Abstract
Objectives
We examined the feasibility of a high-dose, 96-hour infusion of ketamine in treatment-resistant depression.
Methods
Ten participants were randomized to receive a 96-hour ketamine infusion, titrated as tolerated to a target rate of 0.6mg/kg/hour, while 10 received a 40-minute ketamine infusion (0.5mg/kg). Both groups received clonidine, titrated to a maximum of 0.6mg orally daily, during the infusion to mitigate side effects of ketamine. Participants were followed for eight weeks to examine potential antidepressant effects.
Results
All 20 participants completed the infusion. Most participants tolerated the infusion well, with minimal psychotomimetic symptoms or blood pressure elevation despite achieving high ketamine concentrations (mean 424ng/ml for 96-hour arm, 156ng/ml for 40-minute arm). There was no rebound hypertension upon discontinuing clonidine. Rapid and sustained improvement in depressive symptoms was observed in both study groups. Higher ketamine concentration was associated with sustained antidepressant response, and was not with greater psychotomimetic side effects, in the 96-hour arm.
Conclusions
This study provides evidence for the feasibility of prolonged ketamine infusions in treatment-resistant depression. Co-administration of clonidine appeared to mitigate ketamine's psychotomimetic effects. Further study is required to investigate the extent to which prolonged ketamine infusions could provide both rapid and sustained improvements in treatment-resistant depression.
Clinicaltrials.gov identifier NCT01179009
Introduction
Approximately 15-30% of individuals with depression have a severe form that is resistant to standard antidepressant treatments (Trevino et al., 2014), leading to extended suffering, excess health care costs, and elevated risk of suicide (Mrazek et al., 2014; Petersen et al., 2004). Ketamine is an anesthetic drug with N-methyl-D-aspartate (NMDA) glutamate receptor antagonist activity, which may provide a novel antidepressant mechanism (Li et al., 2010). Accordingly, research groups have tested the antidepressant effects of a brief infusion of ketamine in both unipolar (Berman et al., 2000; Murrough et al., 2013; Zarate et al., 2006) and bipolar depression (Diazgranados et al., 2010), finding a significant reduction in depressive symptoms lasting approximately one week using 0.5mg/kg ketamine infused over 40- minutes. This focus on brief, low-dose infusions (Lai et al., 2014) stems from ketamine's propensity to have significant neuropsychiatric side effects, including dissociation, confusion, and even overt psychosis (Morgan et al., 2004). The psychotomimetic effects are highly correlated with plasma ketamine concentrations (Bowdle et al., 1998; Newcomer et al., 1999). In addition to psychotomimetic side effects, a safety concern with ketamine is sympathomimetic effects, including increased blood pressure (Luckenbaugh et al., 2014). To provide a more sustained antidepressant effect while mitigating these safety concerns, serial brief infusions have been proposed (Liebrenz et al., 2009; Rasmussen et al., 2013).
By contrast, groups studying ketamine for chronic pain have tested prolonged, high-dose ketamine infusions of 4-14 days to provide sustained pain relief (Niesters et al., 2014). The rationale is that a prolonged blockade of NMDA receptors causes long-term changes in signal transduction leading to sustained clinical improvement (Farber et al., 1995; Sigtermans et al., 2009). To reduce or prevent side effects with ketamine, especially psychotomimetic and sympathomimetic effects, many such studies co-administered the alpha-2 agonist clonidine (Handa et al., 2000; Nitta et al., 2013; Schwartzman et al., 2009; Sigtermans et al., 2009). The basis for alpha-2 agonist co-administration stems from preclinical research on ketamine and other NMDA antagonists (Farber et al., 1995) showing that the cholinergic pathway arising out of the basal forebrain, as well as glutamatergic pathways arising out of the thalamus, become hyperactive with systemic NMDA blockade via loss of GABAergic inhibition (see Figure in appendix for depiction of the neurocircuitry of ketamine's effects). These two excitatory pathways converge on the same downstream corticolimbic neurons, thus hyperactivating them and leading to cognitive deficits and complex behavioral effects (Farber, 2003; Newcomer et al., 1999). Alpha-2 agonist co-administration dampens the cholinergic pathway (Farber et al., 2002), relieving some of the excessive stimulation of the downstream corticolimbic neurons, thereby reducing pathologic effects of NMDA antagonists (Jevtovic-Todorovic et al., 1998; Newcomer et al., 1998). Thus, clonidine co-administration may allow for prolonged ketamine infusions for treatment-resistant depression.
Accordingly, we carried out a pilot randomized controlled trial in which we compared a 96-hour ketamine infusion against a 40-minute ketamine infusion. In this study, all subjects also received clonidine, an alpha-2 agonist, to minimize side effects of ketamine. The primary goal was demonstrating the feasibility of this research, so our focus was on safety and tolerability. We examined the rates, types, and severity of cognitive and behavioral side effects commonly seen with ketamine, with a focus on psychotomimetic side effects, as well as blood pressure changes during the infusion. We predicted that clonidine would mitigate these psychotomimetic and sympathomimetic side effects without interrupting ketamine's antidepressant effects. We further hypothesized that both groups would show rapid antidepressant effects but only the 96-hour treated group would show sustained antidepressant effects. We also explored whether side effects and sustained antidepressant effects were related to ketamine concentration.
