Abstract
IMPORTANCE
Questions remain about the role and durability of bariatric surgery for type 2 diabetes mellitus (T2DM).
OBJECTIVE
This study compared the remission of T2DM following surgical and non-surgical treatments.
DESIGN
Randomized Controlled Trial
SETTING
University of Pittsburgh Medical Center, in the United States.
PARTICIPANTS and INTERVENTIONS
Outcomes were assessed 3 years after treating 61 obese participants with T2DM who were randomized to either an intensive lifestyle weight loss intervention for 1 year followed by a lower lifestyle weight loss intervention (LLLI) for 2 years or surgical treatments [Roux en Y gastric bypass (RYGB) or Laparoscopic adjustable gastric banding (LAGB)] followed by LLLI in years 2 and 3.
MAIN OUTCOME MEASURES
Primary endpoints were partial and complete diabetes remission and secondary endpoints included diabetes medications and weight change.
RESULTS
Body mass index was <35kg/m2 for 26 (43%) participants, 50 (82%) were women, and 13 (21%) African American. Mean (SD) values for weight were 100.5 (13.7) kg, age 47.3 (6.6) years, hemoglobin A1c level 7.8% (1.9%), and fasting plasma glucose 171.3 (72.5) mg/dL. Partial or complete T2DM remission was achieved by 40% (n=8) of RYGB, 29% (n=6) of LAGB, and no LWLI participants (p=0.0037). The use of diabetes medications was reduced more in the surgical groups than the lifestyle alone group; with 65% of RYGB, 33% of LAGB, and 0% of LWLI going from using insulin or oral medication at baseline to no medication at year 3 (p<0.0001). Mean (SE) reductions in percent body weight at 3 years was the greatest after RYGB 25.0% (2.0), followed by LAGB 15.0% (2.0) and lifestyle treatment 5.7% (2.4) (p<0.01).
CONCLUSIONS
Among obese participants with T2DM, bariatric surgery with 2 years of an adjunctive LLLI resulted in more disease remission than did lifestyle intervention alone.
INTRODUCTION
It remains to be established whether or not bariatric surgery is a durable and effective treatment for type 2 diabetes (T2DM), and how bariatric surgery compares to intensive lifestyle modification and medication management with respect to T2DM-related outcomes. Non-surgical treatments alone have not generally resulted in the complete amelioration of diabetes or its potential long-term complications.1 As demonstrated in many observational studies2–5 and several small randomized controlled trials (RCTs) of short duration6–9, diabetes is greatly improved after bariatric surgery. To date, only one reported RCT with at least 3 years of follow up has shown that bariatric surgery (gastric sleeve, gastric bypass) was superior to intensive medical therapy for glycemic control, medication use, and quality of life measures.9 Thus more information is needed about the longer-term effectiveness and risks of all types of bariatric surgical procedures compared to lifestyle and medical management for those with T2DM and obesity. In addition, little is known about the relative utility of surgical versus non-surgical treatments for those with lower body mass index (BMI; weight in kilograms divided by height in meters squared) between 30 and 35 kg/m2 (Class I obesity) who are typically not included in surgical studies that are intended for weight loss outcomes alone.10
Earlier results from the trial reported here highlighted the significant challenges to completing a larger and more definitive RCT to determine the best treatment for T2DM in the setting of obesity. The one year results from this trial show that gastric bypass was the most effective treatment, followed by gastric banding for both T2DM remission and weight loss.11 In this longer-term study we report 3 year results examining the efficacy of two types of bariatric surgery (Roux-en-Y gastric bypass [RYGB], laparoscopic adjustable gastric banding [LAGB]) and an intensive lifestyle weight loss intervention (LWLI) for one year, followed by a low level lifestyle intervention (LLLI) for all 3 treatment groups in years 2 and 3 that was modeled after the Look AHEAD (Action for Health in Diabetes) trial.12 This report addresses the primary question of comparative efficacy of surgical and non-surgical treatments for T2DM remission and reports other glycemic control outcomes, weight change, lipids, blood pressure, and body composition. These results contribute to addressing questions about the relative efficacy of different surgical versus non-surgical treatments for T2DM in lower BMI individuals.
METHODS
STUDY DESIGN
The rationale, design, and methods of this study including details on recruitment, inclusion, assessment, randomization, and intervention during the first year of follow up have been reported.11 Briefly, the trial was a three-arm RCT stratified by gender and baseline BMI, conducted at an academic medical center that compared the efficacy for treating T2DM of two common surgical procedures (RYGB and LAGB) plus low level lifestyle intervention (LLLI) in years 2 and 3 of follow up with an intensive lifestyle weight loss intervention (LWLI) in year 1 followed by 2 years of LLLI. Adults 25 to 55 years old with a BMI of 30 to 40 kg/m2 were eligible and the diagnosis of T2DM was confirmed by fasting plasma glucose (FPG) level of greater than 125 mg/dL (to convert to millimoles per liter, multiply by 0.0555) and/or treatment with glucose-lowering medications.11 The 61 individuals who were treated (20 RYGB, 21 LAGB, 20 LWLI)11 and were thus eligible for the second phase of the trial and are included in analyses reported here. The study protocol was reviewed and approved by the Institutional Review Board at the center.
