Abstract
Membrane integrity is critical for cell survival, defects of which cause pathological symptoms such as metabolic diseases. In this study, we used ethanol sensitivity of the nematode Caenorhabditis elegans to identify genetic factors involved in membrane integrity. In C. elegans, acute exposure to a high concentration (7% v/v) of ethanol changes membrane permeability, as measured by propidium iodide staining, and causes paralysis. We used the timing of complete paralysis as an indicator for alteration of membrane integrity in our genetic screen, and identified ptr-6 as a gene that confers ethanol resistance when mutated. PTR-6 is a patched-related protein and contains a sterol sensing domain. Inhibition of two PTR-encoding genes, ptr-15 and ptr-23, and mboa-1, encoding an Acyl Co-A: cholesterol acyltransferase homolog, restored ethanol sensitivity of the ptr-6 mutant, suggesting that these ptr genes and mboa-1 are involved in the maintenance of membrane integrity and permeability. Our results suggest that C. elegans can be used as a model system to identify factors involved in metabolic diseases and to screen for therapeutic drugs.
Keywords: Caenorhabditis elegans, alcohol, Patched-related, membrane integrity
PROPER composition and organization of cellular membranes are important for signaling pathway, cell polarity, and membrane structural stability. Phospholipid composition and thus membrane integrity is often altered in Alzheimer’s disease and metabolic diseases such as atherosclerosis and hyperlipidemia (Gillies and Robinson 1988; Muller et al. 1990; Engelmann et al. 1992). Although changes in membrane integrity have been studied intensively in artificial membranes, isolated tissues, and unicellular organisms (Harrison and Vickers 1990; Jung and Levin 1999; Kusumi et al. 2005), the genetic and molecular mechanisms regulating membrane integrity at the multicellular organismal level are largely unknown.
Under experimental conditions, membrane integrity can be altered by chemical stresses such as alcohols and detergents. Classical studies found that ethanol influences the fluidity of cell membranes (Chin and Goldstein 1977; Johnson et al. 1979; Dombek and Ingram 1984). Therefore, we reasoned that genetic screens using ethanol as a reagent to alter membrane integrity could lead to isolation of mutants involved in the regulation of membrane integrity. In previous studies, we established a genetic system to identify genes involved in the ethanol response of Caenorhabditis elegans and isolated ethanol-resistant mutants (Kwon et al. 2004; Hong et al. 2008). Realizing that our previous genetic screens might have uncovered mutants involved in the regulation of membrane integrity, we decided to revisit the previously isolated ethanol-resistant mutations. In this study, we analyze the previously isolated ys20 mutant, which we identify as a mutation in the ptr-6 gene. Through further genetic analysis, we propose that cholesterol metabolism is involved in membrane integrity. Our study suggests that the nematode C. elegans can be utilized as a model system to screen for therapeutic treatments for diseases such as atherosclerosis.
Materials and Methods
Strains and culture
C. elegans worms were grown on nematode growth media (NGM) agar plates seeded with OP50 bacteria at 20° as described (Brenner 1974). Mutant strains were obtained from the Caenorhabditis Genetic Center or EMS mutagenesis (Hong et al. 2008). Wild type was the N2 Bristol strain. The strains used were as follows: ptr-6(ys9), ptr-6(ys20), ptr-20(ok2988), ptr-10(ok2106), N2;Ex[Pptr-6::GFP, pRF6(rol-6(su1006))], ptr-6(ys20);Ex[Pdpy-7::ptr-6::GFP, pRF6(rol-6(su1006))], ptr-6(ys20);Ex[Pmyo-3::ptr-6::GFP, pRF6(rol-6(su1006))], N2;Ex[mboa-1 (+), pRF6(rol-6(su1006))] rde-1(ne219);kzIS9[Plin-26::nlp::GFP, Plin-26::rde-1, pRF6(rol-6(su1006)], and rde-1(ne219);kvIS20[Phlh-1::rde-1, Psur-5::nls::GFP].
DNA constructs
To examine the ptr-6 expression pattern, 2 kb of the 5′ upstream region of the gene was combined with GFP by PCR fusion (Hobert 2002). For tissue-specific rescue experiments, we used the GATEWAY system (Invitrogen). The ptr-6 genomic DNA (gDNA) was cloned into the pJL1007 vector, which is a middle entry vector containing the multi-cloning sites and GFP region from pPD95.77. ptr-6 gDNA was amplified by PCR with C. elegans gDNA as the template. Entry vectors for the promoters were obtained from the Promoterome library (Dupuy et al. 2004). For the mboa-1 overexpression experiment, genomic DNA containing 2 kb of the 5′ upstream region and the entire coding region of the mboa-1 gene was amplified by PCR.
The primers used were as follows: ptr-6(RNAi)-F AAACTGCAGATCATTGCTGTCCAGGGTTT; ptr-6(RNAi)-R AAGGTACCAATGGGTCAGAATGGAGCAG; PF-A_Pptr-6 AGTTTGTCCTGACAGTTTTCTTA; PF-B_Pptr-6 AGTCGACCTGCAGGCATGCAAGCTCATCTGGAAATTTTAAGAGACTC; PF-C_GFP AGCTTGCATGCCTGCAGGTCG; PF-D_GFP AAGGGCCCGTACGGCCGACTA; PF-D*_GFP GGAAAAAGTTATGTTTGGTATA; ptr-6 (pJL1007)-F AAG CTT ATG CGA TGC CGG ATT CCA ACT CTT G; ptr-6 (pJL1007)-R TCT AGA CGA GGA TAC ATT TAC CGG CAA ATT AAT TGC; mboa-1-F(O/E) GCCGAAACTGTACAGGGTTTCT; and mboa-1-R(O/E) TATCAAGTAACTAATATTTATTTTTTCTG.
Statistical analysis
In ethanol resistance assays, statistical significance was determined by a log-rank analysis using OASIS (Yang et al. 2011). In staining experiments and the freezing–thawing assay, statistical significance was determined using one-way ANOVA and Bonferroni’s multiple-comparison post-test.
