Abstract
Objective
Diabetes is associated with left ventricular diastolic and systolic dysfunction. South Asians may be at particular risk of developing LV dysfunction due to a high prevalence of diabetes. We investigated the role of diabetes and hyperglycaemia in LV dysfunction in a community-based cohort of older South Asians and White Europeans.
Research Design and Methods
Conventional and Doppler echocardiography was performed in 999 participants (542 Europeans, 457 South Asians aged 58-86 years) in a population-based study. Anthropometry, fasting bloods, coronary artery calcification scoring, blood pressure and renal function were measured.
Results
Diabetes, and hyperglycaemia across the spectrum of HbA1c had a greater adverse effect on LV function in South Asians than Europeans (NT-proBNP beta±SE 0.09±0.04, p=0.01 versus -0.04±0.05, p=0.4, p for HbA1c/ethnicity interaction 0.02), diastolic function (E/e’ 0.69±0.12, p<0.0001 versus 0.09±0.2, p=0.6, p interaction 0.005, and systolic function (s’ -0.11±0.06, p=0.04 versus 0.14±0.09, p=0.1, p interaction 0.2). Multivariable adjustment for hypertension, microvascular disease, LV mass, coronary disease and dyslipidaemia only partially accounted for the ethnic differences. Adverse LV function in diabetic South Asians could not be accounted for by poorer glycaemic control or longer diabetes duration.
Conclusions
Diabetes and hyperglycaemia have a greater adverse effect on LV function in South Asians than Europeans incompletely explained by adverse risk factors. South Asians may require earlier, and more aggressive treatment of their cardiometabolic risk factors to reduce risks of LV dysfunction.
Keywords: systolic function, diastolic function, heart failure, ethnicity
Diabetes is a major risk factor for cardiovascular disease and heart failure(1) with increasing importance as a consequence of increasing diabetes prevalence and ageing populations. However the existence of a distinct diabetes related cardiomyopathy, specifically as a consequence of hyperglycaemia, is controversial (2–4). Understanding the underlying mechanisms driving the association between diabetes and left ventricular function is greatly assisted by studying ethnic minority groups where risk factor clustering markedly differs.
South Asians across the world have a substantially elevated risk of diabetes. In comparison with European origin populations, diabetes prevalence is four fold greater; by the age of 80 one in two South Asians will have developed diabetes compared to only one in five Europeans (5) . We have reported that diabetes increases the risk of stroke and ischemic heart disease to a greater extent in South Asians than Europeans (6), for reasons that are unclear. It is unknown whether diabetes also exerts a more adverse impact of LV function and risk of heart failure in South Asians.
The comparative risk of heart failure (HF) has not been measured directly, but could be as much as 5-fold higher in South Asians(7) and there is growing evidence that survival, severity and aetiology of HF also differ by ethnicity(8). Both LV systolic dysfunction (LVSD) and diastolic dysfunction (LVDD) are independent risk factors for development of HF and cardiac death(9,10). Heart failure in middle-age is rare but emerges with older age and with population ageing. Studying risk factor associations with subclinical disease may shed light on underlying risks and mechanisms, and avoids the problem of medication for heart failure altering the nature of their outcomes.
Ethnic differences in prevalence and severity of LV dysfunction have been reported (8,11–13) however, these reports are from selected populations with established disease, or with all morbidities excluded; and therefore cannot fully explore the role of diabetes and hyperglycaemia in accounting for ethnic differences in risk(14–17).Given the elevated rates diabetes and adverse cardiovascular disease (CVD) risk factors in South Asians, previous studies excluding individuals with known CVD or risk factors (13) may have therefore resulted in biased comparisons.
We therefore aimed to test the hypotheses that diabetes and the spectrum of hyperglycaemia would be associated with measures of left ventricular dysfunction, and that these relationships would differ by ethnicity. We also sought to establish whether established risk factors could account for any ethnic differences observed in these associations.
Research design and methods
Study Population
A population based sample of 1206 men and women of white European and South Asian ethnicity were recruited from the Southall and Brent REvisited (SABRE) cohort. SABRE recruited between 1988-1991 between the ages of 40-69 years from West London primary care registers(18). Random selection of participants was made from 5-year age- and sex-stratified primary care physician lists and workplaces in the London districts of Southall and Brent. This paper presents a cross-sectional analysis derived from the 20 year follow-up data. All surviving participants were invited to participate in the follow-up study; the only exclusion criterion was if patients were not able to give informed consent. At the follow up clinic in 2008-2011, participants were aged 59-86 years. Ethics committee approval was obtained and all participants gave written informed consent. Ethnicity was assigned by clinic staff and confirmed by participants. All South Asians were first generation migrants from the Indian subcontinent, the majority were of Punjabi origin. Participants were fasted for 12hrs and refrained from medication on the morning of their visit. They completed a questionnaire detailing health behaviours, morbidity and medications.
