Abstract
The dynamic balance between microtubule extension and actin contraction regulates mammalian cell shape, division and motility, which has made the cytoskeleton an attractive and very successful target for cancer drugs. Numerous compounds in clinical use to reduce tumor growth cause microtubule breakdown (Vinca alkaloids, Colchicine-site, Halichodrins) or hyperstabilization of microtubules (Taxanes, Epothilones). However, both of these strategies indiscriminately alter the assembly and dynamics of all microtubules, which causes significant dose-limiting toxicities on normal tissues. Emerging data is revealing that posttranslational modifications of tubulin (detyrosination, acetylation) or microtubule-associated proteins (Tau, Aurora kinase) may allow for more specific targeting of microtubule subsets, thereby avoiding the broad disruption of all microtubule polymerization. Developing approaches to reduce tumor cell migration and invasion focus on disrupting actin regulation by the kinases SRC and ROCK. Since the dynamic balance between microtubule extension and actin contraction also regulates cell fate decisions and stem cell characteristics, disrupting this cytoskeletal balance could yield unexpected effects beyond tumor growth. This review will examine recent data demonstrating that cytoskeletal cancer drugs affect wound healing responses, microtentacle-dependent reattachment efficiency and stem cell characteristics in ways that could impact the metastatic potential of tumor cells, both beneficially and detrimentally.
Background
Microtubules (MT) consist of polymers of α-tubulin and β-tubulin that originate through nucleation with γ-tubulin localized at the centrosome in the cell center. The growing MT polymer of α/β-tubulin extends outward from the centrosome toward the plasma membrane (Fig. 1 – green arrows). A cortical network of actin filaments supports the cytoplasmic face of the plasma membrane, and contraction of these filaments with myosin motor proteins provides an inward force (Fig. 1 – red arrows). Continuous pressure of MTs against the contracting actin cortex moves the MT polymerization origin at the centrosome toward the cell center, by pushing against all edges of the cell. Elegant seminal experiments showed that this balancing of MT forces allows the centrosome to find the center of a plate well, even when it is not enclosed within a cell(1). To enable cell division, duplication of the MT centrosome allows the mitotic spindle to form as a scaffold for chromosome segregation, as well as providing a new cell center for the two resulting daughter cells. These critical roles in chromosome segregation and cell division have made MTs the most commonly targeted cytoskeletal system for cancer therapeutics(2).
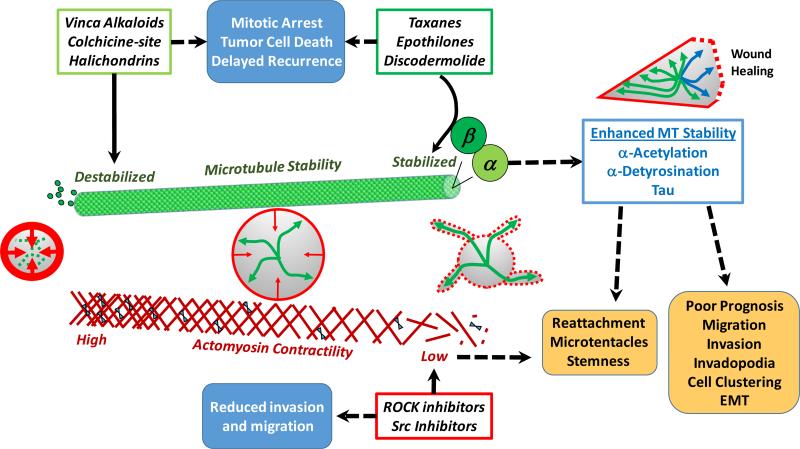
Figure 1.
