ABSTRACT
O6-methylguanine-DNA methyltransferase (MGMT) is a DNA repair enzyme involved in chemoresistance. We have shown that MGMT abundance is regulated by canonical Wingless (Wnt) signaling and that inhibition of Wnt signaling restores chemosensitivity in preclinical cancer models. These findings have direct therapeutic implications for the treatment of cancers with high MGMT expression.
KEYWORDS: β-catenin, chemoresistance, MGMT, temozolomide
Cytotoxic chemotherapy remains the mainstay for the treatment of advanced forms of cancers. Cytotoxic drugs have well proven antitumorigenic effects but their efficiency is limited by tissue toxicity, restricted pharmacokinetic effects, and by the presence or development of cancer drug resistance.1 It is estimated that 90% of patients with metastatic cancers fail to respond to treatment as a result of cancer cells overcoming the cytotoxicity triggered by chemotherapy.2 The cell killing activity of most chemotherapeutic drugs relies on modifications of DNA that cause lesions during cell replication since rapidly dividing tumor cells often have inadequate DNA repair mechanisms. The failure to repair the altered DNA is sensed by the cell apoptotic machinery with subsequent activation of apoptosis. This strategy renders cancer cells sensitive to chemotherapy.2 One large group of DNA damaging antineoplastic agents are collectively called DNA alkylators. These drugs alkylate DNA at the guanosine base, resulting in base mispairing, DNA breakage, and cell cycle arrest, followed by cell death through induction of apoptosis. O6-methylguanine-DNA methyltransferase (MGMT) is a small evolutionary conserved DNA repair enzyme that is expressed in normal tissues and has a fundamental role in cell physiology and maintenance of genome integrity.3 MGMT protects against mutagenesis and malignant transformation by efficiently removing O6-guanosine alkylation adducts in a one-step reaction that restores the guanosine residue in a DNA molecule to its normal form without causing DNA strand breaks. Expression of MGMT in tumors provides resistance to DNA alkylating agents, unless expression is lost by gene promotor methylation or through direct inhibition of protein activity by MGMT inhibitors. The enzymatic action of MGMT is stoichiometric since one MGMT molecule is needed to remove one alkyl group from DNA. During transfer of the alkyl-group from O6-guanosine on DNA to a cysteine residue in the protein, MGMT is irreversibly inactivated and subjected to proteosomal degradation.4 This makes MGMT an ideal candidate for the development of targeted drugs that would restore chemosensitivity to DNA alkylators. Unfortunately, although several inhibitors of MGMT have been developed their systemic clinical use has been limited, mainly by potentiation of the hematologic toxicity of DNA alkylators.5 As an alternative approach to inhibit MGMT we therefore searched for cellular regulators that can be used to reduce the expression of MGMT and resensitize tumors to anticancer chemotherapy. In our recent study, we used computational analysis on available patient tumor datasets and demonstrated that high MGMT expression correlates with elevated activity of the Wingless/β-catenin (Wnt/β-catenin) signaling pathway. Analysis of tumor tissues and cell lines from glioma, medulloblastoma, neuroblastoma, and colon cancer revealed co-localization of MGMT and active β-catenin, which was also reflected in cell lines derived from these tumors.6 The canonical Wnt signaling pathway is fundamental for both embryonic development and adult stem cell maintenance, and mutations or aberrant activation of key molecules controlling the activity of this cascade are prevalent in several cancers of different origin.7 A crucial mediator of canonical Wnt signaling is β-catenin, which when activated translocates from the cytoplasm into the nucleus where it guides the binding of members of the T cell factor/lymphoid enhancer factor (Tcf/Lef) family of transcription factors to DNA.7 The activity of canonical Wnt/β-catenin signaling is regulated by the destruction complex consisting of Axin, adenomatous polyposis coli gene product (APC), the glycogen synthase kinase-3 α/β (GSK-3) and casein kinase 1 (CK1). In the absence of Wnt ligands, the 2 scaffolding proteins APC and Axin bind β-catenin. The kinases GSK-3 and CK1 then sequentially phosphorylate a set of conserved Ser and Thr residues on β-catenin resulting in proteasomal degradation of β-catenin7 (Fig. 1).
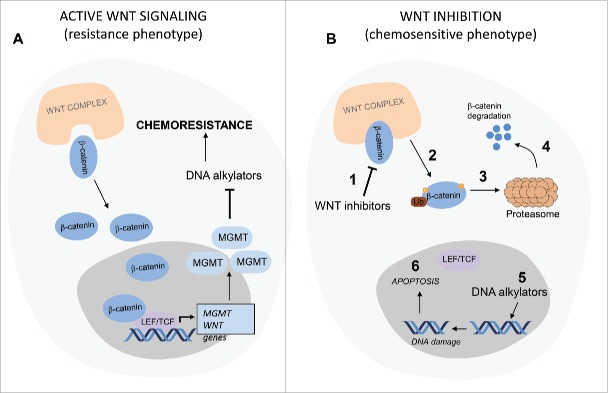
Figure 1.