Behavior assay
To immerse C. elegans worms in 1-ml ethanol solutions of defined concentrations, ∼20 young worms were washed off agar plates with ethanol dissolved in M9 buffer at known concentrations and placed in clear 55-mm petri dishes. Moving worms were scored over time. We used this ethanol assay to isolate and identify mutation. Another method used was to pick ∼20 young adult worms in clear wells containing 200-μl ethanol solutions of defined concentrations and score for motility over time until all individuals ceased movement. Ethanol sensitivity measured by picking worms was not significantly different from the result by immersing. With all data, we used the picking method. Each experiment was repeated three times independently. For every strain, a control experiment using M9 buffer alone was done to check basal locomotion activity. It was normal until the 20-min time point.
The sodium azide sensitivity test was done similarly to the ethanol test. We put 20 young adult worms in clear dishes containing 25 mM sodium azide with M9. Acute osmotic stress assay was performed using 500 mM NaCl plates as described (Solomon et al. 2004).
Freezing and thawing assay
To check the survival ratio after freezing and thawing quantitatively, we used a previously defined liquid freezing solution with defined glycerol concentrations (Brenner 1974). The freezing solution samples were divided into four groups and frozen after 0, 10, 20, and 30 min of incubation at room temperature, respectively. After 1 week in the −80 freezer, we thawed the samples at room temperature. We allocated the solution in three NGM plates and then measured the numbers of total animals and live animals.
Identification of ptr-6 as a gene that confers ethanol resistance
Ethanol-resistant mutants were previously isolated by EMS mutagenesis (Hong et al. 2008). The ys9 and ys20 mutations were genetically mapped by SNP mapping (Wicks et al. 2001). Both mutants were located in the middle region [II: 23.3II: 1.2 map unit (m.u.)] of chromosome II. By testing F1 heterozygotes from crossing ys9 and ys20 homozygotes, we found that these two mutations do not complement each other and that they are alleles of the same gene, previously named jud-1 (Hong et al. 2008). By whole-genome sequencing of the ys9 and ys20 mutants and comparison with the wild-type N2 sequence, we obtained a list of single-nucleotide changes in ys9 and ys20. We selected the candidate genes that contained the detected mutations and were located within the SNP mapping region (II: −3.3∼II: 1.2 m.u.). Because ys9 and ys20 were isolated from independent mutagenesis, background mutations were filtered by subtracting identical nucleotide changes found in both ys9 and ys20. ptr-6 was the only gene mutated in both ys9 and ys20, with mutations in different regions of ptr-6. Rescue experiments were performed by injecting a wild-type version of ptr-6 into the ys20 mutant.
Microinjection and microscopy
Microinjection of DNA into the gonads of adult hermaphrodites was carried out according to standard procedures (Mello et al. 1991). Young adult hermaphrodites were picked to a 2% agarose pad using halocarbon oil. After injection into their gonads, worms were quickly recovered with M9 buffer. The pRF4 plasmid, which contains the dominant rol-6 (su1006) gene, was used as an injection marker (100 ng/μl), and Psur-5::GFP and Pact-5::GFP were used as markers for transgenic worms used in behavior assays. Most plasmids were injected at the concentration of 100 ng/μl. Plasmid DNAs used for injection were extracted with the QIAGEN plasmid midi kit (catalog no. 12145) or the Axygen midi prep kit (catalog no. AP-MD-P-25). To observe the transgene expression patterns, transgenic animals were mounted on 5% agar pads with 2.5 mM levamisole, and fluorescence was observed using an Axioplan 2 microscope or LSM700 confocal microscope (Zeiss).
Feeding RNA interference method
In most cases, clones in the C. elegans RNA interference (RNAi) library from the Ahringer Library (Cambridge, UK) were used. Bacteria containing the RNAi clone targeting the gene of interest were streaked and cultured in LB containing ampicillin. Transcriptional activation was induced by adding 1 mM IPTG. For ptr-6 that did not have proper RNAi clones in the Ahringer Library, 748 bp of genomic fragment was cloned into pPD129.36 (L4440) with PstI and BamHI. Bleached embryos or L1 larvae were cultured on the RNAi plates until they reached the L4-to-young-adult stages, when they were harvested for analysis of ethanol sensitivity. ptr-6(ys20) animals were used for the RNAi experiments. In tissue-specific RNAi experiments, we used NR222 (hypodermis) and NR350 (muscle) strains, which are rde-1 background and rescued in each specific tissue (Qadota et al. 2007).
