Abstract
Aims
Heart muscle contraction is regulated via the β-adrenergic response that leads to phosphorylation of Troponin I (TnI) at Ser22/23, which changes the Ca2+-sensitivity of the cardiac myofilament. Mutations in thin filament proteins that cause Dilated Cardiomyopathy (DCM) and some mutations that cause Hypertrophic Cardiomyopathy (HCM) abolish the relationship between TnI phosphorylation and Ca2+-sensitivity (uncoupling). Small molecule Ca2+-sensitisers and Ca2+-desensitisers that act upon troponin alter the Ca2+-sensitivity of the thin filament but their relationship with TnI phosphorylation has never been studied before.
Methods and Results
Quantitative in vitro motility assay showed that 30 μM EMD57033 and 100 μM Bepridil increase Ca2+-sensitivity of phosphorylated cardiac thin filaments by 3.1 and 2.8-fold respectively. Additionally they uncoupled Ca2+-sensitivity from TnI phosphorylation, mimicking the effect of HCM mutations. EGCG decreased Ca2+-sensitivity of phosphorylated and unphosphorylated wild-type thin filaments equally (by 2.15±0.45 and 2.80±0.48-fold respectively), retaining the coupling. Moreover, EGCG also reduced Ca2+-sensitivity of phosphorylated but not unphosphorylated thin filaments containing DCM and HCM-causing mutations, thus the dependence of Ca2+-sensitivity upon TnI phosphorylation of uncoupled mutant thin filaments was restored in every case. In single mouse heart myofibrils, EGCG reduced Ca2+-sensitivity of force and kACT and also preserved coupling. Myofibrils from the ACTC E361G (DCM) mouse were uncoupled; EGCG reduced Ca2+-sensitivity more for phosphorylated than unphosphorylated myofibrils, thus restoring coupling.
Conclusion
We conclude that it is possible to both mimic and reverse the pathological defects in troponin caused by cardiomyopathy mutations pharmacologically. Re-coupling by EGCG may be of potential therapeutic significance for treating cardiomyopathies.
Introduction
Hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) are common inherited diseases. HCM is detected in 1 in 500 of the adult population and the causative mutations are overwhelmingly in the proteins of the cardiac muscle contractile apparatus 1, 2. At least 30% of cases of idiopathic DCM are of genetic origin, and DCM with no additional complications such as conduction disease is usually caused by mutations in the contractile apparatus 3, 4. In many cases, the cardiomyopathies result from missense mutations in one of the proteins of the muscle thin filament (actin, tropomyosin, troponin I, C or T). Investigations of the mechanisms that cause HCM and DCM have generally found that mutations in the muscle thin filament cause abnormalities in the Ca2+-regulatory system of the muscle 5–7.
HCM is closely associated with enhanced myofilament Ca2+-sensitivity although the process by which chronically high Ca2+-sensitivity leads to the symptoms of HCM: hypertrophy, fibrosis and arrhythmias, is uncertain. In DCM, the situation is more complex. It has been suggested that DCM-causing mutations are associated with a reduced Ca2+-sensitivity 8 but comprehensive surveys now show that DCM-causing mutations in thin filament proteins can increase or decrease Ca2+-sensitivity 5, 9. However, the DCM phenotype is always linked to the absence of modulation of Ca2+-sensitivity by troponin I (TnI) phosphorylation (uncoupling) and this has been proposed to be causative of the DCM phenotype 9, 10. In addition, the coupling between TnI phosphorylation and change of Ca2+-sensitivity seems to be lost due to HCM mutations in thin filament proteins when studied by in vitro motility assay (IVMA) techniques and in myofibrilar assays 11–15, but in permeabilized muscle this phenomenon is not observed 16. Thus, in HCM the relationship between TnI phosphorylation and Ca2+-sensitivity is not clear.
Uncoupling appears to be closely associated with mutations in thin filament proteins that cause cardiomyopathies. The decrease in Ca2+-sensitivity upon phosphorylation of TnI and the corresponding increase in the rate of Ca2+-dissociation from troponin C (TnC), is a key component of the lusitropic response to β1-adrenergic stimulation in the heart. The uncoupling reported in DCM and HCM, is likely to impact on cardiac reserve pathologically, and indeed, most studies of mouse models with cardiomyopathy mutations in thin filament proteins report a blunted response to adrenergic stimulation in vivo (reviewed in 17).
A number of small molecules have been found that alter myofilament Ca2+-sensitivity by binding to troponin and act as either Ca2+-sensitisers or Ca2+-desensitisers. The effect of these reagents on Ca2+-sensitivity is well documented, but the effect of small molecules on the coupling between Ca2+-sensitivity and TnI phosphorylation has not previously been considered.
EMD57033 and Bepridil are well established Ca2+-sensitisers acting directly upon TnC 18–20. Epigallocatechin-3-Gallate (EGCG), the principal polyphenol isolated from green tea, is reported to be a Ca2+-desensitiser that also acts via a binding site on TnC 21–23. Structural studies indicate that the regulatory Ca2+ binding site (site II in the N-terminal lobe of TnC) is closely coupled both to the binding of the TnI switch peptide (147-163), critical for neutralising the inhibitory action of TnI, and the cardiac specific N-terminal peptide of TnI (1-30) that contains the phosphorylatable serines 22 and 23 24–28. We have proposed that DCM-causing mutations in thin filament proteins uncouple phosphorylation from the change in Ca2+-sensitivity by disrupting this coupled allosteric system 9, 17. It is likely that Ca2+-sensitisers and desensitisers binding to TnC would also have an effect on the coupling between Ca2+-sensitivity and TnI phosphorylation by modulating the coupled system.
We have therefore investigated how EMD57033, Bepridil and EGCG affect Ca2+-regulation and its modulation by phosphorylation in native human heart thin filaments and how these reagents interact with mutations in thin filament proteins associated with HCM or DCM. Using IVMA and single myofibril contractility we have confirmed the Ca2+-sensitising effects of EMD57033 and Bepridil and demonstrated that they also uncouple Ca2+-sensitivity from the TnI phosphorylation level, thus mimicking the effects of HCM-causing mutations. In contrast, EGCG decreases Ca2+-sensitivity in native thin filaments whilst retaining the modulation of Ca2+-sensitivity by TnI phosphorylation. Moreover, EGCG has the unique ability to restore the coupling to uncoupled HCM and DCM mutant thin filaments and myofibrils, thus antagonising the disease-causing defect. This property of EGCG may be of therapeutic significance for treating some cardiomyopathies.
Methods
Sources of contractile proteins
Donor heart tissue, used as control, and end-stage failing heart tissue from explanted hearts were obtained from the Sydney Tissue Bank Sydney, Australia 1,2. Ethical approval for collection and use of tissue samples was obtained from the St Vincent’s Hospital, Sydney and Brompton, Harefield and NHLI Research Ethics Committees.
Troponin was isolated from 100 mg of human heart muscle using an anti-cardiac troponin I (TnI) monoclonal antibody affinity column as described by Messer 29. Troponin containing the TNNC1 G159D mutation was purified from explanted heart samples as previously described 30,9, 31 . Recombinant TNNT2 K280N and TNNI3 K36Q was introduced into donor heart troponin by exchange as described 29, 32. Wild-type α-tropomyosin (Tpm1.1) and the mutants E40K, E54K and E180G were expressed in a baculovirus/Sf9 system with a protocol based on that of Akkari et al. 9, 33, 34. Native, E361G and E99K mutant mouse cardiac actins were extracted from transgenic mouse hearts as described by Song et al. 35.
Manipulation and measurement of TnI phosphorylation level
Troponin isolated from human heart samples has a high level of phosphorylation, which was reduced by treatment with shrimp alkaline phosphatase (Sigma P9088). Recombinant TnI was phosphorylated by treatment with protein kinase A (PKA) catalytic subunit (Sigma, P2645-400) as previously described. 9, 32. To dephosphorylate mouse heart troponin, mice were treated with Propranolol as described 10. TnI phosphorylation levels in myofibrils and isolated troponin was measured by Phosphate affinity SDS-PAGE as described 9, 36, results are shown in Supplement C.
Quantitative in vitro motility assay
Thin filaments were reconstituted with 10 nM rabbit skeletal or mouse cardiac muscle α-actin (labelled with TRITC phalloidin), tropomyosin (40-60 nM) and troponin (60 nM) to study Ca2+-regulation of filament motility by the quantitative in vitro motility assay 29, 37. Thin filament movement over a bed of immobilised rabbit fast skeletal muscle heavy meromyosin (100 μg/ml) was compared in paired motility chambers in which troponin varied by a single factor (mutation, phosphorylation state or treatment with drug). Filament movement was recorded and analysed as previously described 38, yielding two parameters, the fraction of filaments moving and the speed of moving filaments.