INTERVENTION
Upon completing 1 year follow up, participants provided informed consent to participate for 2 more years with annual visits and the addition of a structured low-level lifestyle intervention (LLLI) for all 3 treatment groups (RYGB +LLLI, LAGB+LLLI, and LWLI+LLLI). Both the initial intensive (LWLI) and later low level (LLLI) lifestyle interventions were modeled after The Diabetes Prevention Program and Look AHEAD and adapted for post-surgical participants. An initial instructional group session was held for participants in both surgical arms (RYGB, LAGB) to provide a lesson on behavioral weight control and orient them to the skills and strategies that had been learned and developed for the intensive lifestyle intervention group (LWLI) in year 1. The LLLI for all 3 treatment groups consisted of twice-monthly contact [one in-person session (~30–45 minutes) and one brief (<10 minutes) telephone contact] and regular refresher group series.13,14 Each intervention contact focused on a specific behavioral topic related to weight loss. If an individual missed an in-person session, all intervention materials were mailed to the subject. If a participant became unable to attend monthly in-person LLLI sessions they received intervention phone calls in place of visits. T2DM management was carried out by the original treating endocrinologist with glucose values monitored by the study physician for safety.
STUDY OUTCOMES
The primary outcomes were measures of T2DM remission, partial and complete according to American Diabetes Association criteria.11,15 Partial remission of T2DM was defined as absence of any medications for diabetes with hemoglobin A1c (HbA1c) level <6.5% and FPG ≤125 mg/dL and complete remission of T2DM as absence of medications with HbA1c <5.7% and FPG ≤ 100 mg/dL.15 At baseline prior to treatment, and annually through 36 months, changes in weight (difference in weight, percentage of weight loss from baseline, BMI, waist circumference), glycemic control (change in FPG and HbA1c), and the use of glucose-lowering medications (categorized; none, insulin only, insulin/other medication, oral/other medication) were assessed. Changes in lipid levels (total cholesterol, triglycerides, and high- and low-density lipoprotein), blood pressure, and body composition (percent body fat, lean mass, and bone mass), were secondary outcomes. Body composition was assessed using dual-energy x-ray absorptiometry (iDXA, GE Lunar, Inc., Madison, WI). Blood pressure was measured twice and averaged at each annual visit.
STATISTICAL ANALYSIS
Statistical analyses were performed using SAS (version 9.3) with the type I error rate fixed at 0.05 (two-tailed). Categorical variables are summarized using frequencies and percentages. Continuous variables with normal distributions are presented as mean (± standard deviation); continuous variables with non-normal distributions are presented as medians and interquartile ranges. Differences in baseline characteristics among the RYGB, LAGB and LWLI groups were examined using the Pearson’s chi-square test or Fisher’s exact test for categorical variables and analysis of variance or Kruskal-Wallis test for continuous variables.
Changes from baseline to 12-, 24-, and 36-months were analyzed using mixed effects models with covariate adjustment for randomization stratification factors (gender and baseline BMI). Change in weight was adjusted for baseline weight. Inferences focused on the overall treatment effect, time, and treatment by time interaction. Pairwise comparisons were made between treatment groups at 36-months. Least-square means were obtained from the models along with their standard errors. Intent-to-treat analyses were conducted using multiple imputation implemented using SAS procedures PROC MI and PROC MIANALYZE. For each outcome, ten datasets were imputed and results were combined. For categorical data with missing values (e.g. T2DM remission, medication use,), no remission or no improvement for the condition at follow up was imputed. The Fisher’s exact test was used to compare differences between groups for T2DM remission and medication category usage.
RESULTS
Study Participants
Of the 61 participants who underwent treatment, 52 (85% overall; 18 (90%) RYGB, 20 (95%) LAGB, 14 (70%) LWLI) were evaluated for the assessments of 3-year safety and efficacy. Of the 52 participants included in the 3 year analyses, 82% were women and 79% were Caucasian. The mean (SD) values for age were 47.3 (6.6) years, BMI 35.7 (3.1) kg/m2, baseline weight 100.5 (13.7) kg and 26 participants (43%) had grade 1 obesity (BMI < 35kg/m2). The mean (SD) baseline hemoglobin A1c level was 7.8% (1.9%), fasting plasma glucose 171.3 (72.5) mg/dL, and mean duration of T2DM was 6.5 (4.8) years. Higher percentages of individuals in the RYGB group had insulin requirements at baseline (RYGB, 50%; LAGB, 38%; LWLI 30%); p=0.01) and the RYGB group had a significantly higher baseline hemoglobin A1c level than LWLI [(RYGB 8.6% (2.1%); LAGB, 7.9% (2.2%); LWLI, 7.0% (0.8%); overall p=0.03, RYGB vs. LWLI p=0.0095] (eTable1 in Supplementary Appendix).