Materials and Methods
We enrolled adults aged 18-65 with major depressive disorder, diagnosed by the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994), with confirmatory clinical evaluation carried out by study psychiatrists, and Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) score ≥22 indicating at least moderate severity. We defined treatment resistance in the current episode as retrospective non-responsive to at least two adequate trials of antidepressant medications in terms of dose and duration, as assessed by clinician assessment, consistent with published methods (Petersen et al., 2005). Exclusion criteria included bipolar disorder, lifetime psychotic disorder, substance abuse/dependence, and medical instability. Medication-related exclusions included dopamine agonists, antagonists or reuptake inhibitors (other than aripiprazole at doses up to 5mg daily); centrally acting pro- or anti-cholinergics; or benzodiazepines or other GABA-acting agents, based on hypotheses regarding NMDA receptor mechanisms (Farber, 2003). Continued use of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors was allowed if the dose was kept constant for at least six weeks leading up to the infusion. The university's institutional review board approved the study, and all participants gave written informed consent prior to any study procedures.
All study participants were started on clonidine 0.1mg orally twice daily, approximately seven-days prior to the infusion. Then, they were admitted for a five-day (four-night) stay at the Washington University Clinical Research Unit (see appendix for details on admission procedures). Participants were randomized 1:1 to receive a 96-hour infusion that contained either (a) ketamine for the entire 96- hours or (b) saline only for the first 95-hours and 20-minutes, followed by 0.5mg/kg ketamine for the final 40-minutes. Participants randomized to 96-hour ketamine started at 0.15mg/kg/hour, with increases as tolerated twice daily until a target infusion rate of 0.6mg/kg/hour was achieved. The uptitration strategy and target infusion rate were based prior ketamine research suggesting that this would achieve a plasma level of 400ng/ml (Newcomer et al., 1999) (Goldberg et al., 2011; Schwartzman et al., 2009; Sigtermans et al., 2009; Webster and Walker, 2006). Based on preclinical and clinical data it was estimated that this plasma level would result in approximately 50% blockade of NMDA receptors (Emnett et al., 2013; Hartvig et al., 1995). Clonidine was increased in both groups as tolerated to 0.2mg orally twice daily starting on the evening of day 1 of the infusion, and then 0.3mg twice daily starting on the evening of day 2. Ketamine and clonidine were stopped on the morning of day 5. Participants were discharged four hours post-infusion, with the exception that the first nine stayed an additional 24-hours so that we could examine rebound hypertension from clonidine discontinuation. Participants and staff were blinded to treatment assignment; the research pharmacy prepared unlabeled, identical-appearing bags of saline and ketamine for each subject. The study statistician generated the random allocation sequence. The trial was stopped after N=20 randomized, which was predetermined based on feasibility goals.
We assessed cognitive and behavioral side effects of the ketamine infusion in two ways. First, participants were assessed daily with the Brief Psychiatric Rating Scale (Flemenbaum and Zimmermann, 1973) four-item positive symptom subscale (BPRS+; scale range 4-28, higher scores indicate greater psychotic symptoms; items are Conceptual Disorganization, Suspiciousness, Hallucinatory Behavior, and Unusual Thought Content), as a scale of psychotomimetic symptoms. Second, participants were assessed with the clinician-administered Clinical and Adverse Events Checklist of 20 ketamine side effects using a 5-point Likert scale (where 0 = symptom absent, 1= minimal, 2= mild, 3 = moderate, 4= severe) (Newcomer et al., 1999), up to four times daily, to measure psychotomimetic symptoms, cognitive symptoms, other behavioral symptoms, and physical symptoms. Vital signs were measured via continuous ECG monitoring as well as blood pressure monitoring hourly for 4 hours after initiation and dose increases of ketamine; blood pressure was also measured every 8 hours (full description of unit orders and procedures is available from the first author).
To measure within-infusion depressive symptoms, participants were assessed daily with a 24-hour version of the MADRS which was modified from a published structured interview (Williams and Kobak, 2008). The 24-hour MADRS was also conducted on post-infusion day 1, as was the Clinical Global Impressions Improvement scale (CGI-I)(Guy, 2000) to examine rapid antidepressant effects (note that the clinicaltrials.gov entry for this study erroneously listed the Hamilton Depression Rating Scale as the outcome measure; this was corrected in 2014). Because of concerns about prolonged infusion-induced hepatitis (Niesters et al., 2014), we also examined liver enzyme levels at the end of the infusion. All participants were assessed at 2, 4, 6, and 8-weeks post-infusion by phone with the MADRS and CGI-I (standard versions encompassing the previous 7 days). These assessments were carried out by a blinded rater who was unaware of the study design, did not do any other assessments, and had no contact with participants during the infusion; nevertheless, this individual guessed correctly in 17/20 cases.