Clinic data and blood/urine measurements
Height, weight, waist and hip circumference and blood pressure were measured under standardised conditions(18) and the waist-to-hip circumference ratio (WHR) was calculated. Fasting samples were analysed for blood glucose, glycosylated haemoglobin (HbA1c), insulin, triglycerides, total and high density lipoprotein (HDL) cholesterol and the total:HDL cholesterol ratio was calculated. Serum N-terminal-pro-brain natriuretic peptide (NT-proBNP) was measured using an Elecsys 2010 electrochemiluminescence analyser (Roche Diagnostics, Burgess Hill, UK) calibrated using the manufacturer’s reagents. A spot early morning urine sample was measured for albumin and creatinine. Microalbuminuria was defined as an albumin:creatinine ratio>2.5mg/mol for men and >3.5mg/mol for women. Established diabetes was diagnosed as patient or GP reported and defined according to the 1999 World Health Organisation guidelines(19). Those without known diabetes underwent an oral glucose tolerance test. Diabetes duration was established by questionnaire. Hypertension was defined as physician diagnosed hypertension or participant reported hypertension in people receiving blood pressure (BP) lowering medication.
Two-dimensional (2D) and Conventional and Tissue Doppler Echocardiography
Transthoracic 2D and Doppler echocardiography was performed as previously described (20) to assess both systolic and diastolic function on 602 Europeans (88%) and 480 South Asians (92%). Left atrial (LA) diameter was measured and indexed to height2.7(LADi). Full Doppler echocardiography could not be performed on 84 individuals with poor acoustic windows (8%) or 40 individuals in atrial fibrillation (4%). Transmitral flow velocity during the early filling phase (E), its deceleration time (DT) (a measure of LV relaxation) and the late filling phase (A) were acquired by pulsed Doppler and averaged from 3 consecutive cycles. Tissue Doppler echocardiography (TDE) was performed on the lateral and septal LV wall. Peak velocities during systole (s′) early diastole (e′) and late diastole (a′) were averaged from 3 consecutive cycles. The s′, e′ and a′ wave velocities taken from the lateral and septal walls were averaged. Intra- and inter-observer reproducibility of echocardiographic measures was assessed by separate scans (on different days) performed in 10 participants selected at random. Intra- and inter-observer reproducibility was excellent for all key measures (intra-class correlation coefficients (ICC) >0.80).
Calculated variables
Doppler variables
The ratio of the transmitral early and late filling phases (E:A) was calculated as a measure of diastolic function. The ratio of early filling and early myocardial velocity (E/e′), was calculated as a non-invasive index of LV filling pressure.
2D echocardiography variables
LV mass was calculated as previously described (21).
LV mass = 0.8 *(1.04 *((IVS(d)+ LVID(d)+ PWT(d))3- LVID(d))3) +0.6
LV mass and LA diameter were indexed to height2.7 (LVMI)(22). Ejection fraction (EF) was calculated as a measure of systolic function.
EF=(Stroke volume(SV)/end- diastolic volume (EDV)) *100
LV dysfunction
To establish the burden of prevalent disease for cross-study comparison, systolic dysfunction was defined as EF<50%. Diastolic dysfunction was assessed on the basis of the tissue Doppler mitral annular velocities and the Doppler measures of mitral inflow. Cut-offs conformed to guidelines for diastolic function (23).
Key measures of function
Key measures were selected a priori and analysed as continuous variables. Using newer echocardiographic measures, which are more precise and less prone to pseudonormalization, our key comparator was s′ for longitudinal systolic function, and E/e′ for diastolic function. NT-proBNP levels indicated LV global dysfunction(24).
Coronary Artery Calcification Score (CACS) and CHD
Coronary artery calcification score was acquired using a Philips 64 slice CT scanner as previously described(25). Scans were read by an experienced radiographer blinded to participant ethnicity and other characteristics. The ICC was >0.9. CACS were combined with CHD information to create a combined clinical/subclinical CHD variable, labelled CACS/CHD. Categories were based on previously accepted cut-points. Category 1 included individuals with minimal CACS (<10 AU) and no CHD: Category 2 included individuals with moderate CACS (10-400AU) and no CHD; Category 3, included individuals with severe CACS (>400AU) and/or prevalent CHD, defined as a coronary event or revascularisation identified by medical record review, and adjudicated by an independent committee (6).
Data analysis
Statistical analyses were performed using Stata 12.0. Of the 1206 (684 Europeans, 522 South Asians) attending clinic, 1082 (602 Europeans, 480 South Asians) underwent 2D and Doppler echocardiography. Of these, 999 (542 Europeans, and 457 South Asians) had full covariate data. Participant characteristics are reported as mean±SD, median (interquartile range) for skewed data and n (%) for categorical data. Ethnic group comparisons were made using analysis of variance (ANOVA) or covariance (ANCOVA). Covariates (age, sex, heart rate, hypertension, ratio of total cholesterol:high density lipoprotein, waist-hip ratio, microalbuminuria, clinical/subclinical coronary heart disease and left ventricular mass) were chosen a priori based on their known influence on LV function (26). For related variables (e.g. BMI and WHR), the single variable that most attenuated the ethnic difference was retained in the final model. Significance was assigned at p<0.05. We have previously shown important ethnicity × diabetes interactions for clinical endpoints (CVD events and stroke)(6), and hypothesised these would be present for subclinical disease. Ethnic differences in systolic and diastolic function were similar in men and women so sexes were combined for analysis.
