Abstract
The precise mechanism by which Ras proteins mediate mitogenic signaling remains obscure. We have identified the p53/p21Cip1 axis as an essential component of the Ras mitogenic pathway. Moreover, cells carrying an inactive p53/p21Cip1/Rb pathway sustain cell proliferation by inducing retroactivation of the MAPK signaling cascade in a Ras-independent manner.
Keywords: cell cycle, MAPK signaling cascade, P21, p53, Ras, Rb
Ras signaling has been studied for more than 40 years.1 Yet our understanding of the molecular mechanisms by which Ras proteins convey mitogenic signals is incomplete, especially beyond their immediate effector pathways, which are primarily the mitogen-activated protein kinase (MAPK) signaling cascade, the phosphatidylinositol 3-kinase/Akt pathway, and the Ral guanine exchange factor route.1,2 Indeed, genetic interrogation of these effectors has indicated that only components of the MAPK signaling cascade—the Raf, Mek, and Erk kinases—can mediate mitogenic signaling, at least in mouse embryonic fibroblasts (MEFs).3 However, how this information is transmitted to the cell cycle machinery remains mostly unknown. Here, we summarize our recent studies aimed at identifying these missing links.
As expected, genetic ablation of the 3 Ras genes Hras, Nras, and Kras in MEFs and keratinocytes completely prevents cell proliferation.3,4 Ectopic re-expression of Ras isoforms restores their proliferative capacity, demonstrating the reversible nature of the quiescent “Rasless” state. Earlier studies have demonstrated that the retinoblastoma protein (Rb) is one of the ultimate targets of Ras mitogenic signaling.5 Indeed, knockdown or inactivation of Rb licenses quiescent Rasless cells to enter the cell cycle.3 In this regard, it is generally accepted that Ras proteins control Rb activity by inducing expression of D- and E-type cyclins, which bind to their catalytic partners cyclin-dependent kinases 4/6 (Cdk4/6) and Cdk2, respectively, and lead to phosphorylation and subsequent inactivation of Rb (Fig. 1A).6 This hypothesis, however, needs to be revised since Rasless cells retain normal levels of D- and E-type cyclins bound to their cognate Cdks.3 Moreover, these cyclin/Cdk complexes lack kinase activity, suggesting that Ras signaling is essential to control their enzymatic activity, presumably via their interaction with Cdk inhibitors (Fig. 1B).3
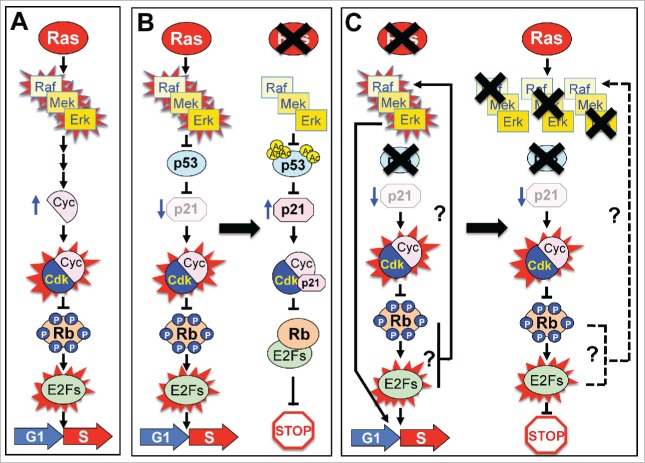
Figure 1.