Primary endpoint
At 3 years, any T2DM remission (partial or complete) was achieved in 40% (n=8) of RYGB, 29% (n=6) of LAGB, and no LWLI (p=0.0037) (Figure 1) while complete remission was achieved in 15% (n=3) of RYGB, 5% (n=1) of LAGB and no LWLI group participants (p=0.21). Continuous, sustained (any; partial or complete) remission for at least 2 consecutive years of the 3-year follow up period was observed in 45% (n=9) of RYGB and 29% (n=6) of LAGB. As shown in Figure 1, there was a decline in any remission among RYGB participants from 60% at year 1 to 45% at year 2 and 40% at year 3, whereas any remission for LAGB participants remained stable at 29% and none for LWLI over the 3-year period (p=0.0876).
Figure 1. Prevalence of any remission (partial or complete) by treatment group and year.
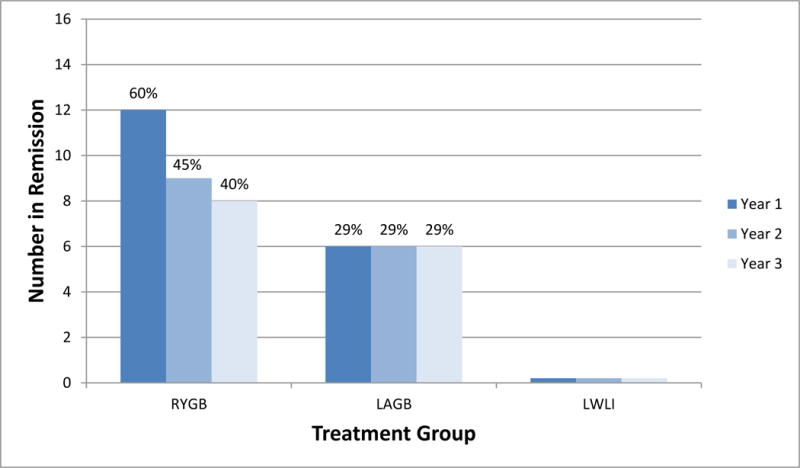
aPartial remission of T2DM - no use of antidiabetics, hemoglobin A1c (HbA1c) level of less than 6.5%, and fasting plasma glucose (FPG) level of125 mg/dL or less
Complete remission of T2DM - no use of antidiabetics, HbA1c level of less than 5.7%, and FPG level of 100mg/dL or less
Missing data at follow-up were assumed to be no remission.
RYGB = Roux-en-Y gastric bypass, LAGB = laparoscopic gastric banding, LWLI = Lifestyle weight loss intervention (intensive)
The test of the difference between treatment group p-values are calculated for each time point as follows: year 1 p = <0.0001, year 2 p<0.0001, year 3 p = 0.0037.
Glycemic control
After 3 years, each of the surgical procedures plus LLLI was superior to lifestyle intervention alone (LWLI + LLLI) in achieving glycemic control (Figure 2). The RYGB group had the greatest change in both hemoglobin A1c [−1.42% (0.34)] and FPG [−66.0 mg/dL (10.9)] from baseline to 3 years (hemoglobin A1c, p<0.0013 RYGB vs. LWLI; FPG, p<0.05 for RYGB vs. both LAGB and LWLI) (Table 1). The LAGB group showed improved hemoglobin A1c averaging 0.80 (0.32) % at 3 years (p=0.0373 LAGB vs. LWLI) and FPG improved an average of 35.2 mg/dL (10.5) (p=0.66). Also examined were the changes in hemoglobin A1c and FPG over time by class (I, II) of obesity. For both measures, there were no significant interactions between obesity class and treatment groups, indicating that the patterns over time between treatment groups did not differ significantly by class of obesity. The use of diabetes medications was reduced more in the surgical groups than the lifestyle alone group; with 65% of RYGB, 33% of LAGB, and 0% of LWLI going from using insulin or oral medication at baseline to no medication at year 3 (p<0.0001). Therefore, at 3 years those in the RYGB group had the largest percentage of individuals (72%) not requiring any medications for T2DM compared to those in the LAGB (45%) and LWLI (0%) groups (Figure 3, p<0.0001).
Figure 2. Hemoglobin A1c (HbA1c) and Fasting Plasma Glucose (FPG) change by treatment group and year.
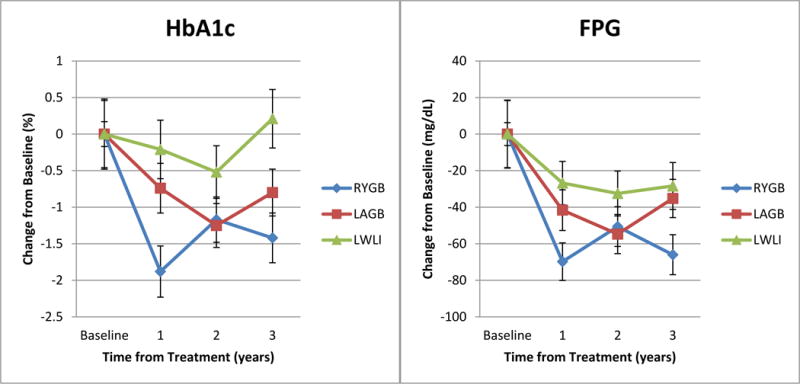
aBars represent standard error
Table 1.