Results
Participant Characteristics
Mean age was around 69 years (table 1). South Asians had a greater prevalence and duration of type 2 diabetes (p<0.0001), more frequent clinical plus subclinical CHD (p<0.0001), microalbuminuria (p=0.001) and hypertension (p<0.0001) than Europeans. They also had more adverse lipid profiles (triglycerides, p=0.02), were hyperinsulinaemic (p=0.01), more centrally obese (WHR, p<0.0001).
Table 1.
Participant demographics and LV functional measures
| European | South Asian | P value | |
|---|---|---|---|
| n | 542 | 457 | |
| Male (n (%)) | 419(77) | 387 (85) | 0.003 |
| Age (y) | 69.8±6.3 | 68.9±6.1 | 0.03 |
| BMI (Kg/m2) | 27.6±4.6 | 26.2±3.7 | <0.0001 |
| WHR | 0.97±0.07 | 1.00±0.07 | <0.0001 |
| CHD/CACs>400 AU | 76(14) | 132(29) | <0.0001 |
| Microalbuminuria (n (%)) | 76(14) | 100(22) | 0.001 |
| Hypertension (n (%)) | 295(54) | 341(75) | <0.0001 |
| SBP (mmHg) | 138±17 | 142±18 | 0.001 |
| DBP (mmHg | 77±10 | 76±10 | 0.2 |
| HR | 68±12 | 67±13 | 0.09 |
| NGT (n%) | 310(57) | 179(40) | <0.0001 |
| IGT/IFG (n%) | 133(25) | 89(19) | |
| Diabetes (n (%)) | 99(18) | 189(41) | |
| Diabetes duration (y) | 4.5(0,9) | 9.0(2,19) | 0.001 |
| Fasting Glucose (mmol/L) | 5.1(4.8,5.6) | 5.3(4.8,6.1) | 0.0001 |
| HbA1c (%) | 5.9(5.6,6.2) | 6.2(5.9,6.9) | <0.0001 |
| HbA1c (mmol/mol) | 41(38,44) | 44(41,52) | <0.0001 |
| Insulin (pmol/L) | 8.4(5.4,13) | 9.7(6.2,15) | 0.01 |
| Fasting Triglycerides (mmol/L) | 1.14(0.9,1.5) | 1.21(0.9,1.7) | 0.02 |
| Cholesterol: HDL (mmol/L) | 3.5(2.9,4.2) | 3.4(2.8,4.2) | 0.6 |
| LVMI-2D (g/m2.7) | 43.7±12 | 42.0±11 | 0.02 |
| LVSD (%) | 118(22) | 93 (20) | 0.6 |
| EF (%) | 61±10 | 62±9 | 0.4 |
| Peak s’ (cm/s) | 7.80±1.4 | 7.46±1.4 | 0.0001 |
| LVDD n(%) | 511(94) | 422(92) | 0.2 |
| E:A | 0.84±0.2 | 0.89±0.3 | 0.005 |
| DT (ms) | 245±51 | 236±50 | 0.006 |
| Peak e’ (cm/s) | 7.21±1.8 | 7.20±1.8 | 0.9 |
| E:e’ | 8.75±2.8 | 9.85±3.1 | <0.0001 |
| LADi (cm/ m2.7) | 0.96±0.2 | 0.99±0.2 | 0.0007 |
| NT-proBNP (pg/ml) | 134(123,149) | 150(136,164) | 0.2 |
| NT-proBNP adjusted for LV mass | 131(118,144) | 156(141,170) | 0.02 |
Data are mean±SD, median (interquartile range) for skewed data , n (%) for categorical data and mean (95%CI) for NT-proBNP. BMI, body mass index; WHR, waist to hip ratio; CACS, coronary artery calcification score; CHD, coronary heart disease; SBP, brachial systolic blood pressure; DBP, Diastolic blood pressure ; HR heart rate; NGT normal glucose tolerance; IGT impaired glucose tolerance; IFG impaired fasting glucose; Total cholesterol: HDL (high-density lipoprotein) ratio; HbA1c, glycated heamoglobin; LVMI, left ventricle mass indexed to height2.7; LVSD,LV systolic dysfunction; Peak s’, mitral annular peak systolic velocity; EF, ejection fraction; LVDD, LV diastolic dysfunction; E:A, Mitral E wave : A wave ratio; DT Mitral E wave deceleration time; Peak e’, mitral annular early peak diastolic velocity; E/e’ estimated filling pressure; LADi, LA diameter indexed to height2.7.
LV measures
NT-proBNP was non-significantly higher in South Asians than Europeans (p=0.2). On adjustment for LV mass this ethnic difference increased and became statistically significant (p=0.02)(Table 1).
South Asians had reduced LV mass index (LVMI) compared to Europeans (p=0.02). While there was no ethnic difference in prevalence of systolic dysfunction (LVSD, p=0.6) as assessed by EF, peak s′ was significantly lower in South Asians than Europeans (p<0.0001) (Table 1). Diastolic dysfunction, largely mild, was common in both ethnic groups (p=0.2, Table 1). E:A and E/e′ were significantly higher in South Asians than Europeans (E:A p=0.005, E/e′ p<0.0001) . DT was significantly reduced in South Asians (p=0.006) and LADI was significantly larger (p=0.0007,Table 1).
LV function and HbA1c within ethnic group
Statistically significant interactions between glycaemic status and ethnicity on key measures of LV function were observed (see below) and therefore associations between glycaemia and function were analysed separately by ethnicity.