Ras mitogenic signaling. (A) Schematic representation of the currently accepted model. (B) Schematic representation of the updated model incorporating our recent results and demonstrating the essential role of the p53/p21Cip1 axis in Ras mitogenic signaling. (Left) Wild type cells, (Right) Rasless cells. (C) Knockdown/inactivation of the p53/p21Cip1/Rb axis induces retroactivation of the MAPK pathway in a Ras-independent manner. (Left) Schematic representation of the Ras signaling pathway illustrating that Rasless cells can proliferate in the absence of p53 as a result of retroactivation of the Raf/Mek/Erk signaling cascade. Similar results were observed in Rasless cells lacking p21 and Rb tumor suppressors (see text). (Right) Unlike Rasless cells, cells lacking Raf kinases (Rafless cells), Mek kinases (Mekless cells), or Erk kinases (Erkless cells) cannot proliferate in the absence of the p53/p21Cip1/Rb axis. Blue arrows indicate variation in expression levels. Red spikes indicate activated proteins. X indicates that the corresponding protein has been genetically ablated. Ac, acetylated lysine residues; P, phosphorylated Ser/Thr residues; Cyc, cyclins; Cdk, cyclin-dependent kinases; Rb, retinoblastoma protein; G1, gap 1 phase; S, S phase of the cell cycle. The dotted line indicates that we do not have experimental evidence for the existence of the retroactivating circuitry observed in Rasless cells in Rafless, Mekless, or Erkless cells. The represented active state of the E2F transcription factors has not been determined experimentally but is deduced from the inactive state of the Rb protein.
To obtain insights into how Ras proteins regulate cell proliferation, we performed an unbiased small hairpin RNA (shRNA) library screen in Rasless cells. This screen unambiguously identified the Trp53/Cdkn1a (p53/p21Cip1) tumor suppressor axis as an essential mediator of Ras mitogenic signaling.7 Indeed, efficient knockdown of either p53 or p21Cip1 expression fully restored the proliferative properties of Rasless cells (Fig. 1B). Further studies revealed that loss of Ras proteins caused widespread transcriptional activation of p53 through a mechanism involving acetylation of 2 specific residues in its DNA binding domain.7 Surprisingly, phosphorylation and/or protein stabilization of p53 was not required. These genetic studies place the p53/p21 axis at the center of the mitogenic pathway that connects Ras signaling with the cell cycle (Fig. 1B).
As indicated above, the MAPK pathway is solely responsible for mediating Ras mitogenic signaling, at least in MEFs and keratinocytes.3,4 Cells lacking Raf, Mek, or Erk kinases (Rafless, Mekless, and Erkless cells respectively) also exit the cell cycle and remain in a quiescent state indistinguishable from that of Rasless cells; however, knockdown of either p53 or p21Cip1 failed to confer proliferative properties on Rafless, Mekless, or Erkless cells (Fig. 1C). Knockdown or inactivation of the retinoblastoma (Rb) tumor suppressor also failed to induce cell proliferation. A solution to this apparent conundrum came when we observed that knockdown of any of the members of the p53/p21Cip1/Rb tumor suppressor axis in Rasless cells resulted in activation of the MAPK signaling pathway (Fig. 1C), indicating that cells must possess a retroactivating circuitry that maintains an active MAPK cascade in the absence of the p53/p21Cip1/Rb axis. Moreover, retroactivation of the MAPK pathway in a Ras-independent manner is essential for cell cycle progression, as demonstrated by the lack of proliferation of cells when we ablated the Raf, Mek, or Erk kinases (Fig. 1C). In other words, inactivation of Rb per se is not sufficient to license cells to cycle; rather, they require an active MAPK pathway regardless of the presence or absence of Ras proteins.
How does knockdown of the p53/p21/Rb axis activate the MAPK pathway? Since Raf proteins are essential for cell proliferation in the absence of an active p53/p21/Rb axis, we assume that the retroactivation circuitry must necessarily go through this family of kinases. It is possible that cells may also have other circuitries to retroactivate the Mek and Erk kinases but, if so, our genetic data indicate that these circuitries are not sufficient to sustain cell proliferation unless they also mediate retroactivation of Raf proteins.8 In any case, and regardless of the precise nature of these circuitries, our genetic studies clearly illustrate activation of the MAPK signaling cascade in the absence of Ras proteins. Interestingly, the absence of p53 also allows cells lacking the activating members of the E2f family of transcription factors—E2f1, E2f2 and E2f3—to proliferate.9 Although it remains to be determined whether such cells also induce retroactivation of Raf/Mek/Erk signaling, this raises the possibility that the E2f transcription factors might be involved in Ras-independent activation of the MAPK cascade (Fig. 1C).