MI Results* for change in continuous variables by intervention condition
| Changes from Baseline to Year 3 P-value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Variable | Intervention Group |
Baseline** MEAN±SE |
Change from Baseline to 1 Year LSMEAN± SE |
Change from Baseline to 2 Years LSMEAN± SE |
Change from Baseline to 3 Years LSMEAN± SE |
Group P-value |
Time P-value |
Group × Time P-value |
RYGB vs LAGB |
RYGB vs LWLI |
LAGB vs LWLI |
| Weight (kg) | RYGB | 99.27±2.99 | −28.8±1.68 | −26.0±2.03 | −24.6±2.12 | <.0001 | 0.0124 | 0.8253 | 0.0004 | <.0001 | 0.0009 |
| LAGB | 100.2±3.06 | −18.6±1.79 | −16.7±2.02 | −14.9±2.05 | |||||||
| LWLI | 102.0±3.19 | −7.35±2.06 | −5.10±2.39 | −5.03±2.53 | |||||||
| Percent Weight Change | RYGB | −29.1±1.64 | −26.3±1.99 | −25.0±2.04 | <.0001 | 0.0135 | 0.8143 | 0.0002 | <.0001 | 0.0011 | |
| LAGB | −18.5±1.74 | −16.5±1.98 | −15.0±1.98 | ||||||||
| LWLI | −7.59±2.00 | −5.59±2.34 | −5.70±2.42 | ||||||||
| Body Mass Index (kg/m2) | RYGB | 35.67±0.61 | −10.2±0.59 | −9.20±0.70 | −8.70±0.72 | <.0001 | 0.0202 | 0.8252 | 0.0002 | <.0001 | 0.0007 |
| LAGB | 35.58±0.75 | −6.41±0.63 | −5.71±0.70 | −5.19±0.69 | |||||||
| LWLI | 35.75±0.73 | −2.38±0.69 | −1.71±0.79 | −1.75±0.82 | |||||||
| Waist Circumference (cm) | RYGB | 110.6±1.83 | −27.3±1.60 | −22.7±1.92 | −21.5±1.93 | <.0001 | 0.0018 | 0.3948 | 0.0007 | <.0001 | 0.0043 |
| LAGB | 114.5±2.59 | −16.4±1.80 | −13.1±2.06 | −12.7±1.98 | |||||||
| LWLI | 111.8±2.13 | −5.65±1.81 | −4.02±2.18 | −4.84±2.19 | |||||||
| Fasting Plasma Glucose (FPG) | RYGB | 191.5±18.33 | −69.8±10.30 | −50.6±10.83 | −66.0±10.94 | 0.0418 | 0.8051 | 0.1318 | 0.0308 | 0.0212 | 0.6697 |
| LAGB | 180.0±18.63 | −41.6±11.12 | −54.7±10.69 | −35.2±10.47 | |||||||
| LWLI | 142.1±6.26 | −26.8±11.85 | −32.5±12.30 | −28.4±12.82 | |||||||
| Hemoglobin A1c (HbA1c) (%) | RYGB | 8.56±0.46 | −1.88±0.35 | −1.17±0.31 | −1.42±0.34 | 0.0137 | 0.0455 | 0.0175 | 0.1554 | 0.0013 | 0.0373 |
| LAGB | 7.87±0.48 | −0.74±0.34 | −1.25±0.30 | −0.80±0.32 | |||||||
| LWLI | 7.03±0.17 | −0.21±0.40 | −0.52±0.36 | 0.21±0.40 | |||||||
| Percent Body Fat | RYGB | 43.27±1.46 | −16.1±1.08 | −12.2±1.31 | −10.7±1.25 | <.0001 | <.0001 | 0.0116 | 0.0013 | <.0001 | 0.1320 |
| LAGB | 44.61±1.23 | −8.07±1.07 | −7.32±1.27 | −5.63±1.19 | |||||||
| LWLI | 44.11±1.05 | −4.41±1.24 | −2.98±1.50 | −3.08±1.44 | |||||||
| Fat Mass(kg) | RYGB | 42.50±1.77 | −23.6±1.30 | −20.1±1.63 | −18.1±1.71 | <.0001 | 0.0007 | 0.2470 | 0.0006 | <.0001 | 0.0223 |