Chemical staining
For the propidium iodide (PI) assay, worms were rinsed with M9 buffer containing 10% (v/v) PI solution with/without 7% (v/v) ethanol. Images were taken by the Axioplan 2 microscope (Zeiss) with the same exposure time. Fluorescence intensity was analyzed by ImageJ software. For CE-bodipy staining, worms were frozen before staining. After thawing, worms were incubated in CE-bodipy solution. Images were taken with an LSM 700 confocal microscope (Zeiss). For quantification, we analyzed the data from 20 animals for each group.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Acute exposure to a high concentration of ethanol alters membrane permeability and causes paralysis in C. elegans
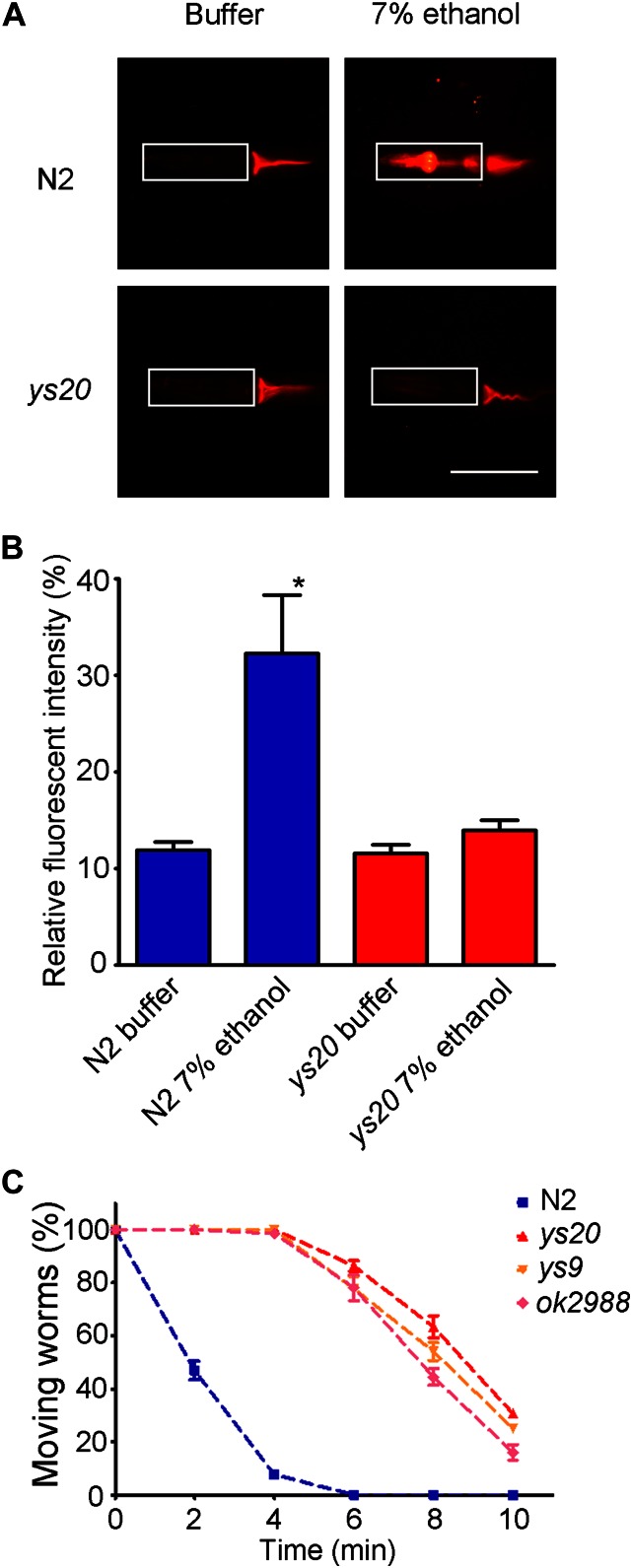
To study the effects of ethanol exposure on biological membranes, we performed PI staining in the nematode C. elegans. PI is an intercalating agent and is highly fluorescent when bound to nucleic acids. Since PI is membrane-impermeable, it cannot stain normal, living cells. Only the intestinal lumen was stained when worms were treated with PI in M9 buffer (Figure 1A) because PI dye ingested by the mouth into the intestinal lumen could not penetrate into cells. When ethanol exposure was simultaneously conducted with PI treatment by immersing worms in a 7% (v/v) ethanol solution for 20 min, the inside of the pharyngeal region was stained as well as the intestinal lumen (Figure 1, A and B). Consistent with the changes in membrane permeability induced by ethanol, C. elegans worms were paralyzed within 10 min upon 7% (v/v) ethanol exposure (Figure 1C). To exclude the possibility that PI staining in ethanol-treated N2 was due only to lethality, we further conducted the recovery experiments. After being treated with 7% (v/v) ethanol and PI dye for 30 min, paralyzed worms were moved to M9 buffer. We found that all of them were recovered and started moving again within 10 min (Supplemental Material, Figure S1), indicating that the viability of the worms after PI treatment was not affected. The correlation of membrane permeability and animal paralysis suggested that a behavioral assay using 7% (v/v) ethanol treatment could be used to identify factors involved in maintaining membrane integrity in C. elegans. Since we had previously isolated ethanol-resistant mutants by random mutagenesis (Hong et al. 2008), we decided to re-analyze the uncloned mutants to see if they corresponded to factors involved in the maintenance of membrane integrity.
Figure 1.
A high concentration of ethanol can affect membrane integrity and induce paralysis. (A) Representative images of a membrane integrity assay using PI upon 7% (v/v) ethanol treatment. Wild-type N2 animals show increased staining inside the pharynx after ethanol treatment. ys20 mutant animals were not stained in the pharynx. Bars, 150 μm. (B) Quantitative analysis of PI staining. *P < 0.01. (C) The Ethanol response of ptr-6(ok2988), ys9 and ys20 mutants to 7% (v/v) ethanol. The ptr-6(ok2988) deletion strain showed an ethanol-resistant phenotype similar to that of the ys9 or ys20 mutations.
ys20 ethanol-resistant mutant is defective in membrane integrity
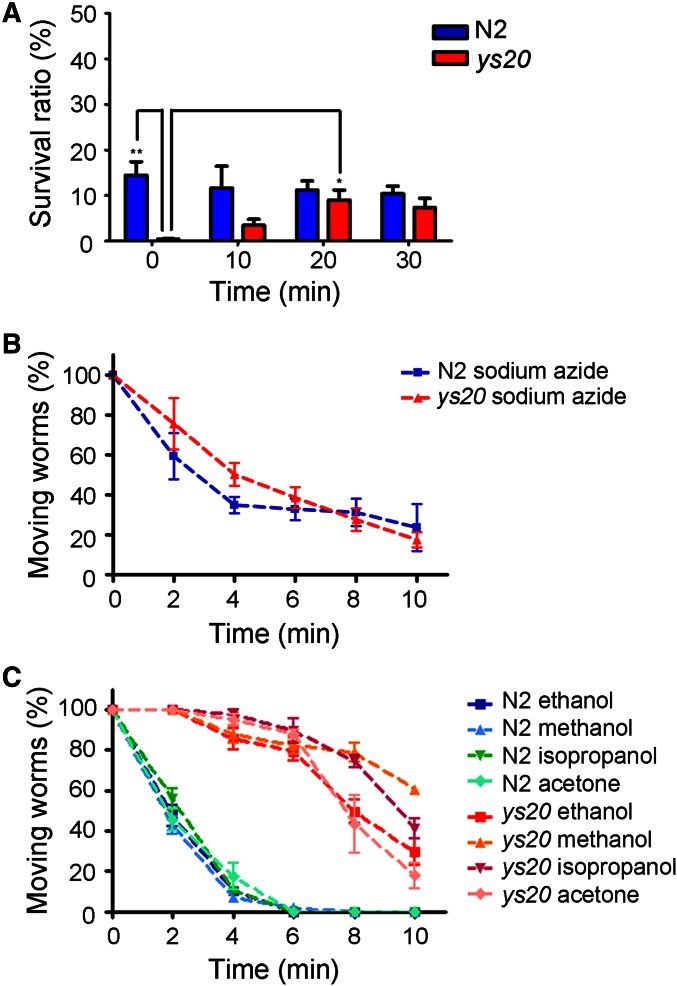
The ys20 allele had been observed to have the strongest resistance to ethanol treatment among the previously isolated ethanol-resistant mutants (Hong et al. 2008). Therefore, we decided to first analyze this mutant further. While ys20 mutant animals showed normal morphology and motility under the dissection microscope in the absence of ethanol, and their growth and reproduction were also normal, PI did not penetrate into the pharyngeal cells of ys20 mutant animals even in the presence of ethanol (Figure 1A). ys20 mutant animals had a low survival rate after freezing and thawing for storage in standard freezing solution containing 15% glycerol, even after extensive outcrossing with wild-type N2 animals (Figure S2) (Hong et al. 2008), again suggesting that ethanol resistance is likely to be related to membrane properties such as rigidity and integrity.