Cytoskeletal cancer therapies alter microtubule-actin balance and tumor cell behavior. Expansion of microtubules (MT) from the cell center (green arrows) is counteracted by contraction of cortical actin filaments beneath the plasma membrane (red arrows). Since cytoskeletal reorganization is critical to cell division and migration, it is a common target for drugs aimed at reducing tumor growth or invasion (blue boxes). MT therapies in clinical use either disrupt MT polymerization (light green box) or hyperstabilize MTs (dark green box) by targeting the β-tubulin subunit. However, the α-tubulin subunit of stabilized MTs (light blue box) has posttranslational modifications (α-acetylation, α-detyrosination) and associated proteins (Tau) that are linked to aggressive tumor cell behaviors and poor patient prognosis (orange boxes). MT-stabilizing therapies yield the same modifications and select for upregulation of Tau. Likewise, disruption of actomyosin contractility (red box) to reduce tumor cell invasion has recently been shown to elevate stem cell characteristics and promote the microtentacle-dependent reattachment of tumor cells. Reflecting a role for MT-actin balance as an environmental sensor, a minor subset of stabilized MTs (blue arrows) orients toward wound edges and actomyosin contraction is reduced (red dotted line) to accelerate directional cell migration. Both of these wound healing responses are simulated by drugs that stabilize MTs or reduce actin contraction. Clarifying how this MT-actin balance governs tumor cell behavior and is affected by existing drug treatments could help improve the development of more precise cytoskeletal cancer therapies. EMT, epithelial-to-mesenchymal transition.
Two general strategies exist to inhibit MT function for cancer therapy, by either disrupting polymer assembly or hyperstabilizing the polymer(2). Both approaches disrupt MT dynamics by tipping the continuous balance of assembly/disassembly toward one end of the spectrum, which results in reduced tumor growth and the induction of apoptosis (Fig. 1 – upper blue box). Intriguingly, of the numerous therapies that are used to target MTs, all bind to the β-tubulin subunit and there are no compounds yet available that target the α-tubulin subunit(2). MT-destabilizing compounds currently in clinical use fall into three classes, based on their natural origin and binding site on the β-tubulin subunit (Fig. 1 – light green box): Vinca alkaloids (Vinblastine, Vincristine), Colchicine-site (Colchicine, Combrestatins) or Halichodrins (Eribulin, E7389). MT-stabilizing compounds are similarly classified according to their source and binding site on β-tubulin (Fig. 1 – dark green box): Taxanes (Paclitaxel, Docetaxel, Cabazitaxel), Epothilones (Ixabepilone, Epothilone-B, Sagopilone) and Discodermalide(2). Since each MT compound also originated as a natural product (2) from either plants (Vinca alkaloids, Taxanes, Colchicine-site), bacteria (Epothilones) or marine sponges (Halichondrins, Discodermolide), this selectivity for the β-tubulin subunit likely reflects the exposure of β-tubulin at the rapidly-growing plus end of the MT polymer. This β-tubulin selectivity has important clinical consequences, since mutation or alternative expression of the seven β-tubulin isotypes allows tumors to develop resistance to MT-directed therapies, with the most prominent being upregulation of the βIII-tubulin isotype which confers resistance to both vinca alkaloids and taxanes(3). In addition, since all current MT therapies broadly target the fundamental polymerization activity of β-tubulin, these drugs affect overall MT dynamics in every cell type, thereby impacting normal tissue functioning(2, 4). The resulting side effects, including neutropenia and peripheral neuropathy, are clinically-significant and have caused dose-limiting toxicities that required clinical trials with Discodermalide to be discontinued(4). While there are ongoing efforts to avoid dose-limiting toxicities through the generation of analogs and conjugates of MT compounds(2, 4), the underlying principle of broadly targeting MT polymerization presents a persistent challenge for specificity toward cancer cells.
Targeting actin polymerization in a similarly broad fashion is not clinically feasible, due to the critical role of actin filaments in the contraction of cardiac and skeletal muscle. Unlike the dimeric polymers of α/β-tubulin, actin filaments are composed of monomers of actin isoforms that polymerize in a double-helical conformation (Fig. 1 – red lines)(5). The α-actin isoform is exclusively expressed in muscle, while the β/γ-actin isoforms are expressed in non-muscle cells. As with MTs, there are numerous natural compounds that either stabilize or depolymerize actin filaments, but structural similarities between different actin isoforms results in toxicities to both muscle and non-muscle cells (6). Likewise, the myosin-II motor protein mediates contraction of actin filaments in both muscle and non-muscle cells, yielding similar challenges to directly targeting myosin-II as a cancer therapy(7). In non-muscle cells, myosin-II assembles in a bipolar arrangement that moves two actin filaments in opposite directions (Fig. 1 – blue triangles), yielding an inward contractile tension of the actin cortex on the cell (Fig. 1 – red arrows)(7). Strategies to target actin for reducing tumor cell migration and invasion (Fig. 1 – lower blue box) have focused around specific kinases (Fig. 1 – red box), such as SRC and Rho-kinase (ROCK), which activate actin contraction by promoting phosphorylation of the regulatory light chain of myosin-II, rather than targeting actin polymerization itself(8, 9). Chemical targeting of SRC tyrosine kinase activity reduces actomyosin contractility, invadopodia formation, and the migration/invasion of many different tumor cell types, and is showing promise in limiting the outgrowth of metastatic lesions(10, 11). Reducing actomyosin contractility through inhibition of ROCK similarly reduces tumor cell migration/invasion and has very recently been shown to reduce the metastatic outgrowth of melanoma(12). As we consider the other potential impacts of targeting actomyosin contractility for cancer treatment, it is nevertheless worth noting that SRC, ROCK and myosin-II coordinate to form and strengthen the E-cadherin intercellular junctions that are a linchpin of epithelial growth control through contact inhibition(8).