Glycated haemoglobin was not related to any measure of LV function in Europeans (Table 2: β(SE), p values :NT-proBNP (-0.04(0.05) p=0.4), E/e’ (0.09(0.2) p=0.6) and peak s’ (0.14(0.09)p=0.1)), but was strongly and adversely associated with functional measures in South Asians (NT-proBNP (0.09(0.04) p=0.01), E/e’ (0.69(0.12) p<0.0001) and peak s’ (-0.11(0.06) p=0.04)). After further adjustment for key cardiometabolic risk factors (heart rate, hypertension, total cholesterol: HDL ratio, WHR, microalbuminuria, CHD/CAC and LV mass) the significant association between HbA1c and E/e’ In South Asians remained (0.52(0.12) p<0.0001) however the associations with peak s’ (-0.06(0.05) p=0.3), and NT-proBNP (0.02(0.04 p=0.5) were attenuated. Full regression results are presented in the online supplement Table S1 for Europeans and Table S2 for South Asians.
Table 2.
Association between measures of LV function and HbA1c stratified by ethnicity
| European | South Asian | ||||
|---|---|---|---|---|---|
| n=542 | n=457 | ||||
| β(SE) | P value | β(SE) | P value | ||
| NT-proBNP (pg/ml) | Model 1 | -0.04(0.05) | 0.4 | 0.09(0.04) | 0.01 |
| Model 2 | -0.08(0.05) | 0.08 | 0.02(0.04) | 0.5 | |
| E/e’ | Model 1 | 0.09(0.2) | 0.6 | 0.69(0.12) | <0.0001 |
| Model 2 | -0.13(0.17) | 0.4 | 0.52(0.12) | <0.0001 | |
| Peak s’ (cm/s) | Model 1 | 0.14(0.09) | 0.1 | -0.11(0.06) | 0.04 |
| Model 2 | 0.16(0.09) | 0.06 | -0.06(0.05) | 0.3 | |
Data are β±SE. Adjustments - model 1: age and sex; model 2: age, sex, Heart rate, hypertension, ratio of total cholesterol:high density lipoprotein, waist-hip ratio, microalbuminuria, clinical/subclinical coronary heart disease and left ventricular mass.
Exploration of interactions between ethnic group and HbA1c/glucose tolerance categories
For most measures of LV function, worsening glycaemic status had a more adverse effect on LV function in South Asians than Europeans, which was statistically significant when tested as an interaction. Across glucose tolerance categories, interaction tests were β(SE), p values 0.14(0.06) p=0.02 for NT-proBNP, 0.46(0.22) p=0.03 for E/e’, and -0.05(0.10) p=0.6 for s’ (figure 1a to 1c). Multivariable adjustment for age, sex, heart rate, hypertension, total cholesterol: HDL ratio, WHR, microalbuminuria, CHD/CAC and LV mass, did not abolish the statistically significant interaction for NT-proBNP (0.14(0.06) p=0.02) but did slightly attenuate the E/e’ interaction (0.39(0.21) p=0.06).
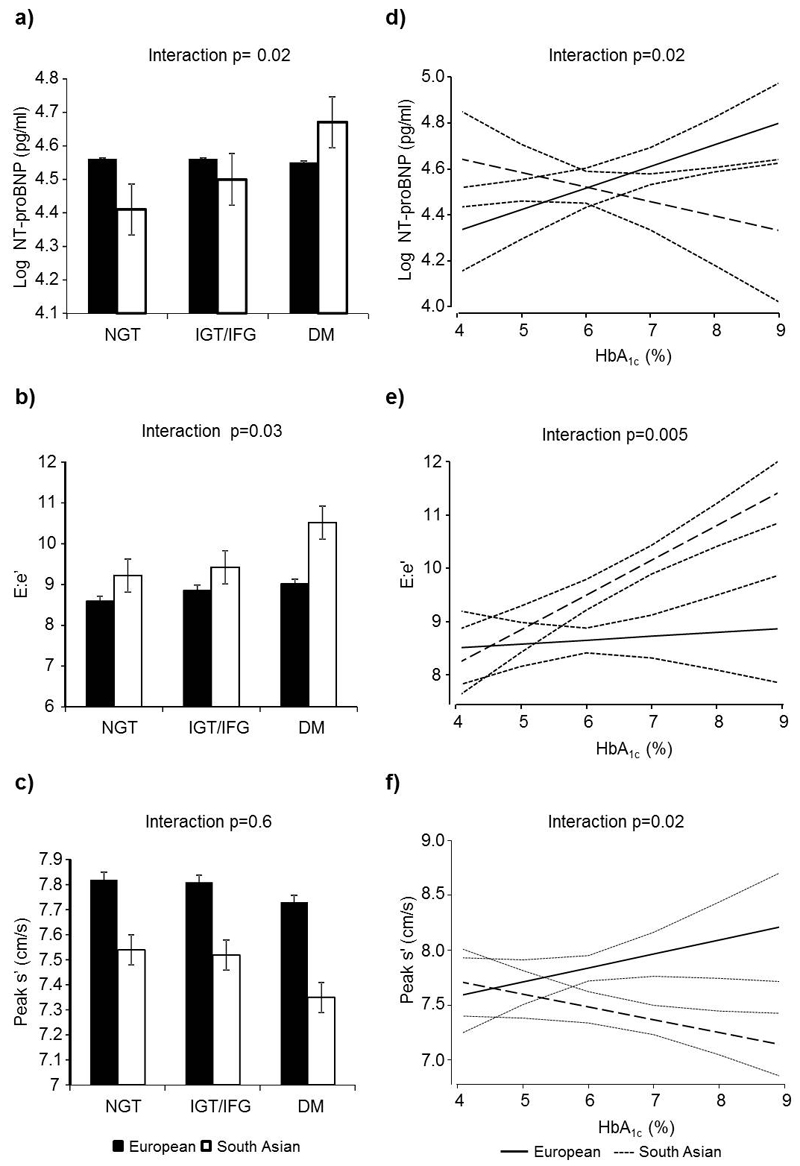
Figure 1.
