ABSTRACT
Oxygen sensing is associated with mitochondrial function. EglN2, which contributes to breast tumorigenesis as a prolyl hydroxylase, has recently been identified as a transcription co-activator through interaction with NRF1 and PGC1α to regulate mitochondrial function under conditions of normoxia and hypoxia. FDXR is the important downstream target gene that mediates this regulation.
Keywords: Breast cancer, EglN2, FDXR, hypoxia, mitochondria
Hypoxia, one of the most important characteristics of the majority of solid tumors, is associated with metabolic reprogramming.1,2 The prolyl hydroxylases EglN1 (also called PHD2), EglN2 (PHD1), and EglN3 (PHD3) are important oxygen sensors that are responsible for the hydroxylation of hypoxia inducible factor α (including HIF1α and HIF2α).3 Hydroxylated HIFα is further recognized by the von Hippel Lindau (VHL) E3 ligase complex and targeted for proteasome-mediated degradation. Under hypoxia, EglNs lose their ability to hydroxylate HIFα, which leads to HIFα stabilization and dimerization with HIF1β (ARNT). This dimeric complex binds to target gene promoters that contain the hypoxia response element (HRE) and regulates their transcription. Many of these genes play important roles in cell proliferation, metabolism, and angiogenesis.3 Therefore, EglNs are well known to regulate a plethora of transcription cascades by serving as oxygen sensors. However, the functional link between oxygen sensing, mitochondrial function, and metabolism remains largely unexplored.
As an estrogen receptor (ER)-inducible gene, EglN2 contributes to breast tumorigenesis. Our previous work demonstrated that depletion of EglN2 decreases cell proliferation and breast tumorigenesis in an orthotopic breast cancer model.4 Mechanistic studies suggest that EglN2 positively regulates cyclin D1 transcription through hydroxylation of FOXO3a in a HIF-independent manner.4,5 Our recent research demonstrates that EglN2 regulates mitochondrial function in ERα-positive breast cancer.6 Depletion of EglN2, but not EglN1 or EglN3, specifically leads to decreased mitochondrial function in ERα-positive breast cancer cells. More strikingly, this regulation is specific for cancer cells, but does not occur in normal human mammary epithelial cells (HMECs) or murine embryonic fibroblasts (MEFs). In addition, the effect of EglN2 on mitochondria may be independent of EglN2 catalytic activity since both wild type (WT) and the catalytic dead EglN2 H358A mutant can increase the oxygen consumption rate (OCR) and mitochondrial DNA (mtDNA) levels in these cells. In accordance with this finding, we found that EglN2 also regulates mitochondrial function under hypoxia and, interestingly, with a more robust effect than under normoxia. Our further experiments suggest that the effect of EglN2 on mitochondrial function is independent of HIF1/2α.
How does EglN2 regulate mitochondrial function?
We found that EglN2 localizes in the cytoplasm as well as the nucleus. More importantly, it is enriched in nuclear and chromatin-bound fractions upon exposure to hypoxia. Integrative analyses of ChIP-Seq and gene expression profiling for EglN2 reveals enrichment of the nuclear respiratory factor 1 (NRF1) transcriptional factor binding motif in genes that are positively regulated by EglN2. Consistent with these findings, further study revealed that binding of EglN2 with NRF1 and PGC1α takes place on chromatin, with a stronger binding pattern under hypoxia. Since the interaction between EglN2 and NRF1/PGC1α and the regulatory activity of EglN2 on mitochondrial function are independent of HIF1/2α, the factors that mediate EglN2 translocation onto the chromatin remain unclear. Our ongoing studies in this field may identify novel therapeutic targets that interfere with EglN2 translocation, therefore inhibiting its transcription activity and decreasing breast tumorigenesis.
We originally hypothesized that by binding with the NRF1 and PGC1α complex EglN2 may regulate canonical NRF1 downstream genes involved in mitochondrial biogenesis such as mitochondrial RNA polymerase (POLRMT), TFAM, TFB1M, and TFB2M.7 However, our results showed that neither EglN2 nor NRF1 depletion affects the transcription of these factors. Instead, we identified several other mitochondrial-related targets from EglN2 ChIP-Seq and microarray analyses. Among them, ferredoxin reductase (FDXR) was the most robustly regulated by both EglN2 and NRF1. FDXR is a mitochondrial flavoprotein that regulates the electron transport from NADPH to cytochrome p450 family members.8 Our experiments demonstrate that FDXR at least partially regulates the effect of EglN2 on mitochondrial function and breast tumorigenesis (Fig. 1). Furthermore, FDXR expression is higher in breast cancer patients compared with normal individuals and its overexpression predicts a worse prognosis in ERα-positive breast cancer. Collectively, these findings show that FDXR regulates mitochondrial function and may be a novel therapeutic target in ERα-positive breast cancer.