Clinical-Translational Advances
To further advance the clinical effectiveness of cytoskeletal cancer drugs, it will be important to understand the opportunities to increase specificity and consider recent findings on how altering MT-actin balance clinically affects tumor cell behaviors aside from growth. While current MT therapies broadly target polymerization through the β-tubulin subunit, post-translational modifications of α-tubulin and microtubule-associated proteins provide the potential to target specific subsets of MTs that are relevant to disease, rather than all MTs indiscriminately.
The majority of MTs are highly dynamic and turnover rapidly, but smaller groups of highly-stabilized MTs (Fig. 1 – light blue box) are identified by posttranslational modifications (α-detyrosination, α-acetylation) or MT-associated proteins (Tau) and play critical roles in tumor cell behavior that are connected to disease progression and survival in cancer patients (Fig. 1 – orange boxes). Dynamic MTs, which continuously expand from the cell center and have a half-life of approximately 5 minutes, contain a tyrosine residue at the c-terminal end of α-tubulin (Tyr-MT)(13, 14). Selective stabilization is observed in MTs where this tyrosine is removed (α-detyrosination), yielding a subset of MTs (Detyr-MTs) that can persist for as long as 16 hours(13, 14). Reflecting the role for MTs as sensors of cell shape, stabilized DeTyr-MTs orient toward wound edges and are required for efficient directional cell migration to heal the wound(15) (Fig. 1 – blue arrows). Clinical studies have shown that DeTyr-MTs are likewise enriched at the invasive fronts of breast tumors in vivo(16) and that elevated α-detyrosination is an indicator of poor prognosis in breast cancer(17). Further supporting a role for α-detyrosination in tumor development, the tubulin tyrosine ligase (TTL) that restores the tyrosine on α-tubulin is suppressed in many cancers and loss of TTL can directly promote tumor formation in mouse models(18). TTL is similarly suppressed during the epithelial-to-mesenchymal transition that occurs during breast cancer progression(16) and DeTyr-MTs are elevated in breast tumor cells with increased stem cell characteristics(19). For these reasons, the tubulin carboxypeptidase (TCP) that catalyzes α-detyrosination is an attractive target to specifically reduce stabilized DeTyr-MTs(20). However, after many years of searching, several promising TCP candidate genes have been identified(21, 22) but the critical TCP has not yet been definitively established.
Acetylation of lysine-40 on α-tubulin also occurs on the stabilized MTs that regulate cell polarity and directional migration (Fig. 1 – light blue box). Recent studies have shown that α-tubulin acetylation predicts poor patient prognosis in head-and-neck cancer(23) and breast cancer(23, 24). Moreover, α-tubulin acetylation promotes the invasion and migration of breast cancer cells and is elevated in metastatic cells from several cancers, including breast(24), pancreatic(25), and head-and-neck(23). Basal-like breast cancers, which are characterized by poor patient survival and high metastasis rates, also demonstrate increased α-tubulin acetylation compared to other breast cancer subtypes(24). Paralleling the observations of stabilized DeTyr-MTs in breast cancer stem-like cells, α-tubulin acetylation can be used as a marker to purify the tumor-initiating subpopulation of pancreatic tumor cells(25). The principal α-tubulin acetyltransferase enzyme (ATAT1) has recently been established(26), providing a target for compounds to selectively target stabilized MTs.