a-c: Bar charts showing mean values for LV functional measures divided by diabetes status and ethnicity. a) NT-proBNP b) E:e’ and c) s’(age and sex adjusted). P values are for ethnicity × glucose tolerance category interactions.
d-f: Predicted LV function (95% CI) by HbA1c in South Asians and Europeans. All values adjusted for age and sex. d) log transformed NT-proBNP e) E/e’ and f) s’. P values are for ethnicity × HbA1c interactions.
Similarly HbA1c also had a significantly greater adverse impact on all three measures of LV function in South Asians than Europeans (Figures 1d-f: interaction β(SE), p values: NT-proBNP 0.14(0.06) p=0.02, E/e’ 0.58(0.21) p=0.005 and s’ -0.24(0.10) p=0.02). These interactions remained statistically significant on multivariate adjustment (interaction p values: NT-proBNP 0.14(0.06) p=0.01, E/e’ 0.62(0.20) p=0.002 and s’ -0.21(0.10) p=0.03). Full interaction details are presented in online Table S3.
The greater adverse effect of hyperglycaemia on LV function in South Asians could be due to their longer duration of exposure. Diabetes duration was almost two-fold greater in South Asians (Table 1). In people with diabetes, diabetes duration associated significantly with higher NT-proBNP (4.4±1.2 (β±SE), p<0.0001), E/e’ (0.08±0.2 p=0.001) and with slightly poorer s’ (-0.01±0.01cm/s, p=0.3). These associations were independent of the effect of HbA1c for NT-proBNP (p=0.001) and E/e’ (p=0.004). However, when we compared NT-proBNP, E/e’ and s’ by ethnicity in those with established diabetes (where diabetes duration can be estimated), we found that adjustment for diabetes duration, and for HbA1c, could account for little of the ethnic difference in observed function (Table 3, Model 2 and online Table S4). In those with established diabetes we also found that the interactions between glycaemic status and ethnicity on key measures of LV function (NT-proBNP 0.24, p=0.05, E/e’ 0.59, p=0.2 and s’ -0.38, p=0.04) remained after further adjusting for diabetes duration (NT-proBNP 0.26, p=0.04, E/e’ 0.77, p=0.1 and s’ -0.45 p=0.02).
Table 3.
Ethnic differences (South Asian – European) in LV function in people with known diabetes
| Mean ethnic difference(95% CI) | P value | ||
|---|---|---|---|
| NT-proBNP (pg/ml) | Unadjusted | 59.8 (2.72,116.9) | 0.04 |
| Model 1 | 75.6 (22.4,128.8) | 0.006 | |
| Model 2 | 62.0 (5.1,118.9) | 0.03 | |
| E/e’ | Unadjusted | 1.92 (0.88,2.96) | <0.0001 |
| Model 1 | 2.16 (1.15,3.17) | <0.0001 | |
| Model 2 | 1.91 (0.86,2.96) | <0.0001 | |
| Peak s’ (cm/s) | Unadjusted | -0.41 (-0.81,-0.01) | 0.05 |
| Model 1 | -0.47 (-0.87,-0.07) | 0.02 | |
| Model 2 | -0.45 (-0.87,-0.03) | 0.04 |
Data are mean ethnic differences (South Asian- European) (95% CI). Adjustments - model 1: age and sex adjusted; model 2: age, sex, glycosylated hemoglobin and diabetes duration.
Discussion
Diabetes and hyperglycaemia across the spectrum of HbA1c into the normal range, have a far more adverse effect on global (NT-proBNP), diastolic (E/e’) and systolic (s’) ventricular function in South Asians than Europeans; hyperglycaemia appeared to have little relationship with LV function in Europeans. The association between worse glycaemic status and LV function in South Asians could largely, (particularly for systolic and global function), be accounted for by concomitant cardiometabolic risk factors such as hypertension, presence of clinical and subclinical CHD, microalbuminuria, dyslipidaemia and central obesity. Previous studies have shown that systolic and/or diastolic LV dysfunction are relatively common in older people (10) and independently predict heart failure (9,27) and total mortality (10,28) in European general population samples: for example mild diastolic dysfunction was associated with a more than 8-fold increase in hazard of all-cause mortality in people aged 45 years or older (10). To our knowledge no previous study has investigated ethnic differences in the relationship between glycaemic status and LV function, but our findings could help to explain the increased risk of heart failure and cardiovascular events in South Asians.
