ABSTRACT
Dysregulated protein kinase B alpha (PKB/AKT1) signaling has been increasingly implicated in melanoma metastasis to distant organs, especially the brain. In a recent study, we expressed activated AKT1 in a non-metastatic melanoma model in vivo and discovered that AKT1 activation decreased tumor latency and elicited lung and brain metastases in this context.
KEYWORDS: AKT, Melanoma, Metastases, Mouse, Pten
Melanoma-related deaths are universally due to complications from metastatic disease and more than half of all melanoma deaths are due to brain metastases. Up to 75% of patients with stage IV melanoma will develop brain metastases and the prognosis for these patients is extremely poor.1 Melanoma brain metastasis continues to pose a significant clinical challenge despite recent therapeutic advances. In a recent study, we investigated the role of activated protein kinase B alpha (AKT1) in promoting the spread of melanoma to distant organs. We discovered that expression of activated AKT1 is sufficient to drive spontaneous lung and brain metastases in a non-metastatic autochthonous mouse model of melanoma.2
Clinical data provide compelling correlative evidence that AKT signaling is involved in melanoma metastasis, especially to the brain.3 A comparison between lung, liver, and brain metastases from human melanoma samples revealed significantly higher levels of phosphorylated AKT and a downstream target, phosphorylated glycogen synthase kinase-3 β (GSK3β), in brain metastases compared with lung and liver metastases. Using a trans-well invasion assay with astrocyte-conditioned media as an attractant, Niessner et al. found that melanoma cells adopted a more invasive phenotype with increased phosphorylated AKT relative to control conditions, demonstrating that aberrant AKT signaling was associated with increased brain-specific invasiveness of melanoma cells.4
In line with increased AKT activity, protein levels of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) were significantly lower in brain metastases compared with lung and liver metastases.3,4 Follow-up studies comparing patient-matched brain versus extracranial melanoma metastases found additional evidence of increased AKT activity as a characteristic of brain metastases; however, PTEN protein levels were similar between the matched metastases.5 Furthermore, Pten silencing combined with expression of BrafV600E in mouse melanocytes in vivo resulted in melanoma formation and development of lymph node and lung metastases, but not brain metastases.6 Activation of AKT in melanoma occurs via several mechanisms including deletion or inactivation of PTEN, which is observed in up to 43% of melanomas; genomic amplification or increased gene expression of AKT; and activating mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA) and AKT isoforms, which occur in 1–5% and 1–2% of melanomas, respectively.7 Moreover, novel mutations in AKT1 confer a survival advantage for melanoma and have been found in the setting of drug-resistant, metastatic disease.8
Using an established mouse model of melanoma that allows postnatal gene delivery to somatic cells, we assessed the ability of Pten silencing or AKT1 activation, either alone or in combination, to promote melanomagenesis and metastasis in the context of mutant BrafV600E and silencing of the cyclin-dependent kinase inhibitor 2a (Cdkn2a) locus. Although either Pten silencing or expression of activated AKT1 promoted melanoma formation in this context, only melanomas with activated AKT1 developed spontaneous brain metastases. Additionally, Pten silencing cooperated with active AKT1 to accelerate both tumor formation and metastasis.2
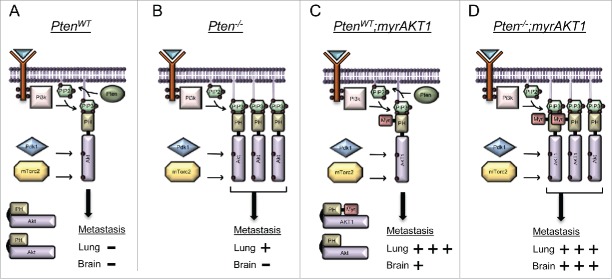
Pten functions as a phosphatase to dephosphorylate phosphoinositide substrates, specifically phosphatidylinositol-3,4,5-trisphosphate (PIP3) to phosphatidylinositol-4,5-bisphosphate (PIP2) (Fig. 1A). Pten is antagonized by phosphatidylinositol-4,5-bisphosphate 3-kinase (Pi3k), which phosphorylates PIP2 to generate PIP3, a plasma membrane phospholipid that binds to the pleckstrin homology (PH) domain of AKT. This binding not only aids in the recruitment of AKT to the membrane but also competes with the intramolecular interactions between the PH domain and the kinase domain thereby promoting an open conformation amenable to phosphorylation by pyruvate dehydrogenase kinase, isozyme 1 (Pdk1) and mammalian target of rapamycin complex 2 (mTorc2). In the absence of Pten, there is an abundance of PIP3 (Fig. 1B). While the myristoylation (myr) signal on AKT1 used in our study assists in membrane targeting (Fig. 1C), binding of PIP3 promotes an open conformation and likely explains the cooperation between myrAKT1 and Pten loss (Fig. 1D).