The potential benefits of advancing beyond a broad targeting of MT polymerization can also be appreciated by considering how MT drugs in current clinical use impact tumor cell behaviors aside from growth. First, MT-stabilizing compounds strongly upregulate the posttranslational modifications of α-tubulin (α-detyrosination, α-acetylation) that are linked to increased tumor cell invasion(27), EMT (16), stem cell characteristics(25), poor patient prognosis(17, 23, 24) and metastasis(23, 24) (Fig. 1 – orange boxes). Second, the location of tumor cells can influence cellular responses to MT-directed drugs. In free-floating microenvironments, tumor cells form microtentacles, which are MT-based plasma membrane protrusions that extend when cells are detached from extracellular matrix (ECM). In contrast, invadopodia are actin-based protrusions that occur on ECM-attached cells and contain proteolytic enzymes that promote ECM degradation and local invasion(12). Since stabilized MTs serve as mechanical sensors for actin-mediated directional cell migration, it is also notable that a small molecule screen for inhibitors of tumor cell invadopodia also discovered that taxane-induced MT stabilization strongly increased invadopodia in melanoma, head-and-neck, ovarian and NSCLC cells(11). Microtentacles, on the other hand, promote the re-attachment of detached tumor cells to endothelial monolayers(16) and retention of circulating tumor cells (CTCs) in the lungs of living mice(28, 29). Microtentacles are supported by stabilized detyrosinated(30) and acetylated MTs(24), so taxane-mediated MT stabilization increases microtentacles and tumor cell reattachment(31). Conversely, MT-destabilizing compounds reduce the ability of microtentacles to promote the aggregation of free-floating tumor cells(30). Viewed in the light of compelling recent results showing that CTCs which form aggregates have up to 50-fold higher metastatic potential(32), it is worthwhile considering how MT-directed therapies could influence CTCs. Taxane treatment significantly increased lung metastases of injected tumor cells and was also linked to elevated stem cell characteristics, but potential effects on CTC clustering or retention were not examined in this earlier study(33). In addition to upregulation of stem cell characteristics, tumor cells become resistant to taxane treatment by overexpressing the Tau MT-associated protein which stabilizes MTs and competes with taxanes for binding to β-tubulin(34). However, Tau also promotes microtentacles and tumor cell lung retention that could account for enrichment of Tau in metastatic tumors(29). Careful monitoring shows that CTCs in breast cancer patients can increase up to 1000-fold during neoadjuvant taxane treatment and that patients with increasing CTC levels have a 25-fold higher recurrence risk(35). Since the current detection limit for clinical imaging is a tumor foci of approximately 10 million cells(36), it will be important to develop methods that are capable of distinguishing whether primary tumor shrinkage during neoadjuvant therapy is occurring due to tumor cell death or tumor cell scattering. This may be particularly relevant in cancers with different modes of dissemination, like ovarian cancer, where more than 4 cycles of neoadjuvant taxane treatment actually leads to a significantly higher recurrence risk (RR=3.05, P<0.001)(37). Nevertheless, evidence remains very strong that breast cancer patients who show a pathological complete response after neoadjuvant therapy with taxanes do have improved survival(38), but some patient subsets show rapid progression(39) and it is not yet clear how to best distinguish responsive patients before neoadjuvant therapy begins(40).
Several routes exist to move beyond broadly targeting MT polymerization. Screening specifically for inhibitors of DeTyr-tubulin identified Parthenolide and Costunolide(20), and these compounds can reduce stabilized MTs, microtentacles and reattachment without affecting overall MT function(41), which could reduce side effects on normal cells. The recent identification of the primary α-tubulin acetyltransferase(26) now provides a clear molecular target to develop compounds that could selectively reduce α-tubulin acetylation. However, there is no guarantee that specifically targeting stabilized MTs will help avoid side effects like neuropathy, since detyrosinated and acetylated MTs are highly enriched in neuronal cell types. Another option is to target specific MT-associated proteins to disrupt critical MT functions, rather than tubulin polymerization itself. Intriguing MT-associated proteins for this focused approach are the Aurora kinases(42), which mediate centrosome duplication and MT spindle positioning. The great interest in targeting these specific MT functions more directly is evident in the ongoing clinical trials from 12 different companies with Aurora kinase inhibitors(43).