Our finding of poorer longitudinal systolic and diastolic function in South Asians compared with Europeans is in accordance with a smaller previous study, although this latter was restricted to individuals with no abnormal risk factors, disease or medication(28), and therefore could not explore the role of glycaemic status on LV function.
In Europeans there was little association between glycated haemoglobin and LV dysfunction and only a modest decline in people with diabetes. Previous studies in European origin populations are conflicting, with some showing marked functional LV impairment when comparing people with and without diabetes (29) and across the glycaemic spectrum (30), whereas others show no differences between people with and without diabetes(31,32) and mixed associations across the glycaemic spectrum(13). A detailed study of the effect of diabetes on LV function noted that while longitudinal systolic function was impaired, radial function was increased compared to those without diabetes(33). These conflicting findings in part could be explained by choice of study population, for example clearer associations are observed in younger than older age groups, exclusions or restrictions to the study population, for example by disease, risk factor or medication status, and the functional measures employed. Few studies have explored the role of associated confounders, and many were too small for detailed analysis.
The greater adverse effect of hyperglycaemia on LV function in South Asians is in keeping with our findings of a greater effect on CVD events (6). This may be a consequence of a longer exposure to hyperglycaemia. We show that diabetes duration is greater in South Asians, but in the absence of repeated longitudinal measures of HbA1c this proposal is speculative. Certainly in people with established diabetes, we show as others do that a longer diabetes duration is associated with adverse LV function; this appears independent of hypertension and CHD(16). Long-standing hyperglycemia is associated with multiple adverse effects including increased oxidative stress, altered energy metabolism, accelerated fibrosis and accumulation of advanced glycation end products (AGEs) in the myocardium and aorta(34,35) that may adversely affect LV function. However, when we took account of the greater diabetes duration in South Asians, acting as a proxy for duration of exposure, we found that neither it, nor the greater level of hyperglycaemia, could account for the more adverse LV function in this ethnic group. This may suggest on the one hand that duration of exposure has little to do with the greater impact of hyperglycaemia on function in South Asians, or alternatively reflect the marked imprecision inherent in estimating diabetes duration.
NT-proBNP is an accepted measure of LV global dysfunction (16) that predicts CVD (36). We are unaware of previous comparisons of NT-proBNP between South Asians and Europeans, and show that unadjusted values are elevated in South Asians. Since NT-proBNP is produced by the ventricles and LV mass differs by ethnicity (20), we additionally adjusted for LV mass (24,37). This further enhanced the ethnic difference in NT-proBNP. Conventionally, EF is often used as a measure of systolic function, however the association between diabetes and EF is inconsistent. While some report lower EF in patients with diabetes (38), numerous studies have found no significant adverse effect of diabetes (39,40) probably because diminished longitudinal function is compensated for by an increase in radial function (41,42). For this reason we employed tissue Doppler s′, as a more sensitive index of systolic function that also predicts future CVD (28). For diastolic function E/e′ was employed as unlike E/A ratio it is not subject to pseudonormalization and also predicts future CVD (43,44). Based on ethnic differences in the relationship between HbA1c and s’ and E/e’ respectively, and previously reported associations between these measures and future CVD (28,44,45), a 1% increment in HbA1c would increase CVD risk in South Asians by 7-10% more than that observed in Europeans.
There are several strengths to this study. The SABRE cohort recruited from primary care without exclusion. As registration with primary care is free, and the gateway to comprehensive health care, it forms a representative sampling frame. Previous studies have either focused on those admitted to hospital, decisions for which may be biased by ethnicity, or excluded people with clinical and subclinical disease, risk factors and medication. If we had excluded such individuals in this study only 10.3% of Europeans and 5.5% of South Asians would have been eligible. As CVD and risk factors differ by ethnicity, such exclusion likely biases the comparison, and results in highly selected, unrepresentative samples, making it difficult to generalize findings. Our measures of LV dysfunction and risk factors are relatively comprehensive, enabling detailed exploration of pathways.
Study Limitations: Our study has several limitations: sample sizes were relatively small and consequently we may have insufficient power to detect some clinical important differences. We only studied older age survivors; this may have introduced a survivor bias. However, detectable subclinical LV disease emerges in older age(10), and we have shown that overall survival rates in this cohort are equivalent by ethnicity (46). While those who survived and attended clinic were healthier at baseline compared to those who did not, these differences were also similar by ethnicity, and are therefore unlikely to have seriously biased comparisons (6). Another limitation is that the majority of South Asians in this study were first generation migrants of Punjabi Sikh origin, and although most South Asian populations worldwide have an increased prevalence of both diabetes and CVD, our findings may not necessarily apply to all people of South Asian ethnicity. Lastly the data presented in this manuscript are based on cross-sectional analyses made at follow up and cannot address issues of causality.
In conclusion, hyperglycaemia may be more detrimental to global, systolic and diastolic LV function in South Asians than Europeans. This does not just apply to diabetes, but occurs across the glycaemic range. Understanding why South Asians are particularly sensitive to the effects of hyperglycaemia and associated risk factors, such as hypertension, dyslipidaemia and microvascular disease, on LV function would help elucidate mechanisms and inform strategies for prevention and treatment. These may need to be implemented earlier in the course of disease in South Asians than Europeans, or South Asian people may require more aggressive treatment for a given cardiometabolic risk factor level. Given the predicted marked increase in diabetes prevalence in South Asians worldwide, further studies on this question are urgently required.