Contraction of isolated myofibrils
Contraction and relaxation of isolated mouse myofibrils was initiated using a fast-solution change system and sensitive force transducer system recently described 10. Further details are in Supplement A.
Results
Relationship between phosphorylation and Ca2+-sensitivity
We measured the Ca2+-dependence of thin filaments containing human heart troponin by the in vitro motility assay (IVMA). The assay yields two parameters, fraction of filaments motile and sliding speed of the motile thin filaments. Both parameters are Ca2+-dependent and Ca2+-activation curves were plotted (Figure 1A). 3.9 μM Ca2+ increased the fraction of filaments motile from 0.03 to 0.83 and the sliding speed from 1.5 μm/sec to 3.0 μm/sec. The EC50 and Hill co-efficient (nH) determined by fitting the Hill equation to the data were similar for both parameters under all conditions; therefore, in this manuscript we have only shown the fraction motile parameter. Full data for both parameters is shown in Supplement B.
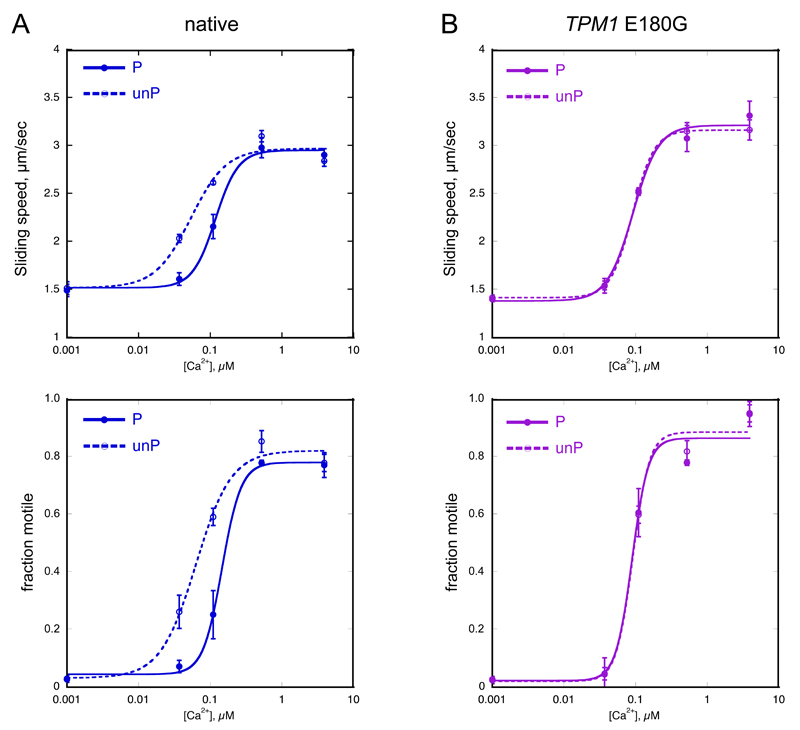
Figure 1. Relationship between EC50 and TnI phosphorylation in native human thin filaments and thin filaments containing HCM-causing mutation TPM1 E180G.
Sliding speed and fraction of filaments motile, measured in the same experiment by in vitro motility assay (IVMA) is plotted against [Ca2+] for representative experiments. Raw data from one experiment is shown here, the mean values of EC50 from replicate experiments is shown in Table 2. Solid lines and points, phosphorylated troponin (P); dotted lines and open points, unphosphorylated troponin (unP). Error bars represent SEM of 4 measurements of motility in the same motility chamber. Blue, native thin filaments; purple, HCM-causing mutation TPM1 E180G present.
A. Native thin filaments: phosphorylation increased EC50 (decreased Ca2+-sensitivity) but had no effect on the maximum sliding speed or fraction of filaments motile at saturating Ca2+.
B. Thin filaments containing TPM1 E180G HCM-causing mutation. The relationship of Ca2+-sensitivity to TnI phosphorylation is uncoupled.
Human heart troponin has a high level of TnI phosphorylation in the 1.4-1.8 mols Pi/mol range 36. This is reduced to less than 0.3 mols Pi/mol by treatment with phosphatase, see Supplement C. The Ca2+-sensitivity of unphosphorylated thin filaments is 1.88±0.10 (p<0.0001, n=16) times greater than the native phosphorylated thin filaments as reported previously 29. nH was significantly reduced from 2.11±0.18 for phosphorylated to 1.72±0.11 for unphosphorylated thin filaments, p=0.017, n=16. The maximum sliding speed was not significantly affected by phosphorylation level (Table 1, Figure 1A).
Table 1. Effect of EMD57033, Bepridil and EGCG on Ca2+-regulation of motility.
| TREATMENT | EC50 | nH | ΔVmax | EC50, TPM1 E54K DCM mutation |
|---|---|---|---|---|
| Native P | 0.16±0.03(4) | 2.08±0.14(4) | 1.40±0.11(4) | 0.12±0.02(3) |
| unP | 0.091±0.014(4)† | 1.51±0.25(4)† | 1.30±0.04(4) | 0.11±0.008(3) |
| +EMD57033 P | 0.057±0.010(4)** | 1.26±0.07(4)* | 1.55±0.08(4) | 0.041±0.002(3)* |
| +EMD57033 unP | 0.053±0.010(4) | 1.34±0.14(4) | 1.54±0.08(4)* | 0.041±0.002(3)* |
| Native P | 0.13±0.007(8) | 2.89±0.48(8) | 2.09±0.14(8) | 0.13±0.014(3) |
| unP | 0.072±0.002(6)†† | 1.96±0.08(6) | 2.13±0.20(6) | 0.13±0.012(3) |
| +Bepridil P | 0.045±0.001(8)** | 1.94±0.08(8) | 2.40±0.15(8)** | 0.060±0.009(3)* |
| +Bepridil unP | 0.045±0.001(6)** | 2.03±0.13(6) | 2.24±0.16(6) | 0.058±0.006(3)** |
| Native P | 0.14±0.03(7) | 1.61±0.19(7) | 1.91±0.18(7) | 0.11±0.01(5) |
| unP | 0.059±0.011(6)†† | 1.50±0.21(6) | 1.92±0.22(6) | 0.11±0.02(5) |
| +EGCG P | 0.26±0.02(7)** | 1.96±0.24(7) | 1.38±0.12(7)** | 0.23±0.03(5)* |
| +EGCG unP | 0.15±0.02(6)**†† | 1.79±0.30(6) | 1.52±0.13(6)* | 0.071±0.005(5)†† |
p<0.05
p<0.01; for presence and absence of reagent
p<0.05
p<0.01; unphosphorylated compared with phosphorylated. Paired t test was used for EC50, nH and ΔVmax (the change in velocity due to Ca2+). EC50 values rounded to 2 significant figures. In brackets is the number of experiments.
We have investigated thin filaments incorporating 6 DCM-causing mutations that have been shown to abolish this relationship (uncouple) in previous studies 9 and 3 HCM mutations that uncouple in vitro (Figure 1B 15, 39).
EMD57033 and Bepridil increase Ca2+-sensitivity and uncouple TnI phosphorylation from changes in Ca2+-sensitivity
EMD57033 and Bepridil substantially increased the Ca2+-sensitivity (Figure 2) and slightly increased the maximum sliding speed of native human thin filaments as expected.
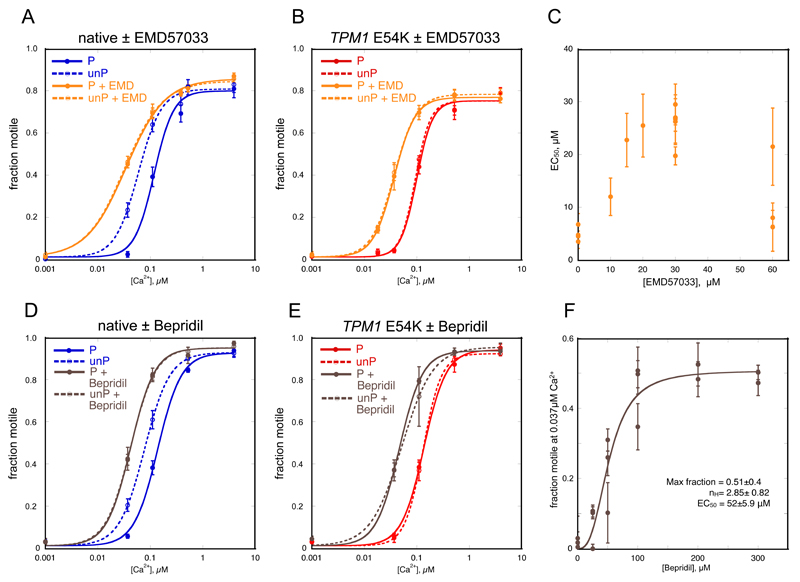
Figure 2. Effect of Ca2+-sensitisers on Ca2+ control of motility.