Next, we examined whether the ys20 mutant was resistant to other chemicals (Figure 2, B and C). Sodium azide, which can act as a mitochondria inhibitor, is a well-known anesthetic in C. elegans (Massie et al. 2003). ys20 animals did not exhibit resistance to sodium azide compared to wild-type animals (Figure 2B). However, ys20 animals showed resistance to other alcohols such as methanol and isopropanol, at different working concentrations from that of ethanol, and also exhibited resistance to acetone treatment (Figure 2C). These data indicate that the resistance phenotype of the ys20 mutation is not specific to ethanol, but broadly applicable to membrane-solubilizing agents. Acetone is not an alcohol, but has a similar property to alcohol in that it can work as a membrane-solubilizing organic solvent (Sikkema et al. 1995). Organic solvents can integrate into the plasma membrane and render the membrane permeable (Isken and de Bont 1998). The defect of ys20 mutants in survival after freezing and thawing may be caused by the impermeability of the membranes of ys20 mutants for glycerol, which is a main organic solvent for freezing. To test this possibility, we divided the N2 and ys20 worms in the freezing solution into four groups, incubated at room temperature for 0, 10, 20, and 30 min, respectively, before freezing, and froze them in the −80 freezer. Interestingly, after thawing, ys20 animals showed an increased survival rate in 20- and 30-min samples compared to 0 min (Figure 2A). These phenotypic analyses suggest that the molecular function of the gene corresponding to the ys20 mutation is related to regulation of membrane integrity.
Figure 2.
The ys20 ethanol-resistant mutant shows membrane-related phenotypes. (A) Survival ratio of wild-type and ys20 mutant animals after freezing and thawing. The x-axis indicates the time of incubation at room temperature before freezing. (B) Time-course sensitivity of N2 and ys20 mutant animals to sodium azide. (C) Time-course sensitivity of N2 and ys20 mutant animals to ethanol, methanol, isopropanol, and acetone.
ys20 is an allele of the jud-1 gene, which corresponds to the ptr-6 gene
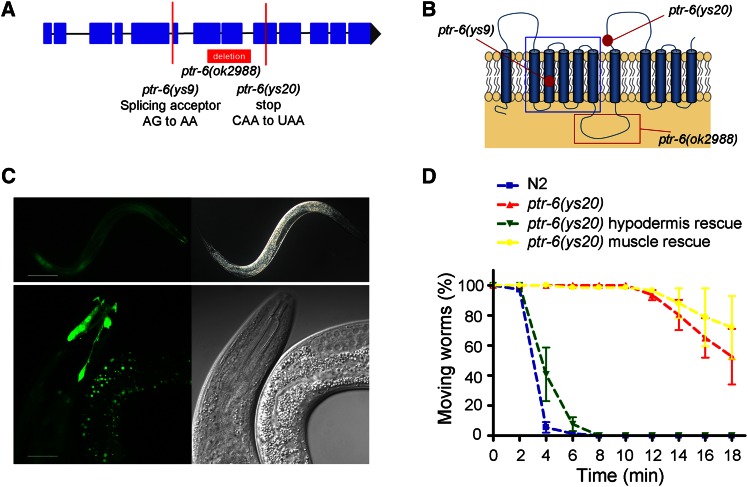
We mapped ys20 to a similar region with ys9, another previously isolated ethanol-resistant mutant, on chromosome II by SNP mapping. A complementation test using ethanol assay showed that these mutations were alleles of the same gene. After we generated a male from ys9, we mated ys9 males with ys20 hermaphrodites and tested ethanol sensitivity of F1 male heterozygotes of the genotype ys9/ys20. While N2 males and ys9 heterozygous males of the genotype ys9/+ showed paralysis within 5 min in 7% ethanol, almost half of the ys9/ys20 heterozygotes showed motility after 8 min, which was similar to the ethanol sensitivity of ys9 and ys20 mutants (Figure S4). ys9 was previously known to be an allele of the uncloned jud-1 gene (Hong et al. 2008). Using rescue experiments with a candidate gene uncovered by whole-genome sequencing, we found that the previously uncloned jud-1 gene corresponded to the ptr-6 gene (Patched-related family member-6) (Figure 3, A and D). PTR-6 has 11 transmembrane domains and a sterol-sensing domain (SSD) (Figure 3B) (Zugasti et al. 2005) and is homologous to human PTCHD3, which defines one of seven paralogous families of SSD proteins. The ptr-6 (ys9) allele has a splicing acceptor mutation in the sixth exon of ptr-6, and the ptr-6 (ys20) allele a nonsense mutation in the nineth exon of ptr-6 (Figure 3, A and B). In addition, ptr-6 RNAi in a wild-type background phenocopied ethanol resistance (Figure S3A). ok2988, a ptr-6 deletion mutant provided by the Caenorhabditis Genetics Center, also showed an ethanol-resistant phenotype similar to the ys9 and ys20 mutants (Figure 1C). Although it was reported that ptr-6 was required for molting together with ptr-1 and ptr-10, we found that knockdown of ptr-1 and ptr-10 mutations did not cause any phenotype related to alcohol sensitivity (Figure S5), suggesting that ptr-6 has a role distinctive from these genes.