Venous blood was obtained daily, centrifuged, plasma stored at −20°C, and later assayed for enantiomeric (i.e., R- and S-) ketamine and active metabolite (norketamine) concentrations at 10am on days 2-5 for the 96-hour group, and at 10am on day 5 (at the end of the infusion) for the 40-minute group. R- and S-ketamine and R- and S-norketamine were determined by HPLC-tandem mass spectrometry using a previously published method (Moaddel et al., 2010).
Statistical Analyses
Spearman correlations were used to explore the relationship between primary outcome variables and additional variables of interest. An Analysis of Variance (ANOVA) model was employed to explore the effects of treatment status on the primary outcome variables while linear regression was used when both the independent and dependent variables were continuous. In exploring the differences between the 40-minute infusion and the 96-hour infusion across categorical variables, Fisher's Exact tests and Chi-Square tests were employed. All analyses were performed using SPSS V. 22. The α value was set at 0.05; two-tailed tests were run.
Results
From 2012-2014, we recruited 22 participants via referrals and clinicaltrials.gov; one withdrew consent prior to the infusion, and one was found to be ineligible (in remission from depression) at the start of the infusion, leaving 20 eligible participants. All 20 individuals completed the infusions and were included in the analyses (appendix shows participant flow from screening through treatment and follow-up). Of the 10 who received the 96-hour ketamine arm, the mean final dose was 0.52mg/kg/hour (SD=0.14); two participants could not tolerate the target infusion rate, and their final dose was ≤0.45mg/kg/hour. Table 1 shows the baseline characteristics, infusion data, and final R- and S-ketamine and norketamine concentrations measured at the end of the infusion. R- and S-ketamine concentrations were very highly correlated (r=0.998, p<0.001) as were R- and S-norketamine concentrations (r=0.98, p<0.001). As the table shows, participants had a high degree of chronicity of depression and high number of previous treatment failures, similar to other clinical trials of ketamine for treatment-resistant depression (e.g., Murrough et al, 2013).
Table 1.
Characteristics of the 96-hour and 40-minute ketamine study arms
|
Total Sample
(N = 20) |
40-Min
Infusion (n = 10) |
96-Hr
Infusion (n = 10) |
Test Value | P | |
|---|---|---|---|---|---|
| Age (mean years, SD) | 44.6 (13.1) | 46.6 (12.8) | 42.5 (13.8) | F[1,19] = 0.47 |
0.50 |
| Gender (n, %) | --------* | 0.63 | |||
| Male | 6 (30) | 2 (20) | 4 (40) | ||
| Female | 14 (70) | 8 (80) | 6 (60) | ||
| Race (n, %) | --------* | 1.00 | |||
| Caucasian | 19 (95) | 10 (100) | 9 (90) | ||
| Asian | 1 (5) | 0 (0) | 1 (10) | ||
| Family History of alcoholism (n, %) | χ2(2.40) | 0.30 | |||
| Yes | 10 (50) | 4 (40) | 6 (60) | ||
| No | 9 (45) | 6 (60) | 3 (30) | ||
| Unknown | 1 (5) | 0 (0) | 1 (10) | ||
| CGI - Severity of Illness (n, %) | χ2(2.00) | 0.37 | |||
| Moderately Ill | 1 (5) | 1 (10) | 0 (0) | ||
| Markedly Ill | 18 (90) | 9 (90) | 9 (90) | ||
| Severely Ill | 1 (5) | 0 (0) | 1 (10) | ||
|
Lifetime Major Depressive Episodes
(n, %) |
--------* | 0.65 | |||
| Single | 8 (40) | 5 (50.0) | 3 (30.0) | ||
| Recurrent | 12 (60) | 5 (50.0) | 7 (70.0) | ||
|
Age of First Major Depressive
Episode (mean years, SD) |
20.1 (6.0) | 22.0 (5.4) | 18.2 (6.1) | F[1,19] = 2.15 |
0.16 |
|
Duration of Current Episode (mean
years, SD) |
12.7 (11.4) | 16.6 (12.8) | 8.7 (8.6) | F[1,19] = 2.61 |
0.12 |
|
Number of Antidepressant Trials
(mean, range) |
17.6 (7->31) | 16.8 (7->23) | 18.4 (12- >31) |
F[1,19] = 0.53 |
0.48 |
|
Current Antidepressant Medications
(during infusion) ** |
n/a | 5 SSRI, 3 SNRI, 1 aripiprazole |
3 SSRI, 2 SNRI, 1 aripiprazole |
n/a | n/a |
|
Baseline Total MADRS Score (mean,
SD) |
33.0 (4.9) | 34.0 (3.8) | 31.9 (5.9) | F[1,19] = 0.90 |
0.35 |
| Baseline HAM-D Score (mean, SD) | 21.9 (4.1) | 21.6 (2.8) | 22.1 (5.3) | F[1,19] = 0.07 |
0.80 |
|
Max. Daily Clonidine Dose (mg)
(mean, SD) |
0.40 (0.12) | 0.33 (0.12) | 0.46 (0.08) | F[1,19] = 8.22 |
0.01 |
|
Day 5 Ketamine Concentrations
(ng/mL) (mean, SD) |
|||||
| Total (S+R) Ketamine | n/a | 156 (43) | 424 (178) | F[1,19] = 21.4 |
<0.0001 |
| S-Ketamine | n/a | 77 (21) | 203 (86) | F[1,19] = 20.5 |
< 0.0001 |
| R-Ketamine | n/a | 79 (21) | 221 (93) | F[1,19] = 22.0 |
< 0.0001 |
| S-Norketamine | n/a | 16 (5) | 135 (55) | F[1,19] = 47.0 |
< 0.0001 |
| R-Norketamine | n/a | 18 (6) | 160 (73) | F[1,19] = 37.7 |
< 0.0001 |
Performed using a Fisher's Exact Test
SSRI=selective serotonin reuptake inhibitor, SNRI=serotonin-norephinephrine reuptake inhibitor. SSRIs, SNRIs, and aripiprazole (up to 5mg daily) were allowed to be continued during the infusion.