A, B, D, E. Fraction of filaments motile, measured by IVMA is plotted against [Ca2+] for representative experiments, details as for Figure 1.
A, D. Effect of Ca2+-sensitisers on Ca2+-regulation of native thin filaments. Blue, native thin filaments; orange, presence of 30 μM EMD57033; brown, presence of 100 μM Bepridil.
B, E. Effect of Ca2+-sensitisers on Ca2+-regulation of thin filaments containing the TPM1 E54K DCM-causing mutation. Red, E54K-containing thin filaments; orange, presence of 30 μM EMD57033; brown, presence of 100 μM Bepridil.
C. EMD57033 dose response curve: EC50 was determined at a range of EMD57033 concentrations. The change in EC50 (± SEM of 4 measurements of motility in the same motility chamber) is plotted for three separate experiments.
F. Bepridil dose response curve: motility was measured at a constant 0.037 μM Ca2+ with increasing concentrations of Bepridil. The increase in fraction of filaments motile (± SEM of 4 measurements of motility in the same motility chamber) is plotted for three separate experiments and the curve represents the fit of the pooled data to the Hill equation. Values of parameters obtained are shown.
The effect of EMD57033 on EC50 for Ca2+-activation of motility was biphasic with a maximum at 30 μM and an EC50 in the range 15-20 μM (Figure 2C). Interestingly, we observed that 30 μM EMD57033 increased the Ca2+-sensitivity of thin filaments containing phosphorylated troponin more than unphosphorylated TnI (mean ratio EC50 P/EC50 P + EMD57033 = 3.07±0.71, p=0.0001, n=4 in phosphorylated compared with a mean ratio of EC50 unP/EC50 unP + EMD57033 = 1.83±0.36, p=0.07, n=4 in unphosphorylated, see Table 1, Figure 2A, Supplement B).
Consequently, in the presence of EMD57033, Ca2+-sensitivity of thin filaments was the same, independent of the level of phosphorylation (EC50 P + EMD57033/EC50 unP + EMD57033 = 1.08±0.03, p=0.06, n=4). EMD57033 increased sliding speed slightly at saturating Ca2+ concentration in phosphorylated thin filaments and in unphosphorylated thin filaments by 18.4±4.1% (p=0.02, n=4). nH was slightly decreased in phosphorylated but not unphosphorylated thin filaments. EMD57033 also increased the Ca2+-sensitivity of thin filaments containing the DCM-causing mutations, TPM1 E40K and E54K. In both these cases, Ca2+-sensitivity was independent of troponin phosphorylation (uncoupled) due to the mutation (Table 1, Figure 2B).
A similar pattern of results was observed with Bepridil. The dose-response curve was cooperative with a calculated EC50 of 52 μM (Figure 2F). At 100 μm, which is the saturated concentration, the increase in Ca2+-sensitivity of phosphorylated donor thin filaments (measured by the mean ratio EC50 P/EC50 P + Bepridil) was 2.8±0.14-fold for fraction motile (p<0.0001, n=6) and 3.09±0.59-fold for sliding speed (p=0.016, n=6), see Supplement B.
Bepridil increased the Ca2+-sensitivity of thin filaments and also uncoupled Ca2+-sensitivity from TnI phosphorylation, as shown by the EC50 ratios of phosphorylated to dephosphorylated troponin (EC50 P/EC50 unP = 1.73±0.03, p<0.0001; EC50 P + Bepridil/EC50 unP + Bepridil = 1.03±0.01, p=0.04, n=6) (Figure 2D, Supplement B). Bepridil increased sliding speed at saturating Ca2+ concentrations significantly in phosphorylated thin filaments (14.8±3.2%, p=0.006, n=6) but did not have a significant effect in unphosphorylated thin filaments (7.2±4.6%, p=0.18, n=6); it had no significant effect on nH. Bepridil also reversibly increased the Ca2+-sensitivity of both phosphorylated and unphosphorylated thin filaments containing the DCM-causing TPM1 E54K mutation and these mutant thin filaments remained uncoupled (Table 1, Figure 2E, Supplement E).
EGCG decreases Ca2+-sensitivity whilst retaining coupling
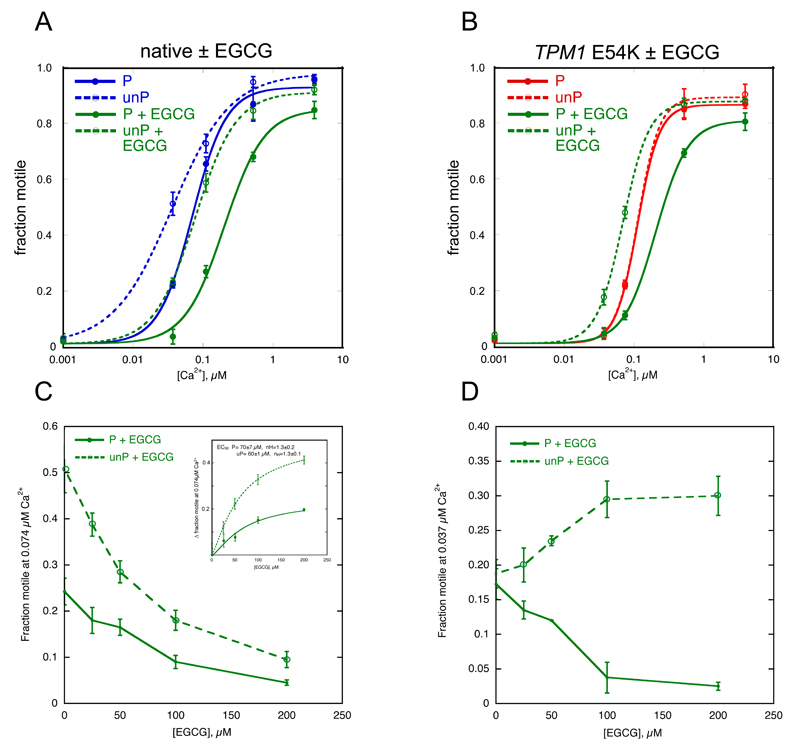
EGCG decreased Ca2+-sensitivity of human cardiac thin filaments measured by IVMA and at saturating concentrations (100 μM) reduced the maximum sliding speed up to 20% but had no significant effect on nH (Table 1, Figure 3, Supplement B). Titration of EGCG at 0.074 μM Ca2+ yielded a cooperative dose-response curve with a calculated EC50 of 70±7 μM for phosphorylated and 60±1 for unphosphorylated (Figure 3C). Unlike EMD57033 and Bepridil, 100 μM EGCG had a similar effect on the EC50 of thin filaments with phosphorylated and unphosphorylated troponin, (mean ratio EC50 + EGCG/EC50 control = 2.15±0.45-fold for phosphorylated and 2.80±0.48-fold for unphosphorylated thin filaments, see Supplement B). Therefore, the coupling of TnI phosphorylation level to changes in Ca2+-sensitivity was retained (EC50 P/EC50 unP = 2.24±0.10, p<0.0001, n=6; EC50 P + EGCG/EC50 unP + EGCG = 1.73±0.16, p=0.006, n=6) (Table 1, Figure 3A, Supplement B).
Figure 3. Effect of EGCG on Ca2+ control of motility.
A, B. Fraction of filaments motile, measured by IVMA, is plotted against [Ca2+] for representative experiments, details as for Figure 1. Raw data from one experiment is shown here, the mean values of EC50 from replicate experiments are shown in Tables 1 and 2.
A. Effect of EGCG on Ca2+-regulation of native thin filaments. Blue, native thin filaments; green, presence of 100 μM EGCG.
B. Effect of EGCG on Ca2+-regulation of thin filaments containing TPM1 E54K DCM-causing mutation. Red, E54K-containing thin filaments; green, presence of 100 μM EGCG.
C, D. Dose response curves, the fraction motile was measured at a constant [Ca2+] with increasing concentrations of EGCG for a representative experiment.
