ABSTRACT
The gap junction proteins connexins play important roles in cell growth and differentiation; however, the underlying mechanism remains largely elusive. We recently identified a channel-independent role of connexins in cell cycle control in which connexin 50 directly interacts with S-phase kinase 2 and prevents its nuclear localization, resulting in p27/p57 protection and cell cycle arrest.
KEYWORDS: Cell cycle, connexin, E3 ligase Skp2, lens cells
Regulation of cell survival and function is dependent on cell-cell communication. An important aspect of communication between adjunct cells is mediated by gap junction channels, which are composed of connexin molecules. Connexin hexamers traffic to the cell surface where they function as stand-alone hemichannels or dock with each other in appositional membranes to form intact gap junctions, allowing the exchange of small molecules (<1.2 kDa). Therefore, gap junctional intercellular communication (GJIC) is considered to be essential in the regulation of important cellular events, such as cell growth, differentiation, survival/apoptosis, homeostasis, and organ development. Importantly, clinical studies have suggested a loss or reduced expression of connexins in primary tumor tissues such as breast, lung, prostate, and many other types of cancer,1,2 implying a critical role of connexins in tumorigenesis and disease. Connexin (Cx) 42 (gene name GJA or GJB) is one of the most-studied connexins because of its ubiquitous expression in mammalian tissues. Cx43 inhibits cell proliferation and suppresses G1/S transition of the cell cycle by increasing the levels of p27, a member of the cell cycle kinase inhibitor (Cip/Kip) family, through binding with another important cell cycle inhibitor, S-phase kinase 2 (Skp2), an E3 ligase.3 The interactive domain of Cx43 with Skp2 was identified in the unique long carboxyl-terminal (CT) domain of Cx43. Although the CT domain of connexin proteins is proposed to regulate the channel function either through a ball-chain gating mechanism or through protein phosphorylation by multiple kinases,4 there is increasing evidence pointing to a non-channel function of connexins that leads to a significant inhibitory effort on the growth and migration of cancer cells.1,2 Indeed, treatment with gap junction inhibitors or expression of channel-dead connexin mutants does not influence the inhibitory effect of Cx43 on Skp2 function.3 However, the detailed mechanisms of such channel-independent functions of connexins in cell growth control remain largely obscure.
We recently characterized a novel mechanism of connexins in regulating cell cycle progression that is unrelated to their roles in forming gap junctions or hemichannels.5 We used the ocular lens as our study model to unravel the mechanistic role of connexins. This single organ contains cells undergoing various stages of growth and differentiation, with highly proliferative cells at the lens equator region and terminally differentiated cells in the center of the lens. Cx50 is one of 3 connexins expressed in the lens and the only one that contributes to lens growth and differentiation, independent of its role in forming channels.6 We showed that the CT domain of Cx50 is sufficient to mediate this action7 and that the V362 amino acid residue is directly involved.8 During lens epithelial fiber cell differentiation, the expression of Cx50 is increased, leading to an increase in the total level of p27 protein and its presence in the nucleus. Given that mRNA level of p27 is not altered during lens cell differentiation, we further identified Skp2, which is responsible for degrading p27 during cell cycle progression, as a primary target of Cx50. Cell cycle regulators normally reside in the nucleus; however, after nascent protein synthesis various post-translational modifications take place in the cytoplasm before these regulators are re-directed to the nucleus. Prolonged stay in the cytoplasm or on the plasma membrane would have adverse effects on cells, including dysregulation of cell cycle progression. The direct physical interaction between Cx50CT and Skp2 that we identified provides concerted evidence that retention of a key cell cycle regulator compromises cell cycle progression, leading to cell cycle arrest. The CT domain of connexin proteins could serve as a structural scaffold site in sequestering intracellular proteins, thus providing a novel means of regulating cell function. Moreover, this action is separate from its role in mediating channel function.
Skp2 mediates ubiquitination and proteasomal degradation of several cell cycle regulators, including p27 and p57. Therefore, Skp2 itself is considered a major indicator for tumorigenesis in many types of cancer.9 p27 and p57 are well studied in the lens system and reported to be key molecules critical for lens cell proliferation, differentiation, and development.10 We further detailed this novel interaction by showing that Cx50 is capable of competing with cyclin A for binding to Skp2. Binding with Cx50 not only retains Skp2 in cytoplasm by physically blocking its nuclear localization signals, thus preventing Skp2 translocation into the nucleus, but also promotes auto-ubiquitination of Skp2 in the cytosol. As illustrated in Fig. 1, this chain of events following the Cx50-Skp2 interaction has a drastic impact on cell cycle progression and subsequently on cell differentiation, ultimately determining the cell fate.
Figure 1.