Supplementary Material
Acknowledgements
We would like to thank SABRE participants and the SABRE team
Funding: Supported by the Wellcome Trust [082464/2/07/2] and British Heart Foundation [SP/07001/23603, PG 08 103]. JM, NC and ADH received support from the UK NIHR BRC scheme.
Footnotes
Author contributions: CP, TT, NC and ADH were involved in the design and interpretation of the data. All authors were involved in the analysis of the data and writing of the manuscript.
Disclosures: No conflicts of interest.
References
- 1.Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation. 1999;100:1132–1133. doi: 10.1161/01.cir.100.10.1132. [DOI] [PubMed] [Google Scholar]
- 2.Rubler S, Dlugash J, Yuceoglu YZ. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 3.Liu JW, Liu D, Cui KZ, Xu Y, Li YB, Sun YM, Su Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic cardiomyopathy. Biochem Biophys Res Commun. 2012;427:441–443. doi: 10.1016/j.bbrc.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 4.Seferovic PM, Milinkovic I, Ristic AD, Seferovic Mitrovic JP, Lalic K, Jotic A, Kanjuh V, Lalic N, Maisch B. Diabetic cardiomyopathy: ongoing controversies in 2012. Herz. 2012;37:880–886. doi: 10.1007/s00059-012-3720-z. [DOI] [PubMed] [Google Scholar]
- 5.Tillin T, Hughes AD, Godsland IF, Whincup P, Forouhi NG, Welsh P, Sattar N, McKeigue PM, Chaturvedi N. Insulin Resistance and Truncal Obesity as Important Determinants of the Greater Incidence of Diabetes in Indian Asians and African Caribbeans Compared With Europeans: The Southall and Brent Revisited (SABRE) cohort. Diabetes Care. 2012 doi: 10.2337/dc12-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, McKeigue PM, Chaturvedi N. The Relationship Between Metabolic Risk Factors and Incident Cardiovascular Disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited)-A Prospective Population-Based Study. J Am Coll Cardiol. 2013;61:1777–1786. doi: 10.1016/j.jacc.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003;89:681–686. doi: 10.1136/heart.89.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shantsila E, Lip GY, Gill PS. Systolic heart failure in South Asians. Int J Clin Pract. 2011;65:1274–1282. doi: 10.1111/j.1742-1241.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- 9.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Newton JD, Blackledge HM, Squire IB. Ethnicity and variation in prognosis for patients newly hospitalised for heart failure: a matched historical cohort study. Heart. 2005;91:1545–1550. doi: 10.1136/hrt.2004.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galasko GI, Senior R, Lahiri A. Ethnic differences in the prevalence and aetiology of left ventricular systolic dysfunction in the community: the Harrow heart failure watch. Heart. 2005;91:595–600. doi: 10.1136/hrt.2003.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Ethnicity-related differences in left ventricular function, structure and geometry: a population study of UK Indian Asian and European white subjects. Heart. 2010;96:466–471. doi: 10.1136/hrt.2009.173153. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 15.Zanchetti A, Cuspidi C, Comarella L, Rosei EA, Ambrosioni E, Chiariello M, Leonetti G, Mancia G, Pessina AC, Salvetti A, Trimarco B, et al. Left ventricular diastolic dysfunction in elderly hypertensives: results of the APROS-diadys study. J Hypertens. 2007;25:2158–2167. doi: 10.1097/HJH.0b013e3282eee9cf. [DOI] [PubMed] [Google Scholar]
- 16.Vinereanu D, Nicolaides E, Tweddel AC, Madler CF, Holst B, Boden LE, Cinteza M, Rees AE, Fraser AG. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond) 2003;105:591–599. doi: 10.1042/CS20030168. [DOI] [PubMed] [Google Scholar]
- 17.Mureddu GF, de SG, Greco R, Rosato GF, Contaldo F. Left ventricular filling in arterial hypertension. Influence of obesity and hemodynamic and structural confounders. Hypertension. 1997;29:544–550. doi: 10.1161/01.hyp.29.2.544. [DOI] [PubMed] [Google Scholar]
- 18.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N. Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41:33–42. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: 1999. [Google Scholar]
- 20.Park CM, March K, Ghosh AK, Jones S, Coady E, Tuson C, Francis D, Mayet J, Tillin T, Chaturvedi N, Hughes AD. Left-Ventricular Structure in the Southall And Brent REvisited (SABRE) Study: Explaining Ethnic Differences. Hypertension. 2013;61:1014–1020. doi: 10.1161/HYPERTENSIONAHA.111.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 23.Rakowski H, Appleton C, Chan KL, Dumesnil JG, Honos G, Jue J, Koilpillai C, Lepage S, Martin RP, Mercier LA, O'Kelly B, et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr. 1996;9:736–760. doi: 10.1016/s0894-7317(96)90076-0. [DOI] [PubMed] [Google Scholar]
- 24.Parekh N, Maisel AS. Utility of B-natriuretic peptide in the evaluation of left ventricular diastolic function and diastolic heart failure. Curr Opin Cardiol. 2009;24:155–160. doi: 10.1097/HCO.0b013e328320d82a. [DOI] [PubMed] [Google Scholar]