Further reflecting the balance between MT expansion and actin contraction, targeting actomyosin contractility produces effects similar to MT stabilization on stem cell characteristics and microtentacles. The kinases SRC and ROCK have clear roles in tumor cell migration and have been proposed as targets to reduce tumor invasion (Fig. 1 – red box)(44). Since both SRC and ROCK promote phosphorylation of myosin-II(8), inhibition of either kinase serves to reduce actomyosin contraction and promotes the formation of microtentacles and increased tumor cell reattachment(28, 45). Further supporting MT-actin balance as a sensor of the local microenvironment, reduced actomyosin contraction accelerates cell migration at wound edges in a MT-dependent manner(46). More strikingly, studies in many different cancers show that reducing actomyosin contractility by inhibiting ROCK activity strongly promotes stem cell characteristics(47-49). Addition of a small molecule ROCK inhibitor (Y-27632) to feeder cultures allows indefinite propagation of breast and prostate cancer cells, as well as matched normal tissue, and limiting dilution experiments demonstrated that ROCK inhibition operates via cell reprogramming rather than selective pressure(47). The effect of Y-27632 to promote stem cell characteristics was recapitulated in colon cancer cells with a direct inhibitor of myosin-II, Blebbistatin, confirming that reprogramming occurs through an effect on actomyosin contractility(49). Studies in human embryonic stem cells used Y-27632 and Blebbistatin together with siRNAs against myosin-II heavy chain and myosin-II light chain to demonstrate that releasing actomyosin contractility allows stem cells to survive in detached and dissociated conditions(50). ROCK activation was also shown to regulate the mechanosensation of ovarian cancer cells which promotes selective growth and migration of ovarian tumor cells in the soft microenvironments that are typical of ovarian cancer metastatic sites(51). Most ROCK inhibitor trials have focused on cardiovascular disease(52) or glaucoma(53). Currently, there is only one active clinical trial using a ROCK inhibitor for cancer, a multi-kinase inhibitor AT13148 that shows its strongest activity against ROCK1, ROCK2, PKA and p70S6K(54). Preclinical data showed AT13148 inhibited the growth of uterine sarcoma, prostate, lung and breast tumor cells(54), Phase I results presented at the 2015 ASCO meeting show that AT13148 is well-tolerated with oral delivery and demonstrates dose-related hypotension as an on-target effect of inhibiting ROCK in smooth muscle(55).
By comparison, 59 clinical trials have been undertaken to date to target the tyrosine kinase SRC for cancer, but clinical results have been largely disappointing(56). Six of these trials have combined SRC inhibitors with taxanes which would be predicted to synergistically disrupt cytoskeletal balance. One of the first completed Phase 2 studies that combines a SRC inhibitor, Saracatinib, with Paclitaxel in ovarian cancer showed that progression-free survival was actually worse with the drug combination, although statistical significance was not reached definitively (P=0.07)(57). Preclinical data continue to indicate that Saracatinib can reduce invadopodia formation and tumor cell extravasation(58), so it is possible that these effects are not detectable with the focus of current clinical trials on tumor growth. Nevertheless, Phase 2 trials with Saracatinib as a monotherapy for cancer were discontinued due to lack of efficacy, but the compound remains under investigation for applications in Alzheimer's disease. Since reducing actomyosin contractility can elevate aggressive tumor cell behaviors, an alternative would be to stimulate actomyosin contraction to restore balance to the cytoskeleton. While there are exceedingly few compounds being tested toward this goal (Fig. 1 - lower left), one very recent high-throughput screen did identify 4-hydroxyacetophenone as a compound capable of restoring cytoskeletal balance by stimulating myosin-II, reducing the invasion and migration of pancreatic tumor cells (59). These pancreatic tumor cells did have very low contractility which was increased by 4-hydroxyacetophenone, but many tumors already have strong actomyosin contractility(60) that would need to be reduced to rebalance the system. Ultimately, defining where tumor cells reside on the mechanical spectrum(61) may help personalize treatment options to rebalance the cytoskeleton rather than simply pushing cells to one extreme of MT polymerization or actomyosin contractility.
Drugs targeting the cytoskeleton are undoubtedly very effective in reducing tumor growth and can extend patient survival. Continuing to improve cytoskeletal cancer therapies will benefit from identifying more specific targets aside from MT polymerization and broad regulators of actin contraction, as well as clarifying how altering MT-actin balance affects metastatic phenotypes, so that cytoskeletal therapies targeting primary tumor growth do not inadvertently increase metastatic risk.