C. Effect of EGCG on donor thin filaments at 0.074 μM Ca2+. The inset plots the change in fraction motility with increasing EGCG concentration and the curve represents the fit of the pooled data to the Hill equation. Values of parameters obtained are shown.
D. Effect of EGCG on thin filaments containing TPM1 E180G tropomyosin at 0.037 μM Ca2+. Initially, motility is the same (see Figure 1); the addition of EGCG reduced motility of phosphorylated and increased motility of unphosphorylated thin filaments, indicating recoupling or the restoration of the phosphorylation-dependent Ca2+-sensitivity difference (see Figure 3B). Details as for Figure 2F.
EGCG restores coupling to HCM and DCM mutant thin filaments and myofibrils
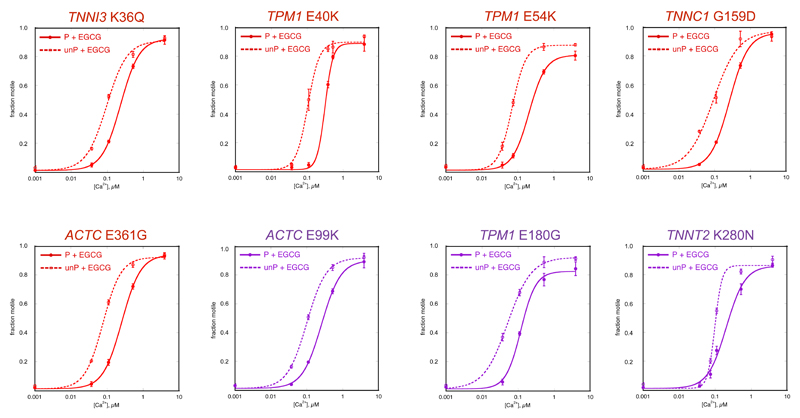
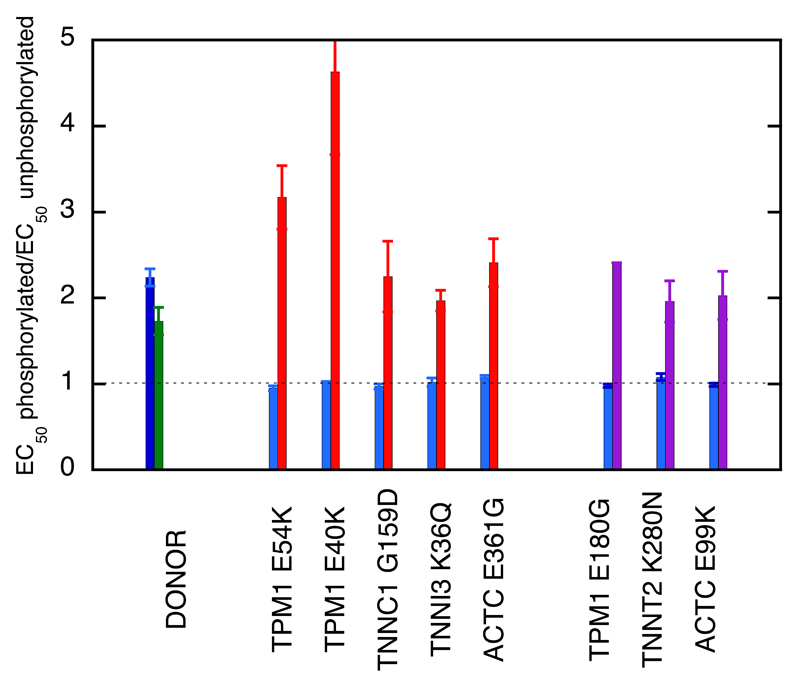
EGCG also affected Ca2+-sensitivity in thin filaments containing mutations associated with HCM or DCM in a phosphorylation-dependent way. This was tested with five DCM-causing mutations and three HCM-causing mutations. EGCG decreased the Ca2+-sensitivity of phosphorylated mutant thin filaments in a similar way to wild-type troponin (Ratio EC50 with/without EGCG was 1.36-2.85). However, with unphosphorylated troponin EGCG tended to have no effect or, in the case of the three tropomyosin mutations, increased Ca2+-sensitivity (Table 2). As a result, the dependence of Ca2+-sensitivity on TnI phosphorylation level was restored. For example, thin filaments containing the DCM-causing TPM1 mutation E54K were uncoupled with EC50 of 0.106±0.013 μM for phosphorylated and 0.114±0.019 μM for unphosphorylated giving a ratio of 0.95±0.03 compared with a ratio of 2.24±0.10 for wild-type thin filaments (Table 1). In the presence of 100 μM EGCG, the EC50 were 0.23±0.03 μM for phosphorylated and 0.071±0.005 μM for unphosphorylated giving a ratio of 3.17±0.37 (p=0.004, n=5) (Table 1, Figure 3B). A dose-response assay for recoupling yielded an EC50 of 58.8±13.3 μM (Figure 3D). Similarly, EGCG was able to reversibly restore coupling of all the thin filament HCM and DCM-causing mutations in this study (Table 2, Figures 4 and 5, Supplement F).
Table 2. Effect of EGCG and TnI phosphorylation on the Ca2+-sensitivity of thin filaments containing HCM and DCM mutations.
| Mutation | EC50 of thin filaments containing phosphorylated troponin μM ± SEM |
EC50 of thin filaments containing unphosphorylated troponin μM ± SEM |
Ratio EC50 P / unP ± SEM | EC50 of thin filaments treated with EGCG containing phosphorylated troponin μM ± SEM |
EC50 of thin filaments treated with EGCG containing unphosphorylated troponin μM ± SEM |
Ratio EC50 P + EGCG / unP + EGCG ± SEM | Ratio EC50 P + EGCG / P ± SEM | Ratio EC50 unP + EGCG / unP ± SEM |
|---|---|---|---|---|---|---|---|---|
| WT | 0.14 ± 0.03(7) | 0.059 ± 0.011(6)†† | 2.24 ± 0.10(6)†† | 0.26 ± 0.02(7)** | 0.15 ± 0.02(6)**†† | 1.73 ± 0.16(6)†† | 2.15 ± 0.45(7) | 2.80 ± 0.48(6) |
| DCM | ||||||||
| TPM1 E54K | 0.11 ± 0.013(5) | 0.11 ± 0.02(5) | 0.95 ± 0.03(5) | 0.23 ± 0.03(5)* | 0.071 ± 0.005(5)†† | 3.17 ± 0.37(5)†† | 2.33 ± 0.53(5) | 0.68 ± 0.10(5) |
| TPM1 E40K | 0.18 ± 0.03(8) | 0.17 ± 0.03(8) | 1.02 ± 0.007(8) | 0.22 ± 0.04(5) | 0.052± 0.01(5)*† | 4.63 ± 0.96(5)† | 1.70 ± 0.43(3) | 0.34 ± 0.05(3) |
| TNNC1 G159D | 0.092 ± 0.004(5) | 0.095 ± 0.005(5) | 0.97 ± 0.03(5) | 0.19 ± 0.03(5)* | 0.088 ± 0.005(5)*† | 2.25 ± 0.41(5)† | 2.13 ± 0.36(5) | 0.93 ± 0.02(5) |
| TNNI3 K36Q | 0.18 ± 0.03(10) | 0.18 ± 0.04(10) | 1.02 ± 0.05(10) | 0.19 ± 0.02(5) | 0.10 ± 0.009(5)*† | 1.97 ± 0.12(5)† | 2.32 ± 0.29(3) | 1.28 ± 0.04(3) |
| ACTC E361G | 0.087 ± 0.002(5) | 0.080 ± 0.002(5) | 1.08 ± 0.02(5) | 0.20 ± 0.02(5)** | 0.081 ± 0.002(5)†† | 2.41 ± 0.28(5)†† | 2.27 ± 0.28(5) | 1.01 ± 0.02(5) |
| HCM | ||||||||
| TPM1 E180G | 0.086 ± 0.013(3) | 0.087 ± 0.011(3) | 0.98 ± 0.02(3) | 0.11 ± 0.01(5)* | 0.048 ± 0.003(5)*† | 2.41 ± 0.32(5)† | 1.36 ± 0.10(3) | 0.51 ± 0.06(3) |
| TNNT2 K280N | 0.11 ± 0.007(5) | 0.10 ± 0.004(5) | 1.08 ± 0.04(5) | 0.20 ± 0.03(5)* | 0.10 ± 0.004(5)† | 1.96 ± 0.24(5)† | 2.15 ± 0.18(3) | 1.09 ± 0.006(3) |
| ACTC E99K | 0.074 ± 0.005(5) | 0.074 ± 0.005(5) | 0.99 ± 0.02(5) | 0.21 ± 0.03(5)** | 0.10 ± 0.007(5)*†† | 2.03 ± 0.21(5)†† | 2.85 ± 0.28(5) | 1.43 ± 0.13(5) |
p<0.05
p<0.01; for presence and absence of EGCG
p<0.05
p<0.01; unphosphorylated compared with phosphorylated using paired t test. Ratios: single value t test compared with 1. ANOVA analysis of this dataset is shown in Supplement E. EC50 values rounded to 2 significant figures. In brackets is the number of experiments.