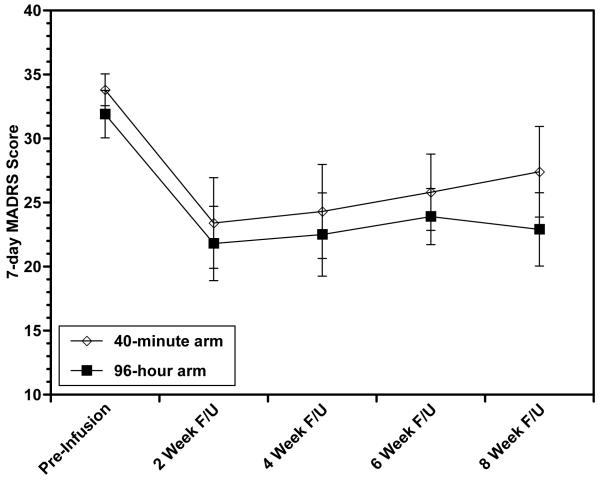
During post-infusion follow-up, one individual (from the 40-minute arm) was removed from the study before week 6 because she received ECT and her remaining data points were imputed; the remaining 19 completed all eight weeks of follow-up. Both groups demonstrated rapid antidepressant effects, as measured by the CGI-I on post-infusion day 1: 7/10 in each arm had had a score in the much or very much improved range. Additionally, both arms showed sustained reduction in depressive symptoms compared to baseline, with no difference between arms in MADRS changes (Figure 1), and 4/10 having a CGI-I of 1-2 at week 8 in the 96-hour arm vs. 2/10 in the 40-minute arm. When we examined results in terms of response (≥50% reduction in MADRS), 4/10 in the 96-hour group and 2/10 in the 40-minute group were responders at week 2; of these, 2/10 in the 96-hour group and 1/10 in the 40-minute group maintained response out to week 8.
Figure 1. Montgomery-Asberg Depression Rating Scale (MADRS) score changes with 96-hour vs. 40-minute ketamine infusion during eight weeks of post-infusion follow-up.
There were no significant differences (at p<0.05) between study arms. Means ± standard error are shown.
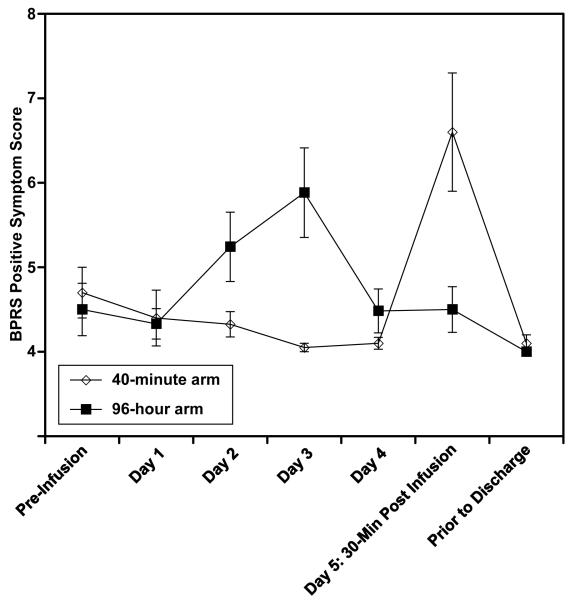
Side effects (Table 2) were common in the 96-hour ketamine arm but were generally mild in intensity and tended to decline during days 4-5 even as the ketamine concentration stayed constant. With regard to psychotomimetic symptoms, a minority of patients showed transient hallucinatory experiences or transient delusional or unusual thoughts. Overall the BPRS+ score stayed near its minimal score of 4 throughout the infusion in both arms (Figure 2). As the figure shows, a small increase in BPRS+ scores in the 96-hour ketamine arm, in days 2-3, subsided as the infusion continued; while in the 40-minute ketamine arm, there was a transient increase attributable to the 40-minutes of ketamine at the end of the infusion.