Figure 3.
ptr-6 is expressed in and acts in the hypodermis to regulate membrane integrity. (A) Gene structure of ptr-6. The ys9 and ys20 mutation sites are indicated as lines. The red box indicates the region deleted in the ok2988 allele. (B) Predicted protein structure of PTR-6 and the sites of the ys9 and ys20 mutations. The red box is the deleted portion of PTR-6 in the ok2988 allele. The blue box indicates the putative SSD domain. (C) ptr-6 expression pattern. A transgenic construct with the ptr-6 promoter fused to GFP is expressed along the hypodermis. Signal intensity was strong in the head region. Bar (upper), 100 μm; bar (lower), 20 μm. (D) ptr-6 tissue-specific rescue experiment. A hypodermis-specific ptr-6 expression line, ptr-6(ys20);[Pdpy-7::ptr-6::GFP], shows altered ethanol sensitivity similar to wild type. In contrast, a muscle-specific expression line, ptr-6(ys20);[Pmyo-3::ptr-6::GFP], did not show any difference compared to the ptr-6(ys20) mutant.
To check the expression pattern of ptr-6, a transcriptional fusion of the ptr-6 promoter and GFP was constructed. ptr-6 was expressed in the hypodermis, especially strongly in the head region (Figure 3C). To test whether ptr-6 in the hypodermis played a role for ethanol sensitivity, we made translational fusions of tissue-specific promoters and the ptr-6-coding region. ptr-6 mutants containing a hypodermis-specific rescue construct (Pdpy-7::ptr-6::GFP) showed a reduced ethanol resistance phenotype, similar to wild-type worms (Figure 3D). To check whether any extrachromosomal array or injection marker itself could diminish ethanol resistance of ptr-6, we tested the muscle-specific expression line (Pmyo-3::ptr-6::GFP) as a negative control. Ethanol sensitivity of muscle-specific expression lines was comparable with that of wild-type animals (Figure 3D). We also found that feeding RNAi of ptr-6 was effective in N2 background (Figure S1A). We wanted to confirm the role of ptr-6 in the hypodermis using tissue-specific RNAi strains. Tissue-specific RNAi strains were generated by tissue-specific rescue of rde-1 in rde-1 mutant background (Qadota et al. 2007). We could observe a ptr-6 RNAi effect for ethanol sensitivity in hypodermis-specific RNAi strains, but not in muscle-specific RNAi strains (Figure S1, B and C).
Other ptr genes are involved in the regulation of membrane integrity
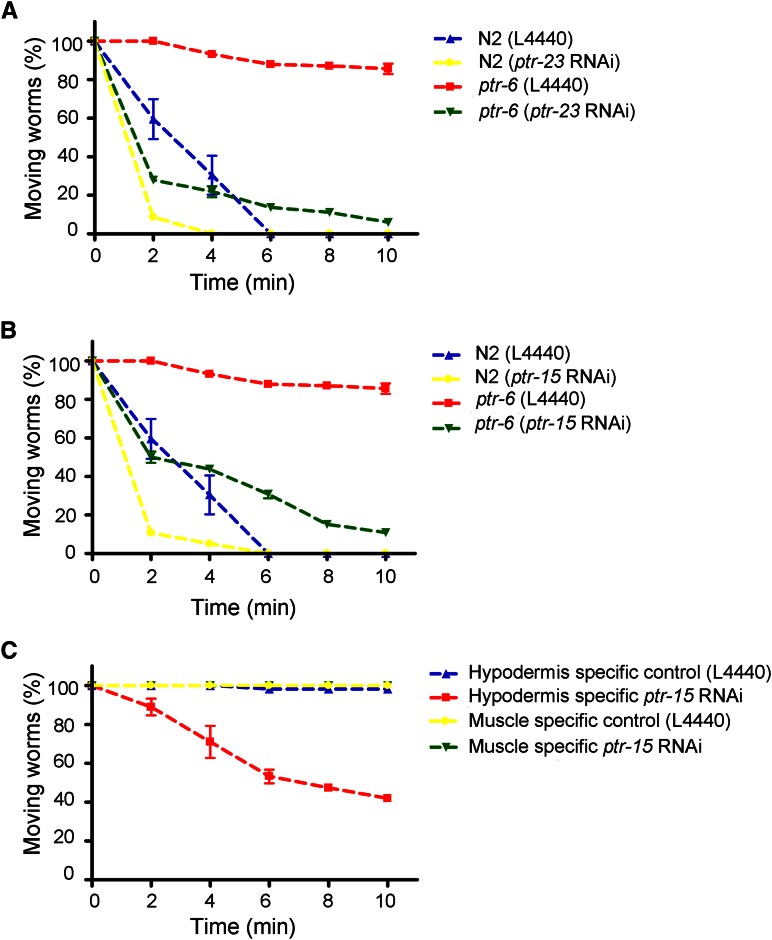
We performed an RNAi screen in search of suppressors of ptr-6(ys20) mutant phenotypes to gain insight into the molecular and genetic mechanisms of ptr-6 function in the membrane. Because hedgehog signaling is not conserved in C. elegans, we wanted to get possible genetic interactors with ptr-6. Based on the fact that PTR proteins are known to contain the sterol-sensing domain (Zugasti et al. 2005), we chose 117 sterol, steroid-related genes as candidates to test from the WormBase database (http://www.wormbase.org) (Table S1). Among them, ptr-15 or ptr-23 RNAi were observed to suppress the ptr-6 ethanol-resistant phenotype (Figure 4, A and B). Knockdown of ptr-15 and ptr-23 in wild-type N2 animals also caused enhancement of ethanol sensitivity, indicating that the endogenous ptr-15 and ptr-23 genes can directly influence ethanol sensitivity and may not need to act through the ptr-6-mediated pathway to exert their effects (Figure 4, A and B). Like ptr-6, ptr-15 function has not yet been studied. Using a hypodermis-specific RNAi experiment, we found that the site of ptr-15 action is the hypodermis (Figure 4C). Although ptr-23 is known to be involved in the osmotic stress response (Rohlfing et al. 2011), suppression of ptr-6 ethanol resistance by ptr-23 RNAi is not related to the osmotic response because ptr-6 mutant animals did not show any differences in the osmotic stress response compared to wild-type animals (data not shown).