| LAGB | 44.49±1.67 | −13.7±1.37 | −12.3±1.64 | −10.3±1.67 | |||||||
| LWLI | 43.78±1.53 | −6.61±1.50 | −4.44±1.88 | −4.71±1.98 | |||||||
| Lean Mass(kg) | RYGB | 53.09±2.39 | −4.78±0.76 | −5.98±0.83 | −5.58±0.81 | <.0001 | 0.3291 | 0.3887 | 0.0042 | <.0001 | 0.0440 |
| LAGB | 52.61±2.22 | −2.55±0.77 | −2.39±0.82 | −2.78±0.79 | |||||||
| LWLI | 52.87±2.06 | −0.10±0.94 | −0.27±1.01 | −0.62±0.99 | |||||||
| Bone Mass(kg) | RYGB | 2.93±0.12 | −0.20±0.03 | −0.28±0.04 | −0.32±0.04 | <.0001 | <.0001 | 0.0004 | 0.0003 | <.0001 | 0.0826 |
| LAGB | 2.78±0.11 | −0.09±0.03 | −0.10±0.03 | −0.15±0.03 | |||||||
| LWLI | 2.77±0.12 | −0.07±0.03 | −0.06±0.04 | −0.07±0.04 | |||||||
| Total Cholesterol(mg/dL) | RYGB | 200.2±9.00 | −32.1±9.18 | −6.56±9.09 | −13.1±8.45 | 0.4416 | 0.0040 | 0.5682 | 0.1620 | 0.9061 | 0.2578 |
| LAGB | 189.5±12.18 | −18.5±9.89 | −10.6±9.58 | 2.48±8.48 | |||||||
| LWLI | 182.0±8.72 | −31.4±10.45 | −18.3±10.35 | −11.6±10.15 | |||||||
| HDL(mg/dL) | RYGB | 41.75±1.95 | 12.85±2.13 | 15.35±2.29 | 16.73±2.35 | 0.0007 | 0.0067 | 0.6593 | 0.0638 | 0.0004 | 0.0611 |
| LAGB | 39.95±2.04 | 5.23±2.27 | 8.06±2.42 | 11.02±2.36 | |||||||
| LWLI | 44.10±3.83 | 3.03±2.36 | 3.96±2.54 | 4.71±2.69 | |||||||
| LDL(mg/dL) | RYGB | 117.8±10.63 | −13.1±7.41 | 5.00±8.47 | −0.50±7.96 | 0.3153 | 0.2085 | 0.1994 | 0.5166 | 0.5423 | 0.2235 |
| LAGB | 90.55±11.04 | 4.66±8.02 | 3.69±9.08 | 6.52±8.27 | |||||||
| LWLI | 105.5±7.45 | −11.2±8.36 | −9.54±9.56 | −7.66±9.42 | |||||||
| Triglycerides(mg/dL) | RYGB | 169.7±27.16 | −107±10.64 | −82.7±17.25 | −95.3±17.11 | 0.0002 | 0.0298 | 0.5780 | 0.0469 | 0.0028 | 0.2225 |
| LAGB | 221.9±44.44 | −86.6±11.44 | −65.8±18.81 | −48.8±17.16 | |||||||
| LWLI | 161.2±24.52 | −35.2±11.88 | −38.5±19.44 | −16.9±20.53 | |||||||
| Systolic Blood Pressure(mmHg) | RYGB | 139.7±2.74 | −17.3±3.58 | −18.7±3.35 | −13.0±4.09 | 0.0063 | 0.0010 | 0.1440 | 0.0033 | 0.0328 | 0.6056 |
| LAGB | 134.5±3.71 | −6.27±3.57 | −7.37±3.49 | 2.72±3.84 | |||||||
| LWLI | 132.0±4.00 | −10.6±3.91 | −3.56±3.63 | −0.24±4.58 | |||||||
| Diastolic Blood Pressure(mmHg) | RYGB | 81.27±2.14 | −7.02±1.82 | −8.52±2.10 | −5.44±1.82 | 0.0102 | 0.0211 | 0.3611 | 0.0005 | 0.3237 | 0.0260 |
| LAGB | 77.12±1.87 | −2.10±1.80 | −1.91±2.22 | 2.65±1.69 | |||||||
| LWLI | 76.28±2.15 | −4.36±1.97 | −3.09±2.30 | −2.87±2.03 | |||||||
Models adjusted for gender, BMI < 35 or ≥ 35 kg/m2, and repeated measures
Baseline values are raw means and standard errors
Figure 3. Diabetes medication usage by treatment group.