Wnt inhibition resensitizes cancer cells to DNA alkylators through MGMT inhibition. (A) In cancer cells with active Wingless (Wnt) signaling, β-catenin translocates to the cell nucleus and activates transcription of Wnt genes including O6-methylguanine-DNA methyltransferase (MGMT) through T cell factor/lymphoid enhancer factor (Tcf/Lef) transcription factors. Abundant MGMT protein removes alkyl groups on DNA that were added by DNA alkylators, leading to chemoresistance. (B) Wnt inhibitors (1) mediate phosphorylation of β-catenin (2) through the Wnt destruction complex. Phosphorylated β-catenin is ubiquitinated (3) and subjected to proteasomal degradation (4). Transcription of MGMT is blocked and DNA alkylators (5) induce DNA damage in replicating tumor cells, resulting in cell cycle arrest and cell death. LEF, lymphoid enhancer factor; MGMT, O6-methylguanine-DNA methyltransferase; TCF, T cell factor; Ub, ubiquitination; Wnt, Wingless.
Using several molecular analysis assays we demonstrated that β-catenin activates the expression of MGMT through direct interaction with Tcf/Lef transcriptional factor binding sites located in the 5´-upstream regulatory region of the MGMT gene. Overexpression or short hairpin-RNA (shRNA)-mediated downregulation of β-catenin resulted in elevated or blocked expression of MGMT, respectively, both in tumor cell lines and preclinical cancer models (Fig. 1A).6 These combined data strongly suggest that MGMT gene expression is regulated by β-catenin.
A number of compounds that inhibit various key molecules in the canonical Wnt signaling pathway have been described.8 However, none of these inhibitors are currently available for clinical use, reflecting the importance of Wnt signaling in tissue homeostasis, stem cell maintenance, and development.8 We investigated the effects of Wnt inhibitors in combination with the DNA alkylator temozolomide on cancer cell lines with high expression of MGMT and demonstrated that these compounds augmented the cytotoxic effect of DNA alkylators.6
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of compounds that dampen inflammation through inhibition of cyclooxygenase-1 and -2. NSAIDs, and especially the selective cyclooxygenase-2 inhibitor celecoxib, are known inhibitors of Wnt signaling.8 Among the compounds we tested, celecoxib was equally effective as the more specific Wnt inhibitors in suppressing MGMT expression. Celecoxib is approved by the US Food and Drug Administration (FDA) and European Medical Agency (EMEA) and has proven antitumorigenic effects in preclinical models, reducing the incidence and severity of various human cancers.6,9,10 Importantly, celecoxib induced synergistic toxicity in tumor cells both with DNA alkylators like temozolomide as with other cytotoxic drugs used as first-line treatment of different cancers.6 In preclinical in vivo tumor models the effect of temozolomide was poor as a single treatment in tumors expressing MGMT. Notably, treatment of mice with celecoxib reduced levels of MGMT within the tumor tissue and this combination treatment induced significant suppression of tumor growth.6 Taken together, our findings suggest that Wnt inhibitors might be used to restore chemosensitivity to DNA-alkylating drugs (Fig. 1B). Importantly, NSAIDs are already clinically approved for various conditions and can readily be tested in combination with DNA-alkylating drugs in cancer patients with high expression of MGMT in their tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from The Swedish Children’s Cancer Foundation, The Swedish Research Council, The Swedish Cancer Society, The Swedish Foundation for Strategic Research (www.nnbcr.com), Märta and Gunnar V Philipson Foundation, The Mary Bevé Foundation, Dämman Foundation, and Erna and Olav Aakres Foundation for Cancer Research.
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13:714-26; PMID:24060863; http://dx.doi.org/ 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol 2005; 205:275-92; PMID:15641020; http://dx.doi.org/ 10.1002/path.1706 [DOI] [PubMed] [Google Scholar]
- 3.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 2004; 4:296-307; PMID:15057289; http://dx.doi.org/ 10.1038/nrc1319 [DOI] [PubMed] [Google Scholar]
- 4.Christmann M, Verbeek B, Roos WP, Kaina B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim Biophys Acta 2011; 1816:179-90; PMID:21745538; http://dx.doi.org/ 10.1016/j.bbcan.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Kaina B, Margison GP, Christmann M. Targeting O(6)-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy. Cell Mol Life Sci 2010; 67:3663-81; PMID:20717836; http://dx.doi.org/ 10.1007/s00018-010-0491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickstrom M, Dyberg C, Milosevic J, Einvik C, Calero R, Sveinbjornsson B, Sanden E, Darabi A, Siesjo P, Kool M, et al.. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun 2015; 6:8904; PMID:26603103; http://dx.doi.org/ 10.1038/ncomms9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192-205; PMID:22682243; http://dx.doi.org/ 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 8.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov 2014; 13:513-32; PMID:24981364; http://dx.doi.org/ 10.1038/nrd4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al.. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946-52; PMID:10874062; http://dx.doi.org/ 10.1056/NEJM200006293422603 [DOI] [PubMed] [Google Scholar]
- 10.Lynch PM, Burke CA, Phillips R, Morris JS, Slack R, Wang X, Liu J, Patterson S, Sinicrope FA, Rodriguez-Bigas MA, et al.. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 2015; 65:286-95; PMID:25792707; http://dx.doi.org/ 10.1136/gutjnl-2014-307235. [DOI] [PubMed] [Google Scholar]