M800102200 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Vander Heiden MG , Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/ 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao P, Tchernyshyov I, Chang T, Lee YS, Kita K, Ochi T, Zeller K, De Marzo AM, Van Eyk JE, Mendell JT, et al. . c-Myc suppression of miR-23 enhances mitochondrial glutaminase and glutamine metabolism. Nature 2009; 458:762-5; PMID:19219026; http://dx.doi.org/ 10.1038/nature07823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348:1625-38; PMID:12711737 [DOI] [PubMed] [Google Scholar]
- 9. Huang D, Li T, Li X, Zhang L, Sun L, He X, Zhong X, Jia D, Song L, Semenza GL, et al. . HIF-1-Mediated Suppression of Acyl-CoA Dehydrogenases and Fatty Acid Oxidation Is Critical for Cancer Progression. Cell Rep 2014; 8:1930-42; PMID:25242319 [DOI] [PubMed] [Google Scholar]