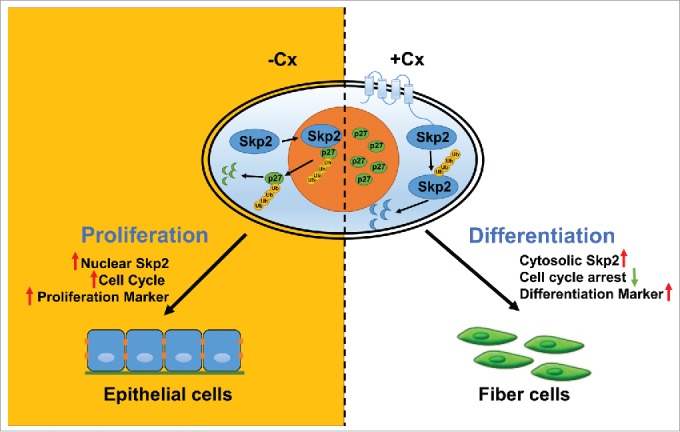
Role of Cx50-Skp2 in lens fiber differentiation. Connexin (Cx) 50 C-terminal domain (CT) interacts with S-phase kinase 2 (Skp2) in the cytosol, leading to cytoplasmic retention of Skp2 and preventing it from translocating into the nucleus. The cytosolic Skp2 is further subjected to auto-ubiquitination and proteolysis. Reduced levels of nuclear Skp2 lead to decreased ubiquitination and accumulation of p27 in the nucleus. The increased levels of p27 lead to cell cycle arrest and promotion of lens epithelial fiber cell differentiation.
In the last 2 decades, ample evidence obtained from our laboratory and others has suggested channel-independent roles of connexins in cell growth and differentiation. Our recent research provided the first mechanism demonstrating a direct relationship between a connexin protein and a key cell cycle regulator. Even though this study was performed under physiological conditions in the lens, the outcome would potentially benefit study of the role of connexins in cancer cells. Disruption of the Skp2-p27 axis and abrogation of entry into S phase of the cell cycle are common features of cancer cells that are frequently observed in human tumors and often correlated with poor prognosis.9 Therefore, enrichment of connexin proteins, or just their interactive fragments, with Skp2 would be a plausible strategy for potential development of new therapies for cancer treatment. Most previous studies have primarily focused on Cx43 because of its broad distribution. Further investigation is warranted to explore whether this regulatory mechanism is preserved among connexin subtypes and how the knowledge obtained would benefit our general understanding of cell growth and differentiation and might be further developed into clinical applications.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work was supported by NIH EY012085 and Welch Foundation Grant AQ-1507.
References
- 1.Zhou JZ, Jiang JX. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions–an update. FEBS Lett 2014; 588:1186-92; PMID:24434539; http://dx.doi.org/ 10.1016/j.febslet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart MK, Simek J, Laird DW. Insights into the role of connexins in mammary gland morphogenesis and function. Reproduction 2015; 149:R279-90; PMID:25792566; http://dx.doi.org/ 10.1530/REP-14-0661 [DOI] [PubMed] [Google Scholar]
- 3.Zhang YW, Kaneda M, Morita I. The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem 2003; 278:44852-6; PMID:12952975; http://dx.doi.org/ 10.1074/jbc.M305072200 [DOI] [PubMed] [Google Scholar]
- 4.De Bock M, Wang N, Decrock E, Bultynck G, Leybaert L. Intracellular cleavage of the Cx43 C-terminal domain by matrix-metalloproteases: a novel contributor to inflammation? Mediat Inflamm 2015; 2015:257471; PMID:26424967; http://dx.doi.org/ 10.1155/2015/257471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Q, Gu S, Yu XS, White TW, Banks EA, Jiang JX. Connexin controls cell-cycle exit and cell differentiation by directly promoting cytosolic localization and degradation of E3 ligase Skp2. Dev Cell 2015; 35:483-96; PMID:26585299; http://dx.doi.org/ 10.1016/j.devcel.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Investigat Ophthalmol Visual Sci 2003; 44:2103-11; PMID:12714649; http://dx.doi.org/ 10.1167/iovs.02-1045 [DOI] [PubMed] [Google Scholar]
- 7.Yu XS, Yin X, Lafer EM, Jiang JX. Developmental regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C terminus of major intrinsic protein (aquaporin-0). J Biol Chem 2005; 280:22081-90; PMID:15802270; http://dx.doi.org/ 10.1074/jbc.M414377200 [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Banks EA, Yu XS, Gu S, Lauer J, Fields GB, Jiang JX. Amino acid residue Val362 plays a critical role in maintaining the structure of C terminus of connexin 50 and in lens epithelial-fiber differentiation. J Biol Chem 2010; 285:18415-22; PMID:20395299; http://dx.doi.org/ 10.1074/jbc.M110.107052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao Z, Huang S. E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front Biosci 2015; 20:474-90; PMID:25553462; http://dx.doi.org/ 10.2741/4320 [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev 1998; 12:3162-7; PMID:9784491; http://dx.doi.org/ 10.1101/gad.12.20.3162 [DOI] [PMC free article] [PubMed] [Google Scholar]