Table 2.
Side effects during the 96-hour infusion
| Symptom | # reporting side effect (and highest level of severity) | |
|---|---|---|
| 96-hr ketamine arm | 40-min ketamine arm | |
| Psychotomimetic | ||
|
| ||
| Visual distortions and hallucinations* |
7/10 (2 minimal, 1 mild, 4 moderate) |
4/10 (4 mild) |
| Unusual thoughts and delusions** |
7/10 (3 minimal, 2 mild, 2 moderate) |
5/10 (1 minimal, 3 mild, 1 moderate) |
|
| ||
| Confusion | ||
|
| ||
| Incoherent | 2/10 (2 moderate) | 1/10 (1 mild) |
| Disorientation | 3/10 (3 minimal) | 2/10 (2 minimal) |
| Decreased | 10/10 (1 minimal, 2 mild, 4 moderate, 3 severe) |
10/10 (4 mild, 6 moderate) |
| Concentration/Memory Loss | ||
|
| ||
| Other Behavioral | ||
|
| ||
| Restlessness | 10/10 (2 mild, 5 moderate, 3 severe) |
8/10 (3 mild, 5 moderate) |
| Anxiety | 10/10 (1 mild, 5 moderate, 4 severe) |
10/10 (1 mild, 7 moderate, 2 severe) |
| Palpitations | 3/10 (1 minimal, 1 mild, 1 moderate) |
4/10 (3 minimal, 1 mild) |
| Insomnia | 9/10 (2 mild, 4 moderate, 3 severe) |
8/10 (1 mild, 7 moderate) |
| Involuntary Movements | 1/10 (1 minimal) | 3/10 (2 minimal, 1 mild) |
|
| ||
| Physiological | ||
|
| ||
| Headache | 9/10 (1 minimal, 5 mild, 2 moderate, 1 severe) |
8/10 (2 mild, 5 moderate, 1 severe) |
| Dizziness | 9/10 (1 minimal, 3 mild, 4 moderate, 1 severe) |
9/10 (1 minimal, 3 mild, 5 moderate) |
| Nausea | 6/10 (1 mild, 5 moderate) | 7/10 (2 mild, 4 moderate, 1 severe) |
| Vomiting | 1/10 (1 moderate) | 1/10 (1 moderate) |
| Diplopia | 3/10 (1 minimal, 1 mild, 1 moderate) |
4/10 (1 minimal, 1 mild, 2 moderate) |
| Sedation | 9/10 (1 mild, 6 moderate, 2 severe) |
9/10 (4 mild, 3 moderate, 2 severe) |
| Nystagmus | 9/10 (5 mild, 4 moderate) | 6/10 (3 mild, 3 moderate) |
| Hypersalivation | 0/10 | 2/10 (1 mild, 1 moderate) |
| Erythema/Rash | 5/10 (3 minimal, 1 mild, 1 moderate) |
4/10 (4 minimal) |
Note: Symptoms were measured 4 times daily using the Brief Psychiatric Rating Scale (Flemenbaum and Zimmerman, 1973) and the Clinical and Adverse Events Checklist (Newcomer et al, 1999). For symptoms which occurred on more than one assessment, the highest severity rating is reported.
Moderate symptoms in 4 participants were: reported closing eyes and seeing images such as Jesus and a bear in curtains, knew it was not real; seeing images of cathedral ceiling; seeing visual patterns (like modernistic painting) when eyes closed; and seeing lights when eyes closed. All were transient.
Moderate symptoms in 3 participants were: suspicious they were moved to another room (40-minute arm); delusion that nurses was inappropriately observing and hurting them (96-hour arm), and felt that “a force” was pulling on their bed (96-hour arm). All were transient, although the delusion about nurses led the patient to ask for the infusion to be stopped temporarily.
Figure 2. Brief-Psychiatric Rating Scale-positive subscale (BPRS+) changes during the 96-hour infusion.
The two groups differed significantly (at p<0.05) in BPRS+ scores at day 3 (96hr arm > 40 min arm, p=0.007) and day 5 30-min post-infusion (40 min arm > 96hr arm, p=0.02). Means ± standard error are shown.
We performed an exploratory analysis examining the relationship of ketamine exposure with sustained antidepressant effects and psychotomimetic side effects (Table 3). It shows that higher ketamine concentrations were associated with better sustained antidepressant response, but not higher side effects, in the 96-hour arm. Ketamine and norketamine concentrations were not correlated with sustained response nor with higher levels of psychotomimetic side effects in the 40-minute arm.
Table 3.