Figure 4.
Other ptr genes are involved in the regulation of membrane integrity. (A and B) Knockdown of ptr-15 or ptr-23 suppressed the ethanol-resistant phenotype of ptr-6. (C) Tissue-specific ptr-15 RNAi experiments using rde-1 rescue lines show that the site of ptr-15 action is the hypodermis.
Among 117 screening candidates, most genes did not affect ethanol sensitivity. It is possible that some family with many paralogous genes in C. elegans such as hedgehog-like ligands or lipid-binding proteins can be redundant for ethanol sensitivity and membrane regulation. Interestingly, nhr-25 can suppress ethanol resistance of ptr-6. It suggests that ptr-6 can affect transcription of a certain gene that is important for membrane regulation.
mboa-1, an acyl-coenzyme A:cholesterol O-acyltransferase homolog, may act through ptr-6 to influence lipid levels and influence ethanol sensitivity
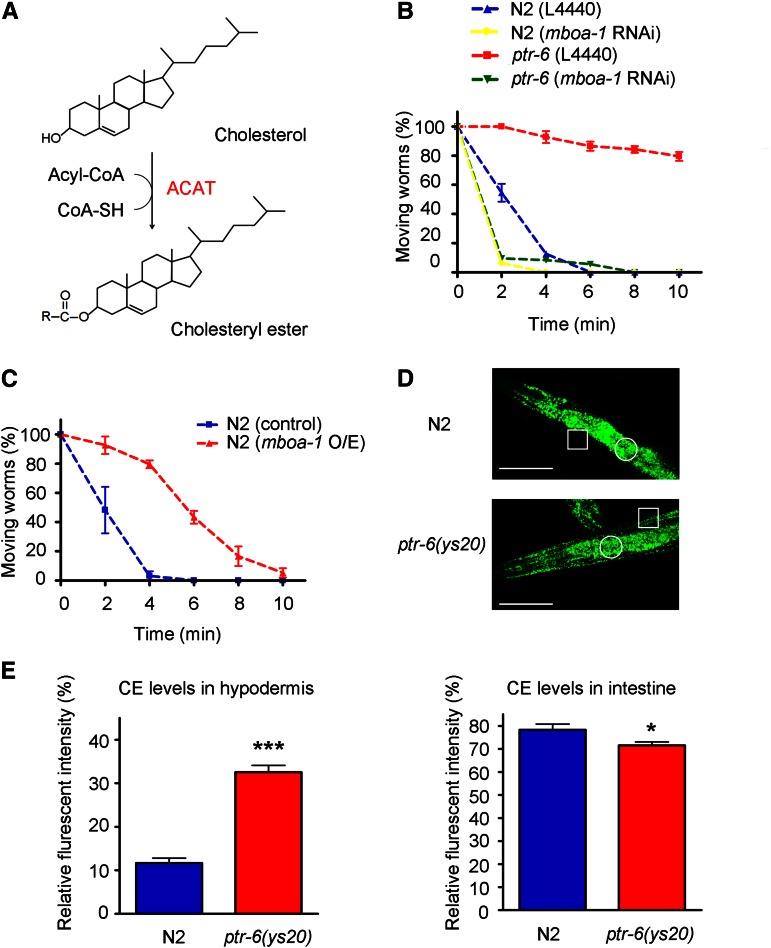
Using further RNAi screening for ptr-6 suppressors among sterol, steroid-related genes, we found that knockdown of mboa-1, a homolog of acyl-coenzyme A: cholesterol O-acyltransferase (ACAT), an enzyme that forms cholesteryl ester from cholesterol, strongly suppresses the ethanol-resistant phenotype of the ptr-6 mutation (Figure 5, A and B). ACAT protects the membrane from an excess of free cholesterol by producing cholesteryl ester (CE), which cannot integrate into or interfere with the cell membranes (Cases et al. 1998). It suggests that balance between the levels of free cholesterol and cholesteryl ester plays an important role in regulating membrane properties. To test this hypothesis, we overexpressed mboa-1 and checked ethanol sensitivity. mboa-1 overexpression in wild-type animals caused an ethanol-resistant phenotype (Figure 5C), suggesting that levels of cholesteryl esters may be critical for ethanol sensitivity. When the amounts of cholesteryl esters in wild-type and ptr-6 mutant animals were analyzed by BODIPY-CE staining, we observed that the amount of cholesteryl esters in the hypodermis was increased in ptr-6 mutants compared to wild-type N2 animals (Figure 5, D and E). In contrast, the amount of cholesteryl esters in the intestine was slightly decreased in ptr-6 mutants. In addition to the fact that PTR-6 is potentially a sterol transporter, these data suggest that PTR-6 acts negatively on the amounts of cholesteryl esters stored in the hypodermis. mboa-1 is known to express in the hypodermal cells (Hunt-Newbury et al. 2007). Although we do not rule out the possibility that mboa-1 in other tissues could play a role in ethanol sensitivity, we speculate that a major site where mboa-1 and ptr-6 interact might be the hypodermis because increased BODIPY-CE staining in ptr-6 is mostly prominent in the hypodermis.
Figure 5.
Suppression of ptr-6 by mboa-1, an ACAT homolog. (A) mboa-1 is a homolog of ACAT, which produces cholesteryl ester using cholesterol and acyl CoA as substrates. (B) mboa-1 RNAi suppresses the ptr-6 ethanol-resistant phenotype. (C) mboa-1 overexpression in the wild-type background causes ethanol resistance. (D) Representative images of cholesteryl ester staining in wild-type and ptr-6 mutant animals. The intensity of images was measured from the hypodermis or intestine. White rectangles highlight the staining signals from the hypodermis and white circles highlight signals from the intestine. (E) Quantitative analysis of the staining results. ***P < 0.001. *P < 0.05. Bars, 150 μm.