Acknowledgments
Grant Support
This work was supported by the NCI of the NIH under award numbers F30CA196075 (K.R. Chakrabarti), T32CA154274 (K.R. Chakrabarti and L. Hessler), and R01CA154624 and R01CA124704 (L. Bhandary and S.S. Martin) and an Era of Hope Scholar Award (BC100675) from the U.S. Department of Defense (S.S. Martin).
Footnotes
Disclosure of Potential Conflicts of Interest
S.S. Martin is listed as a co-inventor on pending patent applications, which are owned by the University of Maryland, related to a medical device to image patient tumor cells, using microtentacles as a measurement of patient prognosis and targeting microtentacles as a therapy. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Holy TE, Dogterom M, Yurke B, Leibler S. Assembly and positioning of microtubule asters in microfabricated chambers. Proc Natl Acad Sci U S A. 1997;94:6228–31. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014;13:275–84. doi: 10.1158/1535-7163.MCT-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–75. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 4.Kingston DG. Tubulin-interactive natural products as anticancer agents. J Nat Prod. 2009;72:507–15. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Kustermans G, Piette J, Legrand-Poels S. Actin-targeting natural compounds as tools to study the role of actin cytoskeleton in signal transduction. Biochem Pharmacol. 2008;76:1310–22. doi: 10.1016/j.bcp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Ouderkirk JL, Krendel M. Non-muscle myosins in tumor progression, cancer cell invasion, and metastasis. Cytoskeleton (Hoboken) 2014;71:447–63. doi: 10.1002/cm.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Rico C, Pincet F, Thiery JP, Dufour S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J Cell Sci. 2010;123:712–22. doi: 10.1242/jcs.047878. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka T, Yashiro M. Rho/ROCK signaling in motility and metastasis of gastric cancer. World J Gastroenterol. 2014;20:13756–66. doi: 10.3748/wjg.v20.i38.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert MA, Yang J. Targeting invadopodia to block breast cancer metastasis. Oncotarget. 2011;2:562–8. doi: 10.18632/oncotarget.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4:ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadok A, McCarthy A, Caldwell J, Collins I, Garrett MD, Yeo M, et al. Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Res. 2015;75:2272–84. doi: 10.1158/0008-5472.CAN-14-2156. [DOI] [PubMed] [Google Scholar]
- 13.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci U S A. 1987;84:9040–4. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988;85:5946–50. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whipple RA, Matrone MA, Cho EH, Balzer EM, Vitolo MI, Yoon JR, et al. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010;70:8127–37. doi: 10.1158/0008-5472.CAN-09-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–7. [PubMed] [Google Scholar]
- 18.Lafanechere L, Courtay-Cahen C, Kawakami T, Jacrot M, Rudiger M, Wehland J, et al. Suppression of tubulin tyrosine ligase during tumor growth. J Cell Sci. 1998;111(Pt 2):171–81. doi: 10.1242/jcs.111.2.171. [DOI] [PubMed] [Google Scholar]
- 19.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, et al. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote attachment. Cancer Res. 2014;74:1250–60. doi: 10.1158/0008-5472.CAN-13-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonrose X, Ausseil F, Soleilhac E, Masson V, David B, Pouny I, et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67:3371–8. doi: 10.1158/0008-5472.CAN-06-3732. [DOI] [PubMed] [Google Scholar]
- 21.Sahab ZJ, Hall MD, Me Sung Y, Dakshanamurthy S, Ji Y, Kumar D, et al. Tumor suppressor RARRES1 interacts with cytoplasmic carboxypeptidase AGBL2 to regulate the alpha-tubulin tyrosination cycle. Cancer Res. 2011;71:1219–28. doi: 10.1158/0008-5472.CAN-10-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez de la Vega M, Sevilla RG, Hermoso A, Lorenzo J, Tanco S, Diez A, et al. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007;21:851–65. doi: 10.1096/fj.06-7330com. [DOI] [PubMed] [Google Scholar]
- 23.Saba NF, Magliocca KR, Kim S, Muller S, Chen Z, Owonikoko TK, et al. Acetylated tubulin (AT) as a prognostic marker in squamous cell carcinoma of the head and neck. Head Neck Pathol. 2014;8:66–72. doi: 10.1007/s12105-013-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, Goloubeva OG, Ioffe OB, et al. alpha-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015;75:203–15. doi: 10.1158/0008-5472.CAN-13-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–56. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