Correlations of ketamine concentrations with post-infusion antidepressant response and with psychotomimetic symptoms during the infusion
| Outcome | |||
|---|---|---|---|
|
| |||
| Sustained (week 8) response |
Psychotomimetic effects (Highest BPRS+ score during infusion) |
||
|
| |||
| CGI-I |
MADRS
change from baseline |
||
|
| |||
| S-ketamine concentration | |||
|
| |||
| 96-hour arm | 0.70* | 0.75* | −0.22 |
| 40-minute arm | 0.11 | 0.17 | −0.49 |
|
| |||
| R-ketamine concentration | |||
|
| |||
| 96-hour arm | 0.70* | 0.75* | −0.22 |
| 40-minute arm | 0.11 | 0.17 | −0.49 |
|
| |||
| S-norketamine concentration | |||
|
| |||
| 96-hour arm | 0.62 | 0.65* | −0.06 |
| 40-minute arm | 0.44 | 0.47 | −0.78* |
|
| |||
| R-norketamine concentration | |||
|
| |||
| 96-hour arm | 0.25 | 0.31 | −0.28 |
| 40-minute arm | 0.17 | 0.19 | −0.77* |
Note: spearman correlations were run. spearman correlation coefficient (ρ) is reported. For CGI-I and MADRS change, a significant positive correlation indicates that higher concentration was associated with higher better response; for BPRS+ change, a significant negative correlation indicates that lower concentration is associated with greater psychotomimetic effects. Ketamine concentrations in the 96-hour arm were steady-state concentrations (average of days 3-5), while concentrations in the 40-minute arm were at the end of the infusion.
indicates p<0.05
Ketamine with clonidine co-administration had a statistically but not clinically significant effect on blood pressure, characterized by lowered systolic and diastolic blood pressure (figure in appendix). No participant suffered from symptomatic orthostatic hypotension or had other adverse effects attributable to clonidine. Additionally, we saw no rebound hypertension after clonidine was stopped (figure in appendix).
Liver enzyme changes from pre- to post-infusion were: AST (SGOT) −2.3 (SD 3.7) in the 96-hour group vs. −0.2 (SD 4.8) in the 40-minute group, with no participants in either group moving from a normal to elevated range; ALT (SGPT) was −4.0 (SD 4.6) in the 96-hour group vs. 2.0 (SD 9.9) in the 40-minute group, with one participant (in the 40-minute group) moving from normal to elevated (26 to 53).
Discussion
This feasibility study examined a 96-hour, high-dose ketamine infusion in treatment-resistant depression, with co-administered clonidine to mitigate ketamine's psychotomimetic side effects. We demonstrated feasibility in terms of adequate recruitment and retention of participants and adherence to the protocol (Leon et al., 2011), as well as good safety and tolerability for both ketamine and clonidine. We did not demonstrate a statistically significant difference in antidepressant treatment response between 96-hour and 40-minute ketamine; however, this pilot study was not powered to detect such differences.
With respect to tolerability and safety, we focused on psychotomimetic side effects, which we posited would be reduced by co-administered clonidine, and blood pressure changes, which could be affected either by ketamine's sympathomimetic effects or by clonidine. Psychotomimetic effects were infrequent, mild, transient, and uncorrelated with ketamine concentration. From this we infer that clonidine co-administration may have mitigated ketamine's psychotomimetic effects, because such side effects are expected to reliably occur at the relatively high ketamine concentrations reached in the 96-hour group (Bowdle et al., 1998; Newcomer et al., 1999). However, we did not formally test this hypothesis by randomization to a 96-hour ketamine infusion without clonidine. Additionally, modest but clinically insignificant blood pressure reduction was seen during the infusion, which is attributable to the use of clonidine. Upon stopping clonidine and ketamine, no rebound hypertension occurred which is in contrast to findings with clonidine in hypertension (Neusy and Lowenstein, 1989). Other side effects were typically mild, and all were transient. No liver toxicity was seen.
With respect to antidepressant effects, we found rapid and sustained responses in this study, with no significant differences between the 96-hour and 40-minute ketamine groups in this small sample. Specifically, there was no difference in terms of either rapid reduction of depression (which was present in 70% of both study arms, similar to prior findings of brief ketamine infusions in depression), nor in sustained reduction of depression (seen in 40% of 96-hour participants and 20% of 40-minute participants). It remains unknown whether there is a superior therapeutic efficacy of prolonged ketamine infusions. We used racemic ketamine, and we examined S- vs. R- ketamine concentrations and their active metabolites (S- and R-norketamine), similar to a prior study in depression (Zarate et al., 2012). We found a significant relationship between ketamine concentration and sustained response in the 96-hour ketamine group, suggesting that a prolonged ketamine infusion must also reach high concentrations to provide sustained antidepressant benefits. A similar finding has been reported in the chronic pain literature (Goldberg et al., 2011). The lack of a correlation of R-norketamine and response in the 96-hour group would be consistent with this agent's lower affinity for the NMDA receptor and is relatively lower concentration in the subjects. It is unknown whether racemic or S-ketamine is superior for depression (Paul et al., 2009). However, if blockade of NMDA receptors is the mechanism of action for both the antidepressant response as well as the psychotomimetic side effects, then both would be expected to have similar effects once the amount of NMDA receptor blockade is equalized. Future research would have to demonstrate that prolonged and/or higher-dose infusions provide a clear benefit over brief infusions, to justify their use in a clinical setting.