Discussion
In this study, we have shown that PTR-6, a Patched-related protein, and MBOA-1, which may act in sterol homeostasis, are involved in maintaining the membrane integrity using C. elegans as a model system. We identified ptr-6 as a gene involved in membrane integrity by unbiased forward genetic screening using ethanol sensitivity as a read-out of the membrane integrity at the organismic level. Then we utilized a targeted candidate approach for the new players and identified two more ptr genes and mboa-1 as genes involved in membrane integrity.
Patched is a well-known principal component of the Hedgehog pathway. In many organisms, the Hedgehog ligand binds to the Patched receptor in the membrane, releasing Smoothened (smo) from the repressive activity of Patched. The Smoothened signal exerts its effects through the Gli/Ci transcription factor. However, Smoothened is absent from the C. elegans genome, leading to the belief that the Hedgehog pathway is not conserved in C. elegans (Hausmann et al. 2009). Also, the C. elegans paralog of Gli/Ci shows a restricted expression pattern in the germ line and seems to be important only for sex determination. Other conserved components of the Hedgehog pathway such as Patched or Hedgehog are not involved in sex determination (Zugasti et al. 2005). Even though the key components of the Hedgehog pathway are not conserved in C. elegans, the number of Patched-related genes is expanded to 23. It is in contrast to the fact that each genome of mice, Drosophila, and humans has only one Patched-related gene. The expansion of Patched-related genes is a feature of Nematoda (Zugasti et al. 2005), and it is thought that they have distinctive cellular functions. Some Patched-related genes are partially redundant in molting and cytokinesis, and some have specific functions in glia cell development or response to osmotic stress (Perens and Shaham 2005; Rohlfing et al. 2011). We focused on the fact that ptr genes contain the sterol-sensing domain. Other unicellular organisms including bacteria have proteins structurally similar to Patched, even though the hedgehog pathway is not conserved in them either. The RND transporter, which is an ancient form of Patched family proteins, contains a sterol-sensing domain and has a role in sterol transport (Ioannou 2001; Hausmann et al. 2009). The human patched receptor is also reported to be involved in cholesterol transport (Bidet et al. 2011). These previous studies and our data suggest that Patched family protein can regulate trafficking of sterol molecules, which in turn can affect plasma membrane properties.
In this study, we used a high-concentration (7% v/v), ethanol-induced paralysis assay to study membrane integrity. Because 7% (v/v)-induced paralysis is an acute and fast response, the consequence from screening is about ethanol delivery mechanism, not ethanol intoxication. This idea is supported by the facts that the ethanol-resistant mutant in intoxication, slo-1, was not isolated by our screening, and that slo-1(eg142) null mutants did not show ethanol resistance in our high-concentration assay (data not shown) (Davies et al. 2003). The different function of ptr-6 in the hypodermis and slo-1 in neurons suggests that the result of drug-resistant mutant screening can generate different targets depending on its concentration and time points. Our previous microarray results based on the same paradigm also support this idea because high concentration of ethanol alters a lot of gene expression, which is involved in lipid metabolism and collagen synthesis (Kwon et al. 2004). Based on our results, we propose the following model for the roles of PTR proteins in membrane integrity: MBOA-1/ACAT synthesizes cholesteryl ester from cholesterol in the hypodermis and PTR-6 acts to regulate the storage of cholesteryl esters in the hypodermis, which leads to changes in membrane permeability. PTR-15 and PTR-23 have roles in regulating membrane permeability in the opposite direction from PTR-6, but it is unclear if they act through the PTR-6-mediated pathway (Figure S6). Among other PTR proteins in C. elegans, only PTR-6 has a potential to contain a coiled-coil motif (Thierry-Mieg and Thierry-Mieg 2006). Consistent with our result that PTR-6 is a potential sterol transporter to the cell membrane, there are reports that coiled-coil motifs mediate trafficking in cellular membrane (Knodler et al. 2011). Cholesterol and cholesteryl esters are highly associated with membrane thickness (Ma et al. 1997) and membrane fluidity (Owen et al. 1982). Furthermore, membrane fluidity can be altered by incubating cholesteryl esters with membranes (Kolena and Kasal 1989), supporting our model.
It is also reported that a lipid environment regulates acute functional tolerance (AFT) to ethanol (Bettinger et al. 2012). We think the detailed mechanisms of AFT to 300 mM ethanol and resistance to 1200 mM would be separable because npr-1, which is resistant in AFT, is not resistant in our 1200-mM assay. This suggests that the interaction between ethanol and lipid in an organism can be dynamic depending on their concentration and site-of-action.
Cellular membranes are highly vulnerable to many diseases, including metabolic diseases such as atherosclerosis and neurodegenerative diseases. It is notable that the integrity of erythrocytes, which are closely related to membrane integrity, is decreased in atherosclerosis and hyperlipidemia (Stubbs and Smith 1984; Muller et al. 1990). Many attempts have been made to attenuate the symptoms of these diseases, and a well-known example is the use of statins (Nicholls et al. 2007), which lowers cholesterol levels in the blood, and ACAT inhibitors (Nissen et al. 2006b). Symptoms can be also attenuated by lowering dietary cholesterol and inhibiting ACAT (Nissen et al. 2006a,b). In this study, we showed that ptr-6 mutants show phenotypes that resemble atherosclerosis symptoms, such as rigid membranes. This suggests that ptr-6 mutant animals can be used as a disease model for atherosclerosis and hyperlipidemia. Interestingly, there was a clinical case where resistance to anesthesia was related to hyperlipidemia in a certain patient (Moore and Smith 2007). According to the report, resistance to anesthesia was secondary to severe hyperlipidemia, which was a side effect of chemotherapy. The C. elegans ptr-6 mutant may provide a cost- and time-worthy model to screen for new therapeutic treatment of such diseases.
Acknowledgments
Mutant worm strains were kindly provided by the Caenorhabditis Genetics Center. The authors thank A. Fire (Stanford University) for vectors. This work was supported by the National Research Foundation of Korea grants funded by the Korean government (no. 2010-0026035 and no. 2013R1A2A03070982).
Footnotes
Communicating editor: B. Goldstein
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.179705/-/DC1.