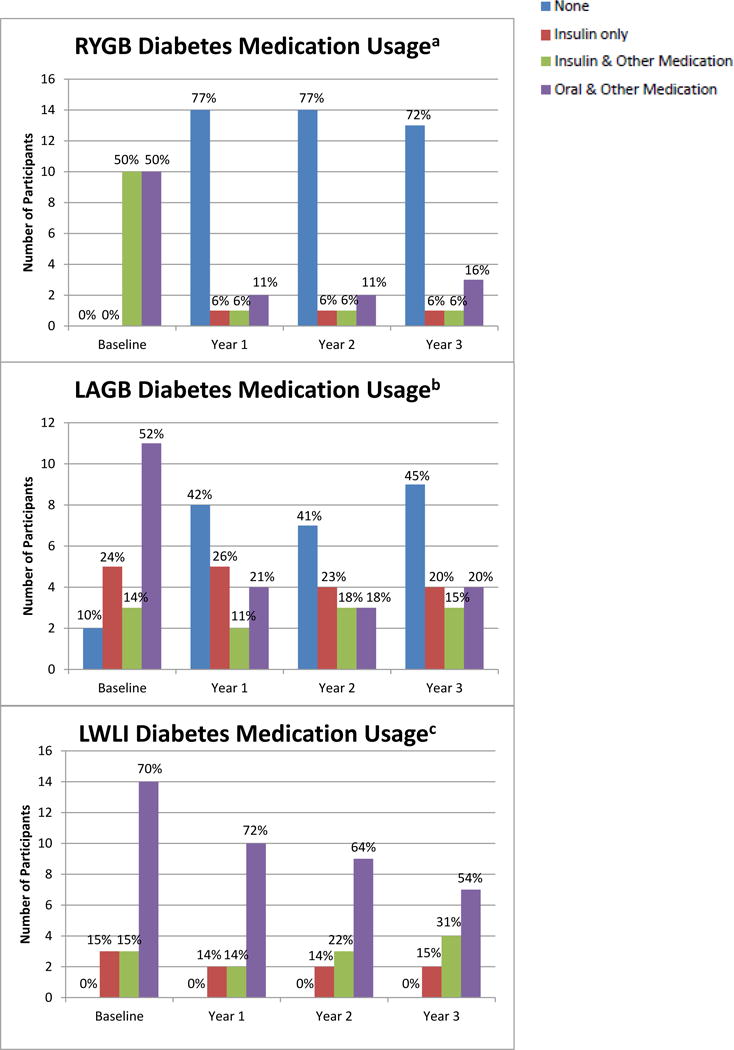
aBaseline n=20; Year 1; n=18; Year 2 n=18; Year 3 n=18
bBaseline n=21; Year 1 n=19; Year 2 n=17; Year 3 n=20
cBaseline n=20; Year 1 n=14; Year 2 n=14; Year 3 n=14*
*One participant was missing medication data
Body weight and composition
At 3 years, modeled reductions in body weight, BMI, and waist circumference were greater after RYGB and LAGB than after lifestyle treatment alone (Table 1). The percent reduction in body weight was also greater after RYGB than after LAGB (p=0.0002). Figure 4 shows the modeled mean (SE) percent weight change from baseline to each of 3 follow up time points by treatment group; RYGB 29.1% (1.6) at year 1 to 25.0%(2.0) at year 3, LAGB 18.5% (1.7) at year 1 to 15.0%(2.0) at year 3, LWLI 7.6%(1.9) at year 1 to 5.7% (2.4) at year 3.
Figure 4. Percent weight change from baseline by treatment groupa.
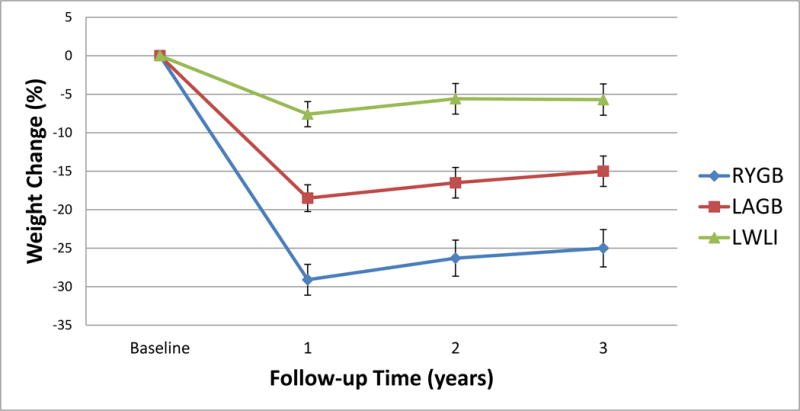
aModeled data, Bars represent standard error (SE)
Modeled change in body composition is shown in Table 1. Change in percent body fat at 3-year follow up was significantly greater in RYGB compared to both LABG and LWLI [−10.7% (1.3), −5.6% (1.2), and −3.1% (1.4), respectively, both pairwise p<0.01]. The change in mean(SE) kilograms of lean mass from baseline to Year 3 was significantly less in LWLI [−0.6 (1.0)] compared to LAGB [−2.8 (0.8), p=0.0440] and RYGB [−5.6 (0.8), p<0.0001], and in LAGB compared to RYGB (p=0.0042). There was also a different pattern in mean bone mass over time between the groups (p=0.0004), showing the reduction in bone mass from baseline to Year 3 to be greater in RYGB compared to both LAGB (p=0.0003) and LWLI (p<0.0001).