Figure 4. Coupling is restored to HCM and DCM mutations by EGCG.
Fraction of filaments motile, measured by IVMA in the presence of 100 μM EGCG is plotted against [Ca2+] for representative single experiments. Details as for Figure 1.
Red lines, thin filaments containing DCM-causing mutations, Purple lines, thin filaments containing HCM-causing mutations.
The mean values of EC50 from replicate experiments are plotted in Figure 5 and summarised in Table 2.
Figure 5. The re-coupling effect of EGCG.
The ratio EC50 phosphorylated/EC50 unphosphorylated (coupling constant) is plotted in the absence (blue) and presence (green, red and purple) of 100 μM EGCG. Green, control troponin; red, DCM-causing mutants; purple, HCM-causing mutants, error bars are ± SEM. All the mutant samples are uncoupled but the coupling constant is restored to control levels by EGCG. Data from Table 2.
The effect of EGCG on contraction of myofibrils
We studied the effect of EGCG on mouse myofibril contractility in basally phosphorylated and dephosphorylated states, obtained by treating mice with propranolol prior to sacrifice (Table 3, Figure 6).
Table 3. Effect of phosphorylation, ACTC E361G mutation and EGCG on Ca2+-regulation of myofibril contraction.
In brackets is the number of experiments. Statistical analysis carried out by un-paired t test (equal variance).
| Fmax, kN/m2 | EC50, μM | nH | kACT, s-1 | tLIN, ms | kREL, S-1 | ||
|---|---|---|---|---|---|---|---|
| WT¶ | P | 100.9±6.3(14) | 0.93±0.06(11) | 10.43±1.84(10) | 4.16±0.43(11) | 50.8±3.5(12) | 35.0±4.0(10) |
| unP | 87.1±6.0(16) | 0.51±0.06(14)††† | 4.74±0.66(13)†† | 4.50±0.24(15) | 67.0±4.2(16)† | 23.2±2.8(11)††† | |
| WT+EGCG | P | 92.5±5.0(15) | 1.85±0.17(9)*** | 4.30±0.85(8)** | 4.44±0.24(15) | 46.7±3.9(12) | 41.0±4.0(12) |
| unP | 92.6±8.9(8) | 1.13±0.13(5)††*** | 5.08±1.24(5) | 3.47±0.55(6) | 88.9±9.9(7)†††* | 22.3±1.2(7)†† | |
| E361G¶ | P | 93.5±8.9(11) | 0.38±0.05(12)§§§ | 5.39±1.26(9)§ | 4.51±0.32(13) | 75.4±6.5(10)§§ | 21.6±2.8(10)§§§ |
| unP | 89.3±8.6(10) | 0.43±0.06 (10) | 4.48±0.70(8) | 4.12±0.32(11) | 70.8±9.7(11) | 21.6±2.1(10) | |
| E361G+EGCG | P | 89.1±4.5(12) | 2.07±0.20(5)*** ‡‡‡ | 7.18±1.54(5) | 3.43±0.20(13)*§ ‡‡ | 49.7±3.3(12)** | 31.0±2.6(12)*§ |
| unP | 96.3±11.1(11) | 1.28±0.10(8)††*** ‡‡‡ | 4.14±0.42(7)† | 3.25±0.26(11)*** ‡‡ | 63.0±4.7(12)†§ | 31.0±2.0(12)**§§ ‡‡ | |
p<0.05
p<0.01
p<0.001; EGCG treated compared with no EGCG.
p<0.05
p<0.01
p<0.001; unphosphorylated compared with phosphorylated.
p<0.05
p<0.01
p<0.001; ACTC E361G compared with wild-type.
p<0.05
p<0.01
p<0.001; ACTC E361G EGCG treated compared with no EGCG wild-type.
Vikhorev et al. 2014 11.
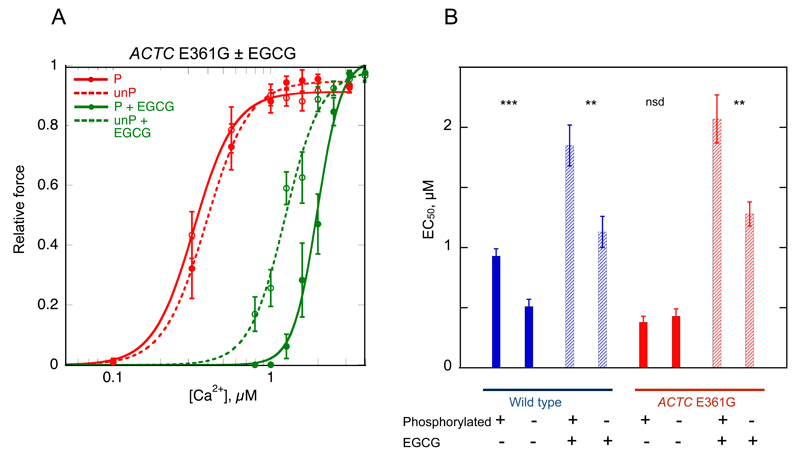
Figure 6. EGCG restores the relationship between Ca2+-sensitivity of force production and TnI phosphorylation in wild-type and ACTC E361G mouse myofibrils.
Phosphorylation level was reduced by propranolol treatment and measured by phosphate affinity SDS-PAGE. Basally phosphorylated wild-type was 1.02±0.03 and ACTC E361G was 1.08±0.01 mols Pi/mol TnI. For propranolol-treated (dephosphorylated) muscle the phosphorylation level was 0.30±0.04 in wild-type and 0.34±0.07 mols Pi/mol TnI for ACTC E361G.
A. Ca2+-activation curves for ACTC E361G myofibrils in the presence (green) and absence (red) of 10 μM EGCG. Solid lines and points, phosphorylated troponin (P); dotted lines and open points, unphosphorylated troponin (unP). The plot shows averaged isometric tension ± SEM from 11-14 myofibrils for experiments performed at SL=2.17 μm. EGCG shifts the activation curve to the right and restores the difference between phosphorylated and unphosphorylated myofibrils.
B. Effects of the ACTC E361G mutation, phosphorylation and EGCG on the mean EC50 (± SEM) for myofibril isometric contraction. Significant differences, calculated by t-test, between phosphorylated and unphosphorylated myofibrils are indicated: ** p < 0.01; *** p < 0.001. Data from Table 3.
We found that 10 μM EGCG decreased Ca2+-sensitivity of isometric force for both phosphorylated and unphosphorylated mouse myofibrils equally (EC50 P/EC50 P + EGCG = 0.50±0.06, p<0.001, n=9, EC50 unP/EC50 unP + EGCG = 0.45±0.07, p<0.00, n=5). Consequently, the effect of phosphorylation on Ca2+-sensitivity in wild-type myofibrils was preserved in the presence of EGCG (EC50 P + EGCG/EC50 unP + EGCG = 1.64±0.24, p<0.001, n=5).
As previously shown 10, in myofibrils from ACTC E361G DCM mice, the Ca2+-sensitivity of force is uncoupled (Table 3, Figure 6). EGCG decreased Ca2+-sensitivity in myofibrils from ACTC E361G mice; however, this effect was greater in phosphorylated myofibrils than unphosphorylated myofibrils. Thus, EGCG restored the phosphorylation-dependent shift in Ca2+-sensitivity for ACTC E361G myofibrils to the same level as in wild-type myofibrils (EC50 P + EGCG/EC50 unP + EGCG = 1.62±0.20, p<0.01, n=5 compared with 1.82±0.24 for wild-type in the absence of EGCG). nH values were similar to those found in wild-type myofibrils (Table 3). The duration of the initial, nearly isotonic, phase of relaxation, tLIN, in phosphorylated and unphosphorylated ACTC E361G myofibrils was restored to wild-type values (Table 3). EGCG decreased the rate of force development (kACT) but did not affect maximum force. kACT was decreased by EGCG in unphosphorylated wild-type myofibrils (decreased by 22.8%, p=0.059, n=15) as well as in phosphorylated and unphosphorylated ACTC E361G (decreased by 23%, p<0.05, n=10 and by 21%, p<0.001, respectively, n=11).