Literature Cited
- Bettinger J. C., Leung K., Bolling M. H., Goldsmith A. D., Davies A. G., 2012. Lipid environment modulates the development of acute tolerance to ethanol in Caenorhabditis elegans. PLoS One 7: e35192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet M., Joubert O., Lacombe B., Ciantar M., Nehme R., et al. , 2011. The hedgehog receptor patched is involved in cholesterol transport. PLoS One 6: e23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S., Novak S., Zheng Y. W., Myers H. M., Lear S. R., et al. , 1998. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273: 26755–26764. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B., 1977. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol. Pharmacol. 13: 435–441. [PubMed] [Google Scholar]
- Davies A. G., Pierce-Shimomura J. T., Kim H., VanHoven M. K., Thiele T. R., et al. , 2003. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 115: 655–666. [DOI] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O., 1984. Effects of ethanol on the Escherichia coli plasma membrane. J. Bacteriol. 157: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy D., Li Q. R., Deplancke B., Boxem M., Hao T., et al. , 2004. A first version of the Caenorhabditis elegans Promoterome. Genome Res. 14: 2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann B., Streich S., Schonthier U. M., Richter W. O., Duhm J., 1992. Changes of membrane phospholipid composition of human erythrocytes in hyperlipidemias. I. Increased phosphatidylcholine and reduced sphingomyelin in patients with elevated levels of triacylglycerol-rich lipoproteins. Biochim. Biophys. Acta 1165: 32–37. [DOI] [PubMed] [Google Scholar]
- Gillies P., Robinson C., 1988. Decreased plasma membrane fluidity in the development of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 70: 161–164. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Vickers S. E., 1990. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 88: 343–352. [DOI] [PubMed] [Google Scholar]
- Hausmann G., von Mering C., Basler K., 2009. The hedgehog signaling pathway: Where did it come from? PLoS Biol. 7: e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hong M., Choi M. K., Lee J., 2008. The anesthetic action of ethanol analyzed by genetics in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 367: 219–225. [DOI] [PubMed] [Google Scholar]
- Hunt-Newbury R., Viveiros R., Johnsen R., Mah A., Anastas D., et al. , 2007. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 5: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y. A., 2001. Multidrug permeases and subcellular cholesterol transport. Nat. Rev. Mol. Cell Biol. 2: 657–668. [DOI] [PubMed] [Google Scholar]
- Isken S., de Bont J. A., 1998. Bacteria tolerant to organic solvents. Extremophiles 2: 229–238. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Lee N. M., Cooke R., Loh H. H., 1979. Ethanol-induced fluidization of brain lipid bilayers: required presence of cholesterol in membranes for the expression of tolerance. Mol. Pharmacol. 15: 739–746. [PubMed] [Google Scholar]
- Jung U. S., Levin D. E., 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Knodler L. A., Ibarra J. A., Perez-Rueda E., Yip C. K., Steele-Mortimer O., 2011. Coiled-coil domains enhance the membrane association of Salmonella type III effectors. Cell. Microbiol. 13: 1497–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolena J., Kasal A., 1989. Effects of cholesteryl esters on the accessibility of LH/hCG receptors and membrane lipid fluidity in rat testes. Biochim. Biophys. Acta 979: 279–286. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Nakada C., Ritchie K., Murase K., Suzuki K., et al. , 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34: 351–378. [DOI] [PubMed] [Google Scholar]
- Kwon J. Y., Hong M., Choi M. S., Kang S., Duke K., et al. , 2004. Ethanol-response genes and their regulation analyzed by a microarray and comparative genomic approach in the nematode Caenorhabditis elegans. Genomics 83: 600–614. [DOI] [PubMed] [Google Scholar]
- Ma J., Folsom A. R., Lewis L., Eckfeldt J. H., 1997. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 65: 551–559. [DOI] [PubMed] [Google Scholar]
- Massie M. R., Lapoczka E. M., Boggs K. D., Stine K. E., White G. E., 2003. Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J., Smith J. H., 2007. A case of resistance to anesthesia secondary to severe hyperlipidemia. Paediatr. Anaesth. 17: 1223–1225. [DOI] [PubMed] [Google Scholar]
- Muller S., Ziegler O., Donner M., Drouin P., Stoltz J. F., 1990. Rheological properties and membrane fluidity of red blood cells and platelets in primary hyperlipoproteinemia. Atherosclerosis 83: 231–237. [DOI] [PubMed] [Google Scholar]
- Nicholls S. J., Tuzcu E. M., Sipahi I., Grasso A. W., Schoenhagen P., et al. , 2007. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 297: 499–508. [DOI] [PubMed] [Google Scholar]
- Nissen S. E., Nicholls S. J., Sipahi I., Libby P., Raichlen J. S., et al. , 2006a Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295: 1556–1565. [DOI] [PubMed] [Google Scholar]
- Nissen S. E., Tuzcu E. M., Brewer H. B., Sipahi I., Nicholls S. J., et al. , 2006b Effect of ACAT inhibition on the progression of coronary atherosclerosis. N. Engl. J. Med. 354: 1253–1263. [DOI] [PubMed] [Google Scholar]
- Owen J. S., Bruckdorfer K. R., Day R. C., McIntyre N., 1982. Decreased erythrocyte membrane fluidity and altered lipid composition in human liver disease. J. Lipid Res. 23: 124–132. [PubMed] [Google Scholar]
- Perens E. A., Shaham S., 2005. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev. Cell 8: 893–906. [DOI] [PubMed] [Google Scholar]
- Qadota H., Inoue M., Hikita T., Koppen M., Hardin J. D., et al. , 2007. Establishment of a tissue-specific RNAi system in C. elegans. Gene 400: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing A. K., Miteva Y., Moronetti L., He L., Lamitina T., 2011. The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLoS Genet. 7: e1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema J., de Bont J. A., Poolman B., 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59: 201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Bandhakavi S., Jabbar S., Shah R., Beitel G. J., et al. , 2004. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 167: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D., 1984. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 779: 89–137. [DOI] [PubMed] [Google Scholar]
- Thierry-Mieg, D., and J. Thierry-Mieg, 2006 AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 7(Suppl 1): S12.1–14. [DOI] [PMC free article] [PubMed]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Yang J. S., Nam H. J., Seo M., Han S. K., Choi Y., et al. , 2011. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One 6: e23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugasti O., Rajan J., Kuwabara P. E., 2005. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res. 15: 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.