Lipids and blood pressure
At 3 years, the RYGB surgical group experienced greater improvements in triglyceride levels (p=0.0028) and high density lipoprotein (HDL) cholesterol levels (p=0.0004) as compared to the lifestyle arm (Table 1). Improvements in systolic and diastolic blood pressure were less consistent, but the RYGB group had the greatest improvements in systolic blood pressure compared to LAGB and LWLI (p<0.05 for both, Table 1) and diastolic blood pressure from baseline to year 3 compared to LAGB (p=0.0005).
Adverse events
There were no complications reported in the LWLI group at any time point up to 3 years and no additional surgical interventions in either of the two surgical groups after year 1 of follow up.11 One infusion port replacement for malposition in a LAGB participant occurred during the first year of follow up. There were no LAGB removals or replacements. All adverse events and complications are shown in Supplemental eTable2.
DISCUSSION
The results of this study show that Roux en Y gastric bypass plus low level lifestyle (RYGB + LLLI) and Laparoscopic adjustable gastric band (LAGB+LLLI), are superior to lifestyle treatment alone for Type 2 diabetes (T2DM) remission and other glycemic control endpoints at 3 years. One important aspect of this study is that over 40% of the sample are individuals with Class I obesity (BMI 30 to less than 35 kg/m2), for whom data in the literature are largely lacking.10 Those who underwent a surgical procedure followed by low level lifestyle intervention were significantly more likely to achieve and maintain glycemic control than were those who received intensive and then maintenance (low level) lifestyle therapy alone, regardless of obesity class. More than two thirds of those in the RYGB group and nearly half of the LAGB group did not require any medications for T2DM treatment at 3 years. Analysis of secondary endpoints also showed favorable results for the RYGB group and to a lesser extent for the LAGB group. Significant reductions in lean and bone mass were observed in the RYGB group that may warrant further investigation. Adverse events were uncommon after the first year. Thus, these results add to a growing body of literature suggesting that bariatric surgery may be a viable treatment option for people with BMI 30–40 kg/m2 for whom medical management is ineffective.
A systematic review and meta-analysis focused on non-morbidly obese people found only 3 trials including individuals with BMIs between 30 and 40 kg/m2 and, in total, they included only 13 people with BMI less than 35 kg/m2. This review demonstrated that surgery was associated with greater weight loss (range, 14.4–24 kg) and glycemic control (range, 0.9–1.43% improvements in hemoglobin A1c levels) during 1 to 2 years of follow-up compared with nonsurgical treatment.10 Further, they concluded that evidence is insufficient to reach conclusions about the appropriate use of bariatric surgery in this lower-BMI (BMI 30–35, Class 1 obesity) population until more data are available about both complications and long-term outcomes of surgery. This report helps fill this gap by contributing 26 additional low BMI participants undergoing surgical and non-surgical treatments to the literature for diabetes remission and weight outcomes. Another systematic review and meta-analysis shows that bariatric surgery when compared with non-surgical treatment leads to greater body weight loss and higher remission rates of T2DM and metabolic syndrome.16 However, these results are limited mostly to two years of follow-up and are based on a relatively small number of studies and individuals. The only longer-term RCT with three-year follow up compared RYGB or sleeve gastrectomy with intensive medication management to an intensive medical management regimen alone in individuals with uncontrolled T2DM9 and demonstrated 38% of RYGB, 24% of sleeve, and 5% of intensive medical treatment groups achieved a HbA1c under 6.0% either on or off glycemic control medications.
Results of the trial reported here add important data to these longer-term results by showing a comparable 40% partial or complete remission of T2DM (including the requirement to be off of any medications to meet the remission definition) with RYGB and 29% remission after LAGB which is a new result. To our knowledge, there has not been any other RCT with three year results that include the LAGB as a surgical treatment alternative. The LAGB is a less invasive, reversible, and low-risk procedure that may have a role for treatment, especially in the lower BMI group. Several small RCTs comparing the LAGB to lifestyle therapy alone in people with BMIs 30–40 kg/m2 have shown superior weight loss and diabetes or metabolic syndrome improvement in the LAGB arm but did not report beyond 2 years of follow up.6,16,17 In two of these prior studies of adults the lifestyle intervention varied from standard diabetes care6 to the use of very low calorie diets17 resulting in mean percentage of baseline weight loss of 1.7% and 5.7%, respectively at 2 years, in the non-surgical arms of each trial, and 20% mean weight lost two years following the LAGB in both trials. There was remission (hemoglobin bA1c < 6.2%, FPG <126, off any medications) of T2DM in 22 (73%) in the surgical group and 4 (13%) of the usual care group in one study.6 In the other trial, the prevalence of metabolic syndrome was reduced significantly in the surgical group at the completion of the 2 year study.17 In the current study the remission rate of T2DM was lower (29%) in the band treatment arm than in these two previously published trials and may reflect differences in study groups at baseline, remission definition differences, or the heterogeneity of response to treatment in those participating in each of these trials. Nevertheless, the mean weight loss (15%) and remission rate for T2DM (29%) in the band arm of this trial was similar to that in the 3 year Longitudinal Assessment of Bariatric Surgery (LABS) Study.18 The LABS Study was an observational, multi-center study that did not standardize post-surgical care, showed significant heterogeneity of weight loss response, and may be more representative of LAGB results in the United States.