Figure 1.
Activation of AKT1 in the context of mutant BrafV600E and Cdkn2a loss promotes lung and brain metastasis, a phenotype that is further enhanced through Pten loss. (A) Intact Pten diminishes endogenous AKT activation through conversion of PIP3 to PIP2. (B) Loss of Pten promotes excess PIP3. Endogenous AKT binds PIP3 via the pleckstrin homology (PH) domain and adopts on open conformation amenable to phosphorylation. Hyperactive AKT results in the emergence of lung metastases. (C) Myristoylation (Myr) leads to AKT1 activation by targeting AKT1 to the plasma membrane. This promotes a high incidence of lung metastasis and the emergence of brain metastases. (D) The combination of Pten loss and myristoylation of AKT1 results in a cooperative event that maximizes AKT activation and promotes a high incidence of both lung and brain metastasis.
Activated AKT phosphorylates a large number of downstream substrates including mTor, leading to its activation. Activated AKT also phosphorylates the mTor inhibitor proteins proline-rich AKT substrate, 40 kDa (Pras40), and tuberous sclerosis complex 2 (Tsc2), inhibiting their function. These combined actions promote cell growth and cell cycle progression. Further analysis of the differences between the tumors in our study revealed activation of mTorc signaling in tumors driven by activated AKT,2 further supporting a role for this pathway in melanoma metastasis. Hyperactive mTORC signaling is disproportionately observed in melanomas (73%, 78/107) compared to benign nevi (4%, 3/67).9 Silencing of serine/threonine kinase 11 (Stk11/Lkb1), a known negative regulator of mTorc signaling, in the context of non-metastatic melanomas resulted in lung metastases.10 Pharmacological targeting of mTor effectively blocked melanoma cell growth in vitro and in animal models.6 Unfortunately, but not surprisingly, mTOR inhibitors have failed to demonstrate clinical efficacy against melanoma as monotherapy and are currently being evaluated in combination with other therapies for this disease.
To our knowledge, this mouse model is the first in vivo autochthonous model of melanoma lung and brain metastasis in an immunocompetent animal. This model system provides a powerful platform to further study the mechanisms of melanoma metastasis and sheds light on the involvement of AKT signaling in this process. Our data highlight the importance of AKT1 activation in promoting melanoma metastasis to the brain and reveal that activation of AKT1 is distinct from Pten silencing in metastatic melanoma progression. Our findings and those of others advance our knowledge of the mechanisms driving melanoma brain metastasis and may provide valuable insights for assessing the risk for development of melanoma brain metastases and for guiding clinical treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by the National Cancer Institute (R01CA121118) and the Melanoma Research Alliance.
References
- 1.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117:1687-96; PMID:20960525; http://dx.doi.org/ 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- 2.Cho JH, Robinson JP, Arave RA, Burnett WJ, Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW, McMahon M, et al.. AKT1 Activation Promotes Development of Melanoma Metastases. Cell Reports 2015; 13:898-905; PMID:26565903; http://dx.doi.org/ 10.1016/j.celrep.2015.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, Woodman SE, Calderone TC, Ju Z, Lazar AJ, et al.. Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clin Cancer Res 2009; 15:7538-46; PMID:19996208; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J, Tabatabai G, et al.. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med 2013; 2:76-85; PMID:24133630; http://dx.doi.org/ 10.1002/cam4.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Chakravarti N, Aardalen K, Lazar AJ, Tetzlaff M, Wubberhorst B, Kim SB, Kopetz S, Ledoux A, Vashisht Gopal YN, et al.. Molecular Profiling of Patient-Matched Brain and Extracranial Melanoma Metastases Implicates the PI3K Pathway as a Therapeutic Target. Clin Cancer Res 2014; 20(21):5537-46; PMID:24803579; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, Depinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009; 41:544-52; PMID:19282848; http://dx.doi.org/ 10.1038/ng.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al.. A landscape of driver mutations in melanoma. Cell 2012; 150:251-63; PMID:22817889; http://dx.doi.org/ 10.1016/j.cell.2012.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Hong A, Kong X, Koya RC, Song C, Moriceau G, Hugo W, Yu CC, Ng C, Chodon T, et al.. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer discovery 2014; 4:69-79; PMID:24265152; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP. mTOR is activated in the majority of malignant melanomas. J Invest Dermatol 2008; 128:980-7; PMID:17914450; http://dx.doi.org/ 10.1038/sj.jid.5701074 [DOI] [PubMed] [Google Scholar]
- 10.Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, Erdelyi I, Platt JT, Huang L, Theodosakis N, et al.. mTORC1 Activation Blocks Braf(V600E)-Induced Growth Arrest but Is Insufficient for Melanoma Formation. Cancer Cell 2015; 27:41-56; PMID:25584893; http://dx.doi.org/ 10.1016/j.ccell.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]