Discussion
In previous studies it was found that mutations in proteins of the cardiac muscle thin filament that are associated with inherited cardiomyopathies (HCM and DCM) alter myofibrillar Ca2+-sensitivity. They also cause the modulation of myofilament Ca2+-sensitivity to become independent of the PKA-dependent phosphorylation of TnI. We have named this phenomenon uncoupling. Moreover, this uncoupling effect may be sufficient to generate the disease phenotype of familial DCM 9, 17. We have investigated whether small molecules might be able to mimic or reverse the molecular effects of mutations.
The Ca2+-sensitisers EMD57033 and Bepridil, known to bind to TnC, induce uncoupling in wildtype thin filaments, thus mimicking the effects of HCM mutations. The Ca2+-desensitiser EGCG has an opposite effect. It preserves coupling in wild-type troponin and restores coupling to thin filaments with HCM and DCM-causing mutations in TnI, TnC, TnT, tropomyosin and actin, thus antagonising the effect of the HCM or DCM mutation. These findings suggest the potential of EGCG for treating the symptoms of inherited cardiomyopathies.
Ca2+-sensitisers mimic the effects of HCM-causing mutations in thin filaments
Both EMD57033 and Bepridil increase Ca2+-sensitivity of phosphorylated thin filaments by 2.8 and 2.9-fold, measured by quantitative IVMA, in common with previous measurements 40, 41. However, it has not been shown before that they increase Ca2+-sensitivity of unphosphorylated thin filaments considerably less than phosphorylated thin filaments. As a consequence, Ca2+-sensitivity becomes independent of the TnI phosphorylation level (uncoupled).
The effect of Ca2+-sensitisers is analogous to the effect of HCM-causing mutations in vitro (see Table 2) where both an increase in the Ca2+-sensitivity and uncoupling of the Ca2+-sensitivity from the TnI phosphorylation are observed. Uncoupling due to HCM mutations was first reported in 2001 13 and several more thin filament mutations have subsequently been demonstrated by in vitro assays 5, 17, as the TPM1 E180G mutation, shown in Figure 1B, demonstrates.
Mechanistic considerations of Ca2+-sensitisers
Since both EMD57033 and Bepridil appear to modulate Ca2+-sensitivity with a minimal effect on the sliding speed in IVMA or maximum isometric force in muscle fibres 10, 42 it is likely that they are acting on troponin rather than the cross-bridge cycling mechanism in our systems.
The Ca2+-sensitivity of cardiac troponin is modulated by the unique N-terminal peptide of TnI (1-30) that contains the PKA phosphorylation sites, Ser22 and 23. When unphosphorylated the peptide interacts with the N-terminal domain of TnC, close to the regulatory Ca2+-binding site and this interaction is lost or reduced when the two serines are phosphorylated 43, 44. The change in Ca2+-sensitivity with TnI phosphorylation is a 2-3 fold reduction.
Bepridil is suggested to displace TnI 1-30 and/or the linker helix TnI 31-70 20, 45. EMD57033 is believed to bind to the C-terminal domain of TnC and also displaces the TnI 31-70 peptide 18, 19, 46. Thus both these compounds could act allosterically by interfering with the modulation of Ca2+-sensitivity due to Ser22/23 phosphorylation, resulting in uncoupling.
Interestingly, in experiments measuring isometric force in cardiac muscle myofibrils, EMD57033 increased Ca2+-sensitivity but only caused uncoupling at short sarcomere lengths. Thus the uncoupling phenomenon may be graded rather than all-or-none. One possibility is that uncoupling may be related to Ca2+-sensitivity if the Ca2+-sensitiser shifts the conformational equilibrium so far towards the N-terminal bound conformation that it cannot be significantly influenced by phosphorylation of the N-terminal peptide of TnI (see Supplement D). However the Ca2+-sensitising property of these compounds does not necessarily involve the same molecular mechanism as uncoupling.
The actions of small molecules that uncouple highlights the crucial role of the allosteric coupling between the TnI 1-30 peptide and ligand binding sites that could be remotely located. Likewise, it is notable that DCM-causing mutations that uncouple are distributed in all the proteins of the thin filament, also demonstrating long-range allosteric interactions 9, 47. We have proposed that these mutations destabilize the unphosphorylated state of the 1-30 peptide 9.
The Ca2+-desensitiser EGCG restores coupling to thin filaments with HCM and DCM mutations
Our observation that EGCG reduces Ca2+-sensitivity 2-3 fold both in human thin filaments and in mouse myofibrils with only small effects on maximum sliding speed, isometric force or Hill coefficient, is in accord with previous measurements in skinned cardiac muscle fibres 21, 23. Moreover we have demonstrated that the phosphorylation-dependent shift in myofilament Ca2+-sensitivity is unaffected by EGCG in both isolated filaments and intact myofibrils, in contrast to the Ca2+-sensitisers (Figures 2, 3 and 6).
EGCG was also able to decrease the Ca2+-sensitivity of phosphorylated thin filaments containing HCM and DCM-causing mutations, however it had different effects on phosphorylated and unphosphorylated thin filaments. With phosphorylated troponin, EGCG decreased Ca2+-sensitivity similarly to wild-type troponin, but EGCG either had no effect or increased Ca2+-sensitivity with unphosphorylated troponin (Table 2). This resulted in the restoration of the coupling of Ca2+-sensitivity change to TnI phosphorylation (Figures 3, 4 and 5).
The ability of EGCG to re-couple all the uncoupled mutations in any of the thin filament proteins equally (actin, tropomyosin, TnI, TnT and TnC; Figures 4 and 5 9, 17) is compatible with a common mechanism for uncoupling and implies that the primary effect of EGCG would be to re-stabilize the unphosphorylated state of the TnI 1-30 peptide. We have previously demonstrated that uncoupling and absolute Ca2+-sensitivity are not related when comparing a series of DCM-causing mutations 9 so the Ca2+-desensitising and re-coupling activities of EGCG may involve separate activities. Indeed, we have found analogues of EGCG that do not affect Ca2+-sensitivity but can still re-couple.
Several studies have located EGCG binding in the C-terminal domain of TnC in the region of the hydrophobic cleft 21, 22, 46. Molecular Dynamics (MD) simulation suggests that it can bind in several interchangeable orientations 46 and unlike EMD57033, EGCG can bind to the C-terminal domain of cTnC even in the presence of TnI 34-71 helix 46. We suggest that it is possible that EGCG can restabilise the TnI – TnC interaction, restoring the Ca2+ response to phosphorylation found in DCM and HCM. MD calculations indicate that the uncoupling mutation cTnC G159D strengthens TnI 34-71 helix binding to the hydrophobic cleft whilst EGCG weakens this interaction 46, compatible with their opposite effects on coupling.
Effects of EGCG on myofibrillar contractility
EGCG decreased Ca2+-sensitivity in phosphorylated and unphosphorylated wild-type myofibrils by ~2 fold but did not change the relaxation parameters tLIN and kREL in phosphorylated myofibrils (see Table 3). The rate of force development (kACT), measured at high Ca2+, was unchanged in myofibrils with phosphorylated TnI and slightly decreased (p=0.059) in unphosphorylated myofibrils. As kACT depends strongly on the Ca2+ concentration 10, we conclude that EGCG shifts the [Ca2+]-kACT relationship towards higher Ca2+ concentration in agreement with IVMA data (Table 1). Thus, unlike EMD57033, EGCG decreases cross-bridge activation kinetics 10.
EGCG reduced Ca2+-sensitivity and kACT in both phosphorylated and unphosphorylated ACTC E361G myofibrils. In addition it restored the lost difference in Ca2+-sensitivity between phosphorylated and unphosphorylated myofibrils and also the difference in the relaxation parameter tLIN (Table 3). The observation that EGCG does not affect the EC50 P/ EC50 unP or tLIN in wild-type myofibrils but changes them in ACTC E361G, suggests that EGCG can restore coupling in ACTC E361G myofibrils independently of its Ca2+-desensitising function.
Although uncoupling can be clearly demonstrated at the level of skinned muscle fibres or myocytes with thin filament mutations causing DCM 35, 48, 49 this does not appear to be true for HCM-causing mutations, despite being indistinguishable at the single filament level. A near-normal response of Ca2+-sensitivity to TnI phosphorylation has been reported for TNNC1 L29Q and TPM1 E180G in transgenic mice 50, 51 and TNNI3 R145W and TNNT2 K280N in human heart myectomy samples 16. Despite this, both DCM and HCM linked mutations in transgenic mice models are associated with an impaired response to β1-adrenergic stimulation and reduced cardiac reserve as would be expected if uncoupled 15, 35 (reviewed by Messer 17). The physiological manifestations of the uncoupling seen in unloaded filaments requires further investigation.