The ability of ketamine to produce an antidepressant response in subjects co-administered clonidine suggests that cholinergic hyperactivity (which is suppressed by alpha-2 antagonists) is not necessary for an antidepressant response. This is arguably similar to previous research showing that antidepressant effects of brief ketamine infusions are not correlated with psychotomimetic effects (Luckenbaugh et al., 2014). Since blockade of NMDA receptors also produces hyperactivity in glutamatergic pathways that putatively project onto non-NMDA receptors in corticolimbic brain regions (Duman and Aghajanian, 2012; Farber, 2003) and alpha-2 agonists are not expected to normalize this excessive activity (Farber et al., 2002; Jevtovic-Todorovic et al., 1998), our findings would be consistent with the proposal that activation of non-NMDA receptors may be the necessary mechanism for NMDA antagonists' antidepressant effects (Akinfiresoye and Tizabi, 2013; Dwyer and Duman, 2013) (See Figure in Appendix). We also note that clonidine's cholinergic-dampening effect in the ketamine-disinhibited circuit (depicted in the figure in the appendix) bears some resemblance to the anticholinergic scopolamine. Scopolamine has been demonstrated to show rapid antidepressant effects (Drevets and Furey, 2010; Furey and Drevets, 2006); conceivably the combination of ketamine and clonidine is having additive antidepressant effects. We speculate as well that a combination of ketamine and scopolamine might have both additive antidepressant effects and reduced side effects compared with ketamine alone.
Strengths of this study were the use of a randomized controlled design to compare the 96-hour infusion to the more standard 40-minute ketamine infusion, and masking of ketamine so that participants and raters were blinded to randomization assignment. However, it should be mentioned that the better-than-average treatment guessing by the rater (guessing corrected 17/20 treatment assignments), together with the tendency for ketamine to cause some cognitive and behavioral changes even when psychotomimetic side effects are minimized, suggests incomplete blinding. Future studies could minimize this potential unblinding risk by masking participants as to the timing and dosing of ketamine administration, or using a placebo with cognitive and behavioral side effects (such as a benzodiazepine) similar to Murrough et al (2013).
In terms of limitations, this was a pilot study carried out mainly to test the feasibility of the methods, and it has limited statistical power due to small sample size. Future research needs to determine the extent to which sustained antidepressant response is achievable with prolonged, high-dose ketamine, and to characterize the optimal parameters of ketamine infusion, including dose and duration, dosing of clonidine, and participant selection. Such research would likely require a better understanding of the mechanism of effects of prolonged ketamine infusions on depression-related neurocircuitry (Li et al., 2010), and biosignatures of response (Naughton et al., 2014). Other limitations were the lack of a scale specifically measuring dissociative symptoms, which prevents us from comparing these side effects to other studies which did use such scales; and, the allowance of co-occurring antidepressant use, which could result in increased heterogeneity of response unrelated to the study medication. As well, final clonidine dose was higher in the 96-hour ketamine group than the 40-minute group; this was likely because the prolonged ketamine infusion provided some sympathetic effects allowing for higher clonidine dosing without inducing hypotension. Also, it was an ethnically homogeneous sample.
Despite these limitations, we posit that prolonged high-dose ketamine infusions could be developed as another treatment option for some treatment-resistant cases. Moreover, this line of research makes the cases that alpha-2 agonist co-administration could improve the tolerability of NMDA-receptor antagonists, allowing for a greater range of dosing.
Supplementary Material
Acknowledgements
this study was supported primarily by the Sidney R. Baer Foundation, Taylor Family Institute for Innovative Psychiatric Research, and the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS). Additional funding came from the Washington University Department of Psychiatry, R01 MH083648, P30 DK56341, R01 DA14211, and R01 DA025931. The study sponsors had no role in the study design or conduct, data analysis, interpretation of results, or preparation of the manuscript. The investigators thank Karen Flavin, R.N., Celeste Herleth, M.D., Christina Friedel, and the nursing staff of the Washington University Clinical Research Unit, for their help in the study.
Dr. Lenze receives research support from the McKnight Brain Research Foundation, Taylor Family Institute for Innovative Psychiatric Research, Takeda, Lundbeck, and Barnes-Jewish Foundation (current), and, Sidney R Baer Foundation and Roche (past). Dr. Farber receives research support from the Taylor Family Institute for Innovative Psychiatric Research, and Barnes-Jewish Foundation (current). Drs. Farber and Olney hold patents pertaining to improved use of NMDA antagonists as therapeutic agents. Dr. Newcomer has received research support from NIH, Foundation2Recovery, and Otsuka American Pharmaceutical, Inc., has consulted for Reviva Pharmaceuticals, Inc. and serves on a Data Safety Monitoring Board for Amgen.