Our results do not resolve whether feedback activation of the MAPK pathway downstream of the p53/p21/Rb axis exists during normal homeostasis as a mechanism to potentiate mitogenic signaling.10 However, under these conditions the retroactivated MAPK pathway is not sufficient to sustain cell proliferation after Ras mitogenic signaling has been switched off. Alternatively, cells may have negative regulatory mechanisms that restore activation of the p53/p21Cip1 axis once they stop receiving mitogenic signals, thus thwarting retroactivation of the MAPK pathway. A final consideration derived from these studies is whether tumor cells lacking the p53 tumor suppressor retain an active MAPK signaling pathway independent of Ras signaling. If so, direct inhibition of Ras proteins may not be sufficient to stop tumor progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer 2003; 3:459-65; PMID:12778136; http://dx.doi.org/ 10.1038/nrc1097 [DOI] [PubMed] [Google Scholar]
- 2.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011; 11:761-74; PMID:21993244; http://dx.doi.org/ 10.1038/nrc3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten M, Dhawahir A, Sum EY Urosevic J, Lechuga CG, Esteban LM, Castellano E, Guerra C, Santos E, Barbacid M. Genetic analysis of Ras signalling pathways in cell proliferation, migration, and survival. EMBO J 2010; 29:1091-104; PMID:20150892; http://dx.doi.org/ 10.1038/emboj.2010.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosten M, Lechuga CG, Barbacid M. Ras signaling is essential for skin development. Oncogene 2014; 33:2857-65; PMID:23831572; http://dx.doi.org/ 10.1038/onc.2013.254 [DOI] [PubMed] [Google Scholar]
- 5.Peeper DS, Upton TM, Ladha MH, Neuman E, Zalvide J, Bernards R, DeCaprio JA, Ewen ME. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 1997; 386:177-81; PMID:9062190; http://dx.doi.org/ 10.1038/386177a0 [DOI] [PubMed] [Google Scholar]
- 6.Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell cycle regulation. Nat Rev Mol Cell Biol 2004; 5:355-66; PMID:15122349; http://dx.doi.org/ 10.1038/nrm1365 [DOI] [PubMed] [Google Scholar]
- 7.Drosten M, Sum EY, Lechuga CG, Simón-Carrasco L, Jacob HK, García-Medina R, Huang S, Beijersbergen R, Bernards R, Barbacid M. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proc Natl Acad Sci USA 2014; 111:15155-60; PMID:25288756; http://dx.doi.org/ 10.1073/pnas.1417549111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Stites EC, Yu H, Germino EA, Meharena HS, Stork PJS, Kornev AP, Taylor SS, Shaw AS. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell 2013; 154:1036-1046; PMID:23993095; http://dx.doi.org/ 10.1016/j.cell.2013.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma N, Timmers C, Trikha P, Saavedra HI, Obery A, Leone G. Control of the p53-p21CIP1 axis by E2f1, E2f2, and E2f3 is essential for G1/S progression and cellular transformation. J Biol Chem 2006; 281:36124-31; PMID:17008321; http://dx.doi.org/ 10.1074/jbc.M604152200 [DOI] [PubMed] [Google Scholar]
- 10.Korotayev K, Chaussepied M, Ginsberg D. ERK activation is regulated by E2F1 and is essential for E2F1-induced S phase entry. Cell Signal 2008; 20:1221-6; PMID:18396012; http://dx.doi.org/ 10.1016/j.cellsig.2008.02.012 [DOI] [PubMed] [Google Scholar]