Clinical relevance of re-coupling by EGCG
Ca2+-antagonists have been suggested as being potentially useful for treatment of HCM 52. EGCG represents a new class of Ca2+-antagonists with a very favourable functional profile. EGCG acts directly on the Ca2+-regulatory system of the thin filament that is also the main target of HCM-causing mutations in sarcomeric proteins 5. By binding to troponin it decreases the enhanced Ca2+-sensitivity characteristic of HCM whilst also reversing the uncoupling effect we observed in HCM mutations. The ability to restore coupling to DCM mutant myofibrils could be beneficial, since this was proposed to be the primary cause of DCM, but the reduction in Ca2+-sensitivity in cases where the mutation reduces Ca2+-sensitivity may be deleterious.
EGCG has a wide range of pharmacological properties; indeed it has been cited as an example of a promiscuous molecule 53. Moreover, low doses of EGCG have a positive inotropic effect in intact heart muscle, due to effects on the ryanodine receptor type 2, Na+-K+ ATPase and Na+-Ca2+ Exchanger, that may override the Ca2+-desensitising effect in the contractile apparatus 54, 55.
Although EGCG is too non-specific to be a viable drug for treating HCM, this study has demonstrated a significant proof of principle: it is possible to directly reverse the molecular mechanism of HCM-causing mutations pharmacologically. The coupling of Ca2+-sensitivity to cTnI phosphorylation has been demonstrated to be a labile property of troponin. HCM or DCM mutations and Ca2+-sensitisers can induce uncoupling, indicating that small perturbations can destabilise troponin 9, 17, 32. It is very unusual to find a reagent that will give a gain of function by apparently restoring the native conformational state in the presence of mutations that destabilised this state.
Our findings provide a starting point for investigating molecules related to EGCG that may be more efficacious and act specifically on troponin 56.
Supplementary Material
Acknowledgements
We are grateful to Cristobal Dos Remedios (University of Sydney, Sydney, Australia) for the donor heart muscle, TNNI3 K36Q DCM mutant and TNNT2 K280N HCM mutant explanted human heart muscle samples. Kristen Nowak, Elyshia McNamara and Royston Ong (University of Western Australia, Perth, Australia) for the baculovirus-expressed mutant TPM1 samples. O’Neal Copeland for technical help and Ian Gould (Chemistry Department, Imperial College London, UK) for help with the structure of troponin and Molecular Dynamics simulations. EMD57033 was a gift from E Merck KGaA, Darmstadt.
Funding
This work was supported by grants from the British Heart Foundation (RG/11/20/29266 and FS/12/24/29568)
Abbreviations
- DCM
Dilated Cardiomyopathy
- EGCG
Epigallocatechin-3-Gallate
- FMAX
Maximum force
- HCM
Hypertrophic Cardiomyopathy
- IVMA
In vitro Motility Assay
- kACT
Rate of force development
- kREL
Rate of fast relaxation phase
- MD
Molecular Dynamics
- nH
Hill Coefficient
- P
Phosphorylated
- PKA
Protein Kinase A
- tLIN
Duration of slow relaxation phase
- TnC
Troponin C
- TnI
Troponin I
- TnT
Troponin T
- unP
Unphosphorylated
Footnotes
Conflict Of Interest Statement
The authors declare no conflict of interest.
References
- 1.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 3.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 4.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 5.Marston SB. How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res. 2011;4:245–255. doi: 10.1007/s12265-011-9266-2. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 7.Knollmann BC, Potter JD. Altered regulation of cardiac muscle contraction by troponin t mutations that cause familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2001;11:206–212. doi: 10.1016/s1050-1738(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- 9.Memo M, Leung M-C, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Mutations in thin filament proteins that cause familial dilated cardiomyopathy uncouple troponin i phosphorylation from changes in myofibrillar ca2+-sensitivity. Cardiovasc Res. 2013;99:65–73. doi: 10.1093/cvr/cvt071. [DOI] [PubMed] [Google Scholar]
- 10.Vikhorev PG, Song W, Wilkinson R, Copeland O, Messer AE, Ferenczi MA, Marston SB. The dilated cardiomyopathy-causing mutation actc e361g in cardiac muscle myofibrils specifically abolishes modulation of ca2+ regulation by phosphorylation of troponin i. Biophys J. 2014;107:2369–2380. doi: 10.1016/j.bpj.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidtmann A, Lindow C, Villard S, Heuser A, Mügge A, Gessner R, Granier C, Jaquet K. Cardiac troponin c-l29q, related to hypertrophic cardiomyopathy, hinders the transduction of the protein kinase a dependent phosphorylation signal from cardiac troponin i to c. FEBS J. 2005;272:6087–6097. doi: 10.1111/j.1742-4658.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong W, Xing J, Ouyang Y, An J, Cheung HC. Structural kinetics of cardiac troponin c mutants linked to familial hypertrophic and dilated cardiomyopathy in troponin complexes. J Biol Chem. 2008;283:3424–3432. doi: 10.1074/jbc.M703822200. [DOI] [PubMed] [Google Scholar]
- 13.Deng Y, Schmidtmann A, Redlich A, Westerdorf B, Jaquet K, Thieleczek R. Effects of phosphorylation and mutation r145g on human cardiac troponin i function. Biochemistry. 2001;40:14593–14602. doi: 10.1021/bi0115232. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Pinto J, Sancho Solis R, Dweck D, Liang J, Diaz-Perez Z, Ge Y, Walker J, Potter J. The generation and functional characterization of knock in mice harboring the cardiac troponin i-r21c mutation associated with hypertrophic cardiomyopathy. J Biol Chem. 2011;287:2156–2167. doi: 10.1074/jbc.M111.294306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W, Dyer E, Stuckey D, Copeland O, Leung M, Bayliss C, Messer AE, Wilkinson R, Tremoleda J, Schneider M, Harding S, et al. Molecular mechanism of the glu99lys mutation in cardiac actin (actc gene) that causes apical hypertrophy in man and mouse. J Biol Chem. 2011;286:27582–27593. doi: 10.1074/jbc.M111.252320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequeira V, Wijnker PJM, Nijenkamp LLAM, Kuster DWD, Najafi A, Witjas-Paalberends ER, Regan JA, Boontje N, ten Cate FJ, Germans T, Carrier L, et al. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013;112:1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messer A, Marston S. Investigating the role of uncoupling of troponin i phosphorylation from changes in myofibrillar ca2+-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li MX, Robertson IM, Sykes BD. Interaction of cardiac troponin with cardiotonic drugs: A structural perspective. Biochem Biophys Res Commun. 2008;369:88–99. doi: 10.1016/j.bbrc.2007.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Li MX, Spyracopoulos L, Beier N, Chandra M, Solaro RJ, Sykes BD. Structure of the c-domain of human cardiac troponin c in complex with the ca2+ sensitizing drug emd 57033. J Biol Chem. 2001;276:25456–25466. doi: 10.1074/jbc.M102418200. [DOI] [PubMed] [Google Scholar]
- 20.Abusamhadneh E, Abbott MB, Dvoretsky A, Finley N, Sasi S, Rosevear PR. Interaction of bepridil with the cardiac troponin c/troponin i complex. FEBS Lett. 2001;506:51–54. doi: 10.1016/s0014-5793(01)02790-9. [DOI] [PubMed] [Google Scholar]
- 21.Liou Y-M, Kuo S-C, Hsieh S-R. Differential effects of a green tea-derived polyphenol (-)-epigallocatechin-3-gallate on the acidosis-induced decrease in the ca(2+) sensitivity of cardiac and skeletal muscle. Pflugers Arch. 2008;456:787–800. doi: 10.1007/s00424-008-0456-y. [DOI] [PubMed] [Google Scholar]