Footnotes
Statement of interest: The other authors report no conflicts.
References
- Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology. 2013;230:291–298. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88:82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biological Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73:1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emnett CM, Eisenman LN, Taylor AM, Izumi Y, Zorumski CF, Mennerick S. Indistinguishable synaptic pharmacodynamics of the N-methyl-D-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol. 2013;84:935–947. doi: 10.1124/mol.113.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- Farber NB, Foster J, Duhan NL, Olney JW. alpha 2 adrenergic agonists prevent MK-801 neurotoxicity. Neuropsychopharmacology. 1995;12:347–349. doi: 10.1016/0893-133X(95)00048-I. [DOI] [PubMed] [Google Scholar]
- Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7:32–43. doi: 10.1038/sj.mp.4000912. [DOI] [PubMed] [Google Scholar]
- Flemenbaum A, Zimmermann RL. Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep. 1973;32:783–792. doi: 10.2466/pr0.1973.33.3.783. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Enantioselective pharmacokinetics of (R)- and (S)-ketamine after a 5-day infusion in patients with complex regional pain syndrome. Chirality. 2011;23:138–143. doi: 10.1002/chir.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Clinical global impressions (CGI) scale. In: Rush AJ, editor. Handbook of Psychiatric Measures. Amrican Psychiatric Association; Washington, DC: 2000. pp. 100–102. [Google Scholar]
- Handa F, Tanaka M, Nishikawa T, Toyooka H. Effects of oral clonidine premedication on side effects of intravenous ketamine anesthesia: a randomized, double-blind, placebo-controlled study. J Clin Anesth. 2000;12:19–24. doi: 10.1016/s0952-8180(99)00131-2. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Valtysson J, Lindner KJ, Kristensen J, Karlsten R, Gustafsson LL, Persson J, Svensson JO, Oye I, Antoni G, et al. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58:165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Wozniak DF, Powell S, Nardi A, Olney JW. Clonidine potentiates the neuropathic pain-relieving action of MK-801 while preventing its neurotoxic and hyperactivity side effects. Brain Res. 1998;781:202–211. doi: 10.1016/s0006-8993(97)01247-x. [DOI] [PubMed] [Google Scholar]
- Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK. Pilot dose-response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry. 2014;15:579–584. doi: 10.3109/15622975.2014.922697. [DOI] [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J Biol Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–1904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65:977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M, Clarke G, O'Leary OF, Cryan JF, Dinan TG. A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord. 2014;156:24–35. doi: 10.1016/j.jad.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Neusy AJ, Lowenstein J. Blood pressure and blood pressure variability following withdrawal of propranolol and clonidine. J Clin Pharmacol. 1989;29:18–24. doi: 10.1002/j.1552-4604.1989.tb03232.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Selke G, Melson AK, Jevtovic-Todorovic V, Olney JW. Guanabenz effects on NMDA antagonist-induced mental symptoms in humans. Society for Neuroscience Abstracts. 1998:525. [Google Scholar]
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta R, Goyagi T, Nishikawa T. Combination of oral clonidine and intravenous low-dose ketamine reduces the consumption of postoperative patient-controlled analgesia morphine after spine surgery. Acta Anaesthesiol Taiwan. 2013;51:14–17. doi: 10.1016/j.aat.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Paul R, Schaaff N, Padberg F, Moller HJ, Frodl T. Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: report of two cases. World J Biol Psychiatry. 2009;10:241–244. doi: 10.1080/15622970701714370. [DOI] [PubMed] [Google Scholar]
- Petersen T, Papakostas GI, Mahal Y, Guyker WM, Beaumont EC, Alpert JE, Fava M, Nierenberg AA. Psychosocial functioning in patients with treatment resistant depression. European psychiatry. 2004;19:196–201. doi: 10.1016/j.eurpsy.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Petersen T, Papakostas GI, Posternak MA, Kant A, Guyker WM, Iosifescu DV, Yeung AS, Nierenberg AA, Fava M. Empirical testing of two models for staging antidepressant treatment resistance. Journal of Clinical Psychopharmacology. 2005;25:336–341. doi: 10.1097/01.jcp.0000169036.40755.16. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Trevino K, McClintock SM, McDonald Fischer N, Vora A, Husain MM. Defining treatment-resistant depression: A comprehensive review of the literature. Ann Clin Psychiatry. 2014;26:222–232. [PubMed] [Google Scholar]
- Webster LR, Walker MJ. Safety and efficacy of prolonged outpatient ketamine infusions for neuropathic pain. Am J Ther. 2006;13:300–305. doi: 10.1097/00045391-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) The British journal of psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.