- 25.Tillin T, Dhutia H, Chambers J, Malik I, Coady E, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, et al. South Asian men have different patterns of coronary artery disease when compared with European men. International Journal of Cardiology. 2008;129:406–413. doi: 10.1016/j.ijcard.2007.07.129. [DOI] [PubMed] [Google Scholar]
- 26.Pearce EN, Yang Q, Benjamin EJ, Aragam J, Vasan RS. Thyroid function and left ventricular structure and function in the Framingham Heart Study. Thyroid. 2010;20:369–373. doi: 10.1089/thy.2009.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogelvang R, Sogaard P, Pedersen S, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 29.Celentano A, Vaccaro O, Tammaro P, Galderisi M, Crivaro M, Oliviero M, Imperatore G, Palmieri V, Iovino V, Riccardi G. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am J Cardiol. 1995;76:1173–1176. doi: 10.1016/s0002-9149(99)80330-0. [DOI] [PubMed] [Google Scholar]
- 30.Holzmann M, Olsson A, Johansson J, Jensen-Urstad M. Left ventricular diastolic function is related to glucose in a middle-aged population. J Intern Med. 2002;251:415–420. doi: 10.1046/j.1365-2796.2002.00979.x. [DOI] [PubMed] [Google Scholar]
- 31.Andren B, Lind L, Hedenstierna G, Lithell H. Left Ventricular Systolic Function in a Population Sample of Elderly Men. Echocardiography. 1998;15:315–324. doi: 10.1111/j.1540-8175.1998.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, Szklo M, Ward BJ. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 33.Leosdottir M, Willenheimer R, Plehn J, Borgquist R, Gudmundsson P, Harris TB, Launer LJ, Bjornsdottir H, Nilsson PM, Gudnason V. Myocardial structure and function by echocardiography in relation to glucometabolic status in elderly subjects from 2 population-based cohorts: a cross-sectional study. Am Heart J. 2010;159:414–420. doi: 10.1016/j.ahj.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol. 2009;103:1463–1466. doi: 10.1016/j.amjcard.2009.01.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Shin MS, Kim YS, Kang WC, Kim BR, Moon J, Chung WJ, Ahn TH, Shin EK. The impact of diabetes duration on left ventricular diastolic function and cardiovascular disease. Postgrad Med J. 2012;88:189–193. doi: 10.1136/postgradmedj-2011-130439. [DOI] [PubMed] [Google Scholar]
- 36.Di AE, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 37.McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, Tunstall-Pedoe H, McMurray JJV, Dargie HJ. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351:9–13. doi: 10.1016/s0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
- 38.Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, Borbely A, et al. Diastolic Stiffness of the Failing Diabetic Heart: Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 39.Annonu AK, Fattah AA, Mokhtar MS, Ghareeb S, Elhendy A. Left ventricular systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitus. J Am Soc Echocardiogr. 2001;14:885–891. doi: 10.1067/mje.2001.112892. [DOI] [PubMed] [Google Scholar]
- 40.Ehl NF, Kuhne M, Brinkert M, Muller-Brand J, Zellweger MJ. Diabetes reduces left ventricular ejection fraction--irrespective of presence and extent of coronary artery disease. Eur J Endocrinol. 2011;165:945–951. doi: 10.1530/EJE-11-0687. [DOI] [PubMed] [Google Scholar]
- 41.Htay T, Mehta D, Heo J, Iskandrian AE. Left ventricular function in patients with type 2 diabetes mellitus. Am J Cardiol. 2005;95:798–801. doi: 10.1016/j.amjcard.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 42.Mustonen JN, Uusitupa MI, Laakso M, Vanninen E, Lansimies E, Kuikka JT, Pyorala K. Left ventricular systolic function in middle-aged patients with diabetes mellitus. Am J Cardiol. 1994;73:1202–1208. doi: 10.1016/0002-9149(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Yip GWK, Wang AYM, Zhang Y, Ho PY, Tse MK, Lam PKW, Sanderson JE. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–826. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 44.Sharp ASP, Tapp RJ, Thom SAM, Francis DP, Hughes AD, Stanton AV, Zambanini A, O'Brien E, Chaturvedi N, Lyons S, Byrd S, et al. Tissue Doppler E/E' ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31:747–752. doi: 10.1093/eurheartj/ehp498. [DOI] [PubMed] [Google Scholar]
- 45.Ballo P, Barone D, Bocelli A, Motto A, Mondillo S. Left ventricular longitudinal systolic dysfunction is an independent marker of cardiovascular risk in patients with hypertension. Am J Hypertens. 2008;21:1047–1054. doi: 10.1038/ajh.2008.244. [DOI] [PubMed] [Google Scholar]
- 46.Lukowicz TV, Fischer M, Hense HW, Doring A, Stritzke J, Riegger G, Schunkert H, Luchner A. BNP as a marker of diastolic dysfunction in the general population: Importance of left ventricular hypertrophy. Eur J Heart Fail. 2005;7:525–531. doi: 10.1016/j.ejheart.2004.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.