- 22.Robertson IM, Li MX, Sykes BD. Solution structure of human cardiac troponin c in complex with the green tea polyphenol, (-)-epigallocatechin 3-gallate. J Biol Chem. 2009;284:23012–23023. doi: 10.1074/jbc.M109.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadano N, Du C, Yumoto F, Morimoto S, Ohta M, Xie M, Nagata K, Zhan D, Lu Q, Miwa Y, Takahashi-Yanaga F, et al. Biological actions of green tea catechins on cardiac troponin c. Brit J Pharmacol. 2010;161:1034–1043. doi: 10.1111/j.1476-5381.2010.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda N, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 25.Solaro RJ, Rosevear P, Kobayashi T. The unique functions of cardiac troponin i in the control of cardiac muscle contraction and relaxation. Biochem Biophys Res Commun. 2008;369:82–87. doi: 10.1016/j.bbrc.2007.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific n-extension of troponin i in cardiac troponin. J Mol Biol. 2007;373:706–722. doi: 10.1016/j.jmb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Gould I, Messer AE, Papadaki M, Marston SB. *modulation of the interaction between troponin i n-terminal peptide and troponin c by phosphorylation studied by molecular dynamics. Biophys J. 2014;106:349a. [Google Scholar]
- 28.Cheng Y, Lindert S, Kekenes-Huskey P, Rao VS, Solaro RJ, Rosevear PR, Amaro R, McCulloch AD, McCammon JA, Regnier M. Computational studies of the effect of the s23d/s24d troponin i mutation on cardiac troponin structural dynamics. Biophys J. 2014;107:1675–1685. doi: 10.1016/j.bpj.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: Dephosphorylation of ser23/24 on troponin i could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007;42:247–259. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Dyer E, Jacques A, Hoskins A, Ward D, Gallon C, Messer A, Kaski J, Burch M, Kentish J, Marston S. Functional analysis of a unique troponin c mutation, gly159asp that causes familial dilated cardiomyopathy, studied in explanted heart muscle. Circ Heart Fail. 2009;2:456–464. doi: 10.1161/CIRCHEARTFAILURE.108.818237. [DOI] [PubMed] [Google Scholar]
- 31.Carballo S, Robinson P, Otway R, Fatkin D, Jongbloed JD, de Jonge N, Blair E, van Tintelen JP, Redwood C, Watkins H. Identification and functional characterization of cardiac troponin i as a novel disease gene in autosomal dominant dilated cardiomyopathy. Circ Res. 2009;105:375–382. doi: 10.1161/CIRCRESAHA.109.196055. [DOI] [PubMed] [Google Scholar]
- 32.Bayliss CR, Jacques AM, Leung M-C, Ward DG, Redwood CS, Gallon CE, Copeland O, Mckenna WJ, Dos Remedios C, Marston SB, Messer AE. Myofibrillar ca2+-sensitivity is uncoupled from troponin i phosphorylation in hypertrophic obstructive cardiomyopathy due to abnormal troponin t. Cardiovasc Res. 2012;97:500–508. doi: 10.1093/cvr/cvs322. [DOI] [PubMed] [Google Scholar]
- 33.Akkari PA, Song Y, Hitchcock-DeGregori S, Blechynden L, Laing N. Expression and biological activity of baculovirus generated wild-type human slow alpha tropomyosin and the met9arg mutant responsible for a dominant form of nemaline myopathy. Biochem Biophys Res Commun. 2002;296:300–304. doi: 10.1016/s0006-291x(02)00852-5. [DOI] [PubMed] [Google Scholar]
- 34.Marston S, Memo M, Messer A, Papadaki M, Nowak K, McNamara E, Ong R, El-Mezgueldi M, Li X, Lehman W. Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients. Hum Mol Genet. 2013;22:4978–4987. doi: 10.1093/hmg/ddt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song W, Dyer E, Stuckey D, Leung M-C, Memo M, Mansfield C, Ferenczi M, Liu K, Redwood C, Nowak K, Harding S, et al. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J Mol Cell Cardiol. 2010;49:380–389. doi: 10.1016/j.yjmcc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Messer A, Gallon C, McKenna W, Elliott P, Dos Remedios C, Marston S. The use of phosphate-affinity sds-page to measure the troponin i phosphorylation site distribution in human heart muscle. Proteomics Clin Appl. 2009;3:1371–1382. doi: 10.1002/prca.200900071. [DOI] [PubMed] [Google Scholar]
- 37.Fraser IDC, Marston SB. In vitro motility analysis of actin-tropomyosin regulation by troponin and ca2+: The thin filament is switched as a single cooperative unit. J Biol Chem. 1995;270:7836–7841. doi: 10.1074/jbc.270.14.7836. [DOI] [PubMed] [Google Scholar]
- 38.Marston SB, Fraser IDC, Wu B, Roper G. A simple method for automatic tracking of actin filaments in the motility assay. J Musc Res Cell Motil. 1996;17:497–506. doi: 10.1007/BF00123365. [DOI] [PubMed] [Google Scholar]
- 39.Bayliss C, Messer A, Leung M-C, Ward D, van der Velden J, Poggesi C, Redwood C, Marston S. *functional investigation of troponin with the homozygous hcm mutation, tnnt2 k280m, obtained from an explanted heart. Cardiovasc Res. 2012;93:S107. [Google Scholar]
- 40.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, Lakatta EG. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- 42.Wolska BM, Kitada Y, Palmiter KA, Westfall MV, Johnson MD, Solaro RJ. Cgp-48506 increases contractility of ventricular myocytes and myofilaments by effects on actin-myosin reaction. Am J Physiol. 1996;270:H24–32. doi: 10.1152/ajpheart.1996.270.1.H24. [DOI] [PubMed] [Google Scholar]
- 43.Ward DG, Brewer SM, Calvert MJ, Gallon CE, Gao Y, Trayer IP. Characterization of the interaction between the n-terminal extension of human cardiac troponin i and troponin c. Biochemistry. 2004;43:4020–4027. doi: 10.1021/bi036128l. [DOI] [PubMed] [Google Scholar]
- 44.Baryshnikova OK, Li MX, Sykes BD. Modulation of cardiac troponin c function by the cardiac-specific n-terminus of troponin i: Influence of pka phosphorylation and involvement in cardiomyopathies. J Mol Biol. 2008;375:735–751. doi: 10.1016/j.jmb.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 45.Kleerekoper Q. Identification of binding sites for bepridil and trifluoperazine on cardiac troponin c. J Biol Chem. 1998;273:8153–8160. doi: 10.1074/jbc.273.14.8153. [DOI] [PubMed] [Google Scholar]
- 46.Botten D, Fugallo G, Fraternali F, Molteni C. A computational exploration of the interactions of the green tea polyphenol (-)-epigallocatechin 3-gallate with cardiac muscle troponin c. PLoS ONE. 2013;8:e70556. doi: 10.1371/journal.pone.0070556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manning EP, Tardiff JC, Schwartz SD. Molecular effects of familial hypertrophic cardiomyopathy-related mutations in the tnt1 domain of ctnt. J Mol Biol. 2012;421:54–66. doi: 10.1016/j.jmb.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin c g159d mutation blunts myofilament desensitization induced by troponin i ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 49.Pinto JR, Siegfried JD, Parvatiyar MS, Li D, Norton N, Jones MA, Liang J, Potter JD, Hershberger RE. Functional characterization of tnnc1 rare variants identified in dilated cardiomyopathy. J Biol Chem. 2011;286:34404–34412. doi: 10.1074/jbc.M111.267211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alves ML, Dias FAL, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RT, Sakthivel S, et al. Desensitization of myofilaments to ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ: Cardiovasc Genet. 2014;7:132–143. doi: 10.1161/CIRCGENETICS.113.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li AY, Stevens CM, Liang B, Rayani K, Little S, Davis J, Tibbits GF. Familial hypertrophic cardiomyopathy related cardiac troponin c l29q mutation alters length-dependent activation and functional effects of phosphomimetic troponin i*. PLoS ONE. 2013;8:e79363. doi: 10.1371/journal.pone.0079363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA, Seidman CE, et al. The l-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–1020. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ingólfsson HI, Thakur P, Herold KF, Hobart EA, Ramsey NB, Periole X, de Jong DH, Zwama M, Yilmaz D, Hall K, Maretzky T, et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS chemical biology. 2014;9:1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, Nonogaki T. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: The measurements of cellular ca2+ and nitric oxide release. Eur J Pharmacol. 2006;552:123–130. doi: 10.1016/j.ejphar.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Feng W, Hwang HS, Kryshtal DO, Yang T, Padilla IT, Tiwary AK, Puschner B, Pessah IN, Knollmann BC. Coordinated regulation of murine cardiomyocyte contractility by nanomolar (-)-epigallocatechin-3-gallate, the major green tea catechin. Mol Pharmacol. 2012;82:993–1000. doi: 10.1124/mol.112.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khandelwal A, Hall JA, Blagg BSJ. Synthesis and structure–activity relationships of egcg analogues, a recently identified hsp90 inhibitor. J Org Chem. 2013;78:7859–7884. doi: 10.1021/jo401027r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.