Figure 1.
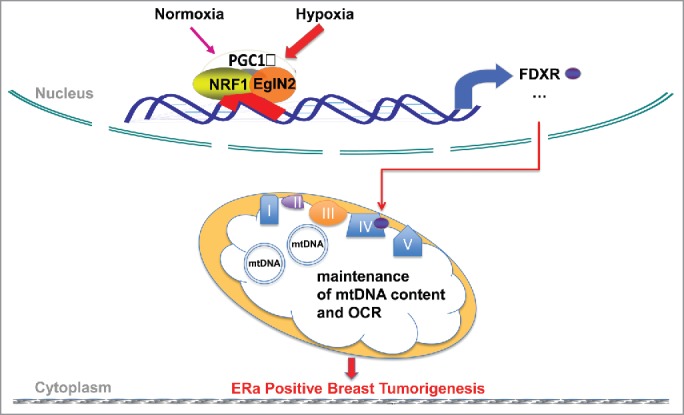
EglN2 forms an activator complex with PGC1a and NRF1 on chromatin and regulates mitochondrial function. EglN2 interacts with PGC1a and NRF1 on chromatin to positively regulate mitochondrial related genes and mitochondrial function in tumor. This trimeric complex interaction becomes much stronger under hypoxia in order to sustain mitochondrial function for maintenance of tumor growth. FDXR is one of their downstream target genes that mediates this regulation.
EglN2 maintains mitochondrial function under hypoxia
HIF mediates adaption to hypoxia by upregulating glycolysis and suppressing mitochondrial function. However, mitochondrial function plays a central role as a major resource of ATP production under hypoxia.9 Our study shows that EglN2 positively regulates mitochondrial function under hypoxia. Therefore, we speculate that on one hand, the tumor develops an adaptive metabolic program under hypoxia by upregulating HIF and glycolysis while suppressing mitochondrial function. On the other hand, since mitochondrial function is still an indispensable ATP production resource under hypoxia, EglN2 serves to maintain mitochondrial function to sustain the basic need for tumor growth.
In summary, our recent studies demonstrate that EglN2 functions as a transcriptional co-activator to regulate mitochondrial function under both normoxia and hypoxia, and that this function is independent of HIF-1/2α and contributes to tumorigenesis. However, it remains unclear how EglN2 can bind with DNA besides acting as a prolyl hydroxylase. Another important unanswered question is the mechanism by which FDXR controls mitochondrial DNA, mitochondrial function, and ERα-positive breast tumorigenesis. These findings implicate the important roles of EglN2 and FDXR in regulating mitochondrial function, which will provide novel therapeutic avenues for the development of strategies to control ERα-positive breast tumorigenesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was partially supported by a grant from the US National Institute of Health/National Cancer Institute (R00CA160351) to QZ, the University Cancer Research Fund from University of North Carolina at Chapel Hill to QZ, a Kimmel Scholar Award from Sidney Kimmel Foundation to QZ, The V Foundation Scholar Award from the V Foundation for Cancer Research to QZ, and a Susan G. Komen Career Catalyst Award to QZ.
Reference
- 1.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4:437-47; PMID:15170446; http://dx.doi.org/ 10.1038/nrc1367 [DOI] [PubMed] [Google Scholar]
- 2.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 2010; 10:267-77; PMID:20300106; http://dx.doi.org/ 10.1038/nrc2817 [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30:393-402; PMID:18498744; http://dx.doi.org/ 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Gu J, Li L, Liu J, Luo B, Cheung HW, Boehm JS, Ni M, Geisen C, Root DE, et al.. Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer Cell 2009; 16:413-24; PMID:19878873; http://dx.doi.org/ 10.1016/j.ccr.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng X, Zhai B, Koivunen P, Shin SJ, Lu G, Liu J, Geisen C, Chakraborty AA, Moslehi JJ, Smalley DM, et al.. Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev 2014; 28:1429-44; PMID:24990963; http://dx.doi.org/ 10.1101/gad.242131.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Wang C, Chen X, Takada M, Fan C, Zheng X, Wen H, Liu Y, Wang C, Pestell RG, et al.. EglN2 associates with the NRF1-PGC1alpha complex and controls mitochondrial function in breast cancer. EMBO J 2015; 34(23):2953-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007; 11:407-20; PMID:17482131; http://dx.doi.org/ 10.1016/j.ccr.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nature Med 2001; 7:1111-7; PMID:11590433; http://dx.doi.org/ 10.1038/nm1001-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Sys Biol 2013; 9:712; http://dx.doi.org/ 10.1038/msb.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]