This study has several strengths that include the delivery of a standardized non-surgical treatment that was intensive in the first year and lower level in subsequent years modeled after The Diabetes Prevention Program (DPP) and the Look AHEAD studies.12,13,19,20 Another strength of this trial was the broad inclusion criteria for T2DM which resulted in recruiting some participants with less disease severity than those recruited into similar RCTs targeting only those with uncontrolled T2DM.7,21,22 Thus, these results may generalize to the broader population of those with obesity and T2DM of varying severity. Thirdly, this trial is the first in the United States to generate 3-year results for the LAGB, an inherently less invasive procedure that may be of particular interest for patients with obesity, but in a lower range of BMI (30 to 35 kg/m2). The limitations of this study include the small sample size from a single site, which may affect generalizability. Nevertheless, a larger and even more definitive study will likely not be completed in the future due to numerous research challenges that have been identified. These include large numbers of potential participants being screened to successfully randomize participants, the need for a multi-center consortium for results to be generalizable, and the ensuing prohibitive costs.11,23,24 A National Institutes of Health 2013 workshop summary concluded that important information about this topic may need to come, in the future, from either combining similar smaller trials or from carefully controlled observational studies.23 Therefore, the data from this relatively small trial are valuable and worthy of careful consideration alone and/or in combination with other RCTs with similar aims/goals.9,21
In conclusion, this study provides further important evidence that at longer-term follow up of 3 years, surgical treatments including RYGB and LAGB are superior to lifestyle intervention alone for the remission of T2DM in obese individuals including those with a BMI between 30 and 35 kg/m2. While this trial provides valuable insights, unanswered questions remain such as the impact of these treatments on long-term micro- and macro-vascular complications and the precise mechanisms by which bariatric surgical procedures induce their effects.
Supplementary Material
Acknowledgments
Disclosure Statement: (1) AC, JJ, MK have support from the NIH/NIDDK, NIH, NIDDK for the submitted work; (2) AC has grants from Nutrisystem, J&J Ethicon, Covidien, and is a consultant for Ethicon, Apollo (outside the submitted work). JJ has grants from Jawbone/BodyMedia, personal fees from Weight Watchers (outside the submitted work). MK has grants from the Obesity Society/Nutrisystem, grants from ASMBS Foundation, and grants from NIDDK (outside the submitted work; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) SB, JD, JE, SP, WL, RN have nothing to declare.
Funding/Support: The study was funded by NIH-NIDDK R01DK095128 and by Magee-Womens Hospital of UPMC (University of Pittsburgh Medical Center) for subsidizing the surgical procedures.
Role of the Sponsor: Design and conduct of the study: This study was funded by a successful competitive grant application to the NIH-NIDDK. The sponsor did not play a role in the collection, management, analysis or interpretation of data, the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01047735
Author Contributions:
Dr. Courcoulas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Courcoulas, Eagleton, Belle, Jakicic.
Acquisition, analysis, or interpretation of data: Courcoulas, DeLany, Eagleton, Neiberg, Lang, Jakicic.
Drafting of the manuscript: Courcoulas, Pierson, Neiberg, Lang, Jakicic.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Neiberg, Lang.
Obtained funding: Courcoulas, Eagleton.
Administrative, technical, or material support: Courcoulas, Eagleton, Pierson, Kalarchian, Jakicic
Study supervision: Courcoulas, Eagleton, Pierson, DeLany, Jakicic.23.
Contributor Information
Anita P. Courcoulas, University of Pittsburgh Medical Center, Department of Surgery.
Steven H. Belle, University of Pittsburgh Graduate School of Public Health, Departments of Epidemiology and Biostatistics.
Rebecca H. Neiberg, Wake Forest School of Medicine, Department of Biostatistical Sciences.
Sheila K. Pierson, University of Pittsburgh Medical Center, Department of Surgery.
Jessie K Eagleton, University of Pittsburgh Medical Center, Department of Surgery.
Melissa A. Kalarchian, Duquesne University, School of Nursing.
James P. DeLany, University of Pittsburgh, Division of Endocrinology and Metabolism, Department of Medicine
Wei Lang, Wake Forest School of Medicine, Department of Biostatistical Sciences.
John M. Jakicic, University of Pittsburgh, Department of Health and Physical Activity, Physical Activity and Weight Management Research Center.
References
- 1.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. doi: 10.1097/00000658-199509000-00011. discussion 350–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 7.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309(21):2250–61. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 11.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707–15. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clin Trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19(10):1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–5. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144(9):625–33. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 18.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–25. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Look ARG, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Look ARG, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term Outcomes of Bariatric Surgery: A National Institutes of Health Symposium. JAMA Surg. 2014 doi: 10.1001/jamasurg.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe BM, Belle SH. Long-term risks and benefits of bariatric surgery: a research challenge. JAMA. 2014;312(17):1792–3. doi: 10.1001/jama.2014.12966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