- 27.Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91:950–60. doi: 10.1016/j.ejcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Balzer EM, Whipple RA, Thompson K, Boggs AE, Slovic J, Cho EH, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29:6402–8. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29:3217–27. doi: 10.1038/onc.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313:1326–36. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2010;121:65–78. doi: 10.1007/s10549-009-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–20. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hekimian K, Meisezahl S, Trompelt K, Rabenstein C, Pachmann K. Epithelial cell dissemination and readhesion: analysis of factors contributing to metastasis formation in breast cancer. ISRN Oncol. 2012;2012:601810. doi: 10.5402/2012/601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Xie H, Ning H, Citrin D, Capala J, Maass-Moreno R, et al. Accuracy of 3D volumetric image registration based on CT, MR and PET/CT phantom experiments. J Appl Clin Med Phys. 2008;9:2781. doi: 10.1120/jacmp.v9i4.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombo PE, Labaki M, Fabbro M, Bertrand M, Mourregot A, Gutowski M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014;135:223–30. doi: 10.1016/j.ygyno.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 38.DeMichele A, Yee D, Berry DA, Albain KS, Benz CC, Boughey J, et al. The neoadjuvant model is still the future for drug development in breast cancer. Clin Cancer Res. 2015;21:2911–5. doi: 10.1158/1078-0432.CCR-14-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821–8. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marino N, Woditschka S, Reed LT, Nakayama J, Mayer M, Wetzel M, et al. Breast cancer metastasis: issues for the personalization of its prevention and treatment. Am J Pathol. 2013;183:1084–95. doi: 10.1016/j.ajpath.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whipple RA, Vitolo MI, Boggs AE, Charpentier MS, Thompson K, Martin SS. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-kappaB inhibition. Breast Cancer Res. 2013;15:R83. doi: 10.1186/bcr3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutertre S, Descamps S, Prigent C. On the role of aurora-A in centrosome function. Oncogene. 2002;21:6175–83. doi: 10.1038/sj.onc.1205775. [DOI] [PubMed] [Google Scholar]
- 43.Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M. Aurora kinase inhibitors: progress towards the clinic. Invest New Drugs. 2012;30:2411–32. doi: 10.1007/s10637-012-9798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narumiya S, Tanji M, Ishizaki T, Rho signaling. ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 45.Bhandary L, Whipple RA, Vitolo MI, Charpentier MS, Boggs AE, Chakrabarti KR, et al. ROCK inhibition promotes microtentacles that enhance reattachment of breast cancer cells. Oncotarget. 2015;6:6251–66. doi: 10.18632/oncotarget.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 49.Ohata H, Ishiguro T, Aihara Y, Sato A, Sakai H, Sekine S, et al. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72:5101–10. doi: 10.1158/0008-5472.CAN-11-3812. [DOI] [PubMed] [Google Scholar]
- 50.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–8. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGrail DJ, Kieu QM, Dawson MR. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J Cell Sci. 2014;127:2621–6. doi: 10.1242/jcs.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7:657–71. doi: 10.2217/fca.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–77. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yap TA, Walton MI, Grimshaw KM, Te Poele RH, Eve PD, Valenti MR, et al. AT13148 is a novel, oral multi-AGC kinase inhibitor with potent pharmacodynamic and antitumor activity. Clin Cancer Res. 2012;18:3912–23. doi: 10.1158/1078-0432.CCR-11-3313. [DOI] [PubMed] [Google Scholar]
- 55.Papadatos-Pastos D, Kumar R, Yap TA, Ruddle R, Decordova S, Jones P, et al. A first-in-human study of the dual ROCK I/II inhibitor, AT13148, in patients with advanced cancers. J Clin Oncol. 2015;33(suppl) abstr 2566. [Google Scholar]
- 56.Creedon H, Brunton VG. Src kinase inhibitors: promising cancer therapeutics? Crit Rev Oncog. 2012;17:145–59. doi: 10.1615/critrevoncog.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 57.McNeish IA, Ledermann JA, Webber L, James L, Kaye SB, Hall M, et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancerdagger. Ann Oncol. 2014;25:1988–95. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 58.Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–70. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 59.Surcel A, Ng WP, West-Foyle H, Zhu Q, Ren Y, Avery LB, et al. Pharmacological activation of myosin II paralogs to correct cell mechanics defects. Proc Natl Acad Sci U S A. 2015;112:1428–33. doi: 10.1073/pnas.1412592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–27. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]