Gait speed is an important physical performance test and is used in gerontologic research as an objective and standardized measure of physical functioning. Physical performance measures were originally developed to address concerns about self-reported functioning, including issues of accommodation and difference in environmental challenges affecting disability. For instance, some may not report having difficulty bathing or walking up stairs because they have a walk-in shower rather than a bathtub or do not encounter stairs in their daily environment. In the disability framework, physical performance tests are indicators of functional limitations and represent essential actions necessary to function independently (Fig 1). Functional limitations are defined as restrictions in basic actions (ie, ambulation or climbing stairs) and are links between impairment, such as poor strength, and disability, such as difficulty transferring from bed to chair. Physical performance tests assessing mobility require a complex integration and coordination of the activities of multiple organ systems (neurologic, musculoskeletal, and cardiopulmonary). As such, they help quantify the impact of comorbid conditions and aging on function, providing a window into an individual’s comorbid burden and physiologic reserve to stress. Slower gait speed and gait variability have been associated with subclinical cerebrovascular disease even in older adults who are apparently high functioning.1,2 Slower gait speed has also been linked to lower aerobic capacity and higher fatigability.3,4 The value of gait speed measurement is that it is simple to perform, is reproducible,5 and has been shown to be strongly associated with falls, hospitalization, disability, and death.6–8
Figure 1.
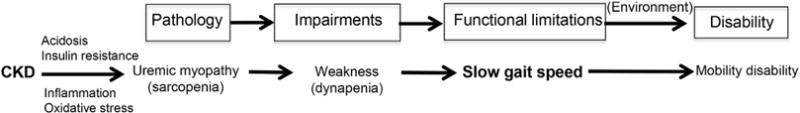
Nagi disability framework. Underneath this framework is the representation of disablement process in kidney disease. Abbreviation: CKD, chronic kidney disease.
Chronic kidney disease (CKD) is a model for accelerated aging leading to sarcopenia (low muscle mass), dynapenia (muscle weakness), and mobility limitation (Fig 1).9–12 Consequently, CKD represents an ideal clinical setting to use physical performance testing. Studies of community-dwelling older adults have indicated that lower kidney function was associated with lower ambulatory muscle density, slower gait speed,11 and faster declines in ambulatory muscle strength over time.13 Patients with stages 2 to 4 CKD had substantially diminished lower-extremity physical performance compared to the general population.14 Regardless of the test, patients with CKD who had worse lower-extremity physical performance were at substantially increased risk of death and dialysis therapy initiation even after accounting for demographics and comorbid conditions.15 This association of slower gait speed with increased risk of mortality persisted even among those free of a history of stroke and mobility disability at baseline.14 Importantly, the addition of a single assessment of gait speed to standard assessments of age, sex, and kidney function significantly improved discrimination of mortality risk between patients with differing life expectancy.14 The evidence in persons with earlier stages of kidney disease strongly supports gait speed as an important measure capturing comorbid burden and assessing risk of mortality. However, less is known about the significance of gait speed in patients with kidney failure treated with long-term dialysis.
In this issue of AJKD, Kutner et al16 assess gait speed in more than 750 long-term dialysis patients from the multicenter ACTIVE-ADIPOSE (A Cohort Study to Investigate the Value of Exercise in ESRD/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD). First, the authors report a remarkably high prevalence of impaired mobility using accepted cutoff points for dismobility and impaired community ambulation from the general population.17 Second, the authors demonstrate associations of slower gait speed with a higher burden of comorbid conditions, higher impairment in activities of daily living, and lower self-reported physical function. Third, the authors show that slower baseline gait speed is associated with subsequent risk of all-cause mortality after adjustment for demographics, comorbid conditions, duration of maintenance dialysis treatment, hemoglobin level, and history of recent falls. Finally, slower gait speed (defined by the cutoff for limited community ambulation of <0.8 m/s)18 was associated with several other adverse outcomes, including a decline in self-reported physical functioning, higher odds of new hospitalization, and onset of new or progressive activities of daily living disability compared with normal gait speed (≥1 m/s) over a 12-month period.
The goal of the current investigation was to demonstrate the utility of gait speed measurement in assessing the risk of adverse health outcomes in a population with kidney disease treated with long-term dialysis with a high burden of comorbid conditions, disability, and rate of hospitalization. One important limitation of the current study is that the outcome of short-term mortality from any cause does not suggest specific interventions for dialysis patients with slow gait speed. Physical performance measures are indicators of important patient-centered outcomes of functional limitations and future disability. Future studies should focus on the associations of gait speed with more specific outcomes of preclinical and clinical mobility disability19 to help guide potential interventions. Another important limitation is that in a population with a high degree of debility, slow gait speed may simply be capturing the sickest patients who are at imminent risk of hospitalization, progressive disability, and death. Among patients treated with long-term dialysis, gait speed may be a consequence of disability rather than a marker of risk of functional decline. The immediate separation of survival curves in this study suggests that ongoing illness and disability may have influenced gait speed (reverse causality), which would inflate observed associations. It would be helpful to know the associations of slower gait speed with outcomes among patients with kidney disease in the early stages of decline before the onset of loss of function and disability when preventative measures may be most effective.
Intervening early in the course of functional decline, before the onset of mobility disability, provides an important opportunity for prevention in the kidney disease population treated with long-term dialysis. Several factors seem to justify the application of gait speed as a screening tool for early detection of functional limitations in the dialysis population. First, falls and disability are highly prevalent and a significant public health problem in this population. Second, there may be a suitable window of opportunity to screen patients before the onset of falls and disability. The current study is an important first step in demonstrating that slow gait speed is associated with new or progressive activities of daily living disability and physical function decline. However, further validation and research in this population are needed to confirm that slow gait speed detects preclinical mobility limitation prior to the onset of disability. Importantly, the decision to screen also depends on the availability and accessibility to treatments that favorably alter the disability process in this population. No trials to date have investigated whether improvements in gait speed through exercise are associated with reduction in risk of falls or disability among dialysis patients.
Gait speed measurement has the potential to serve as an important adjunct to current standard assessment of self-reported function. It is easy to perform reliably and, unlike self-report, is unencumbered by issues of accommodation and interindividual differences in environmental challenges influencing disability. Furthermore, exercise trials have demonstrated that gait speed and walking capacity are modifiable across the spectrum of kidney disease.20,21 The strong link between gait speed, daily function, and survival across a wide range of populations has supported its distinction as a credible clinical outcomes assessment measure in clinical trials.22 Current Centers for Medicare & Medicaid Services clinical performance measures for dialysis facilities addressing physical function include an annual KDQOL-36 (Kidney Disease Quality of Life-36) self-reported physical function assessment.23 Despite the introduction of this measure in 2008, it is unknown if there have been tangible improvements in patient-centered outcomes. Pragmatic trials are urgently needed to demonstrate whether the addition of gait speed to standard clinical practice can detect functional limitation leading to interventions that may prevent disability. For example, a cluster randomized trial could be designed comparing dialysis facilities using self-reported physical function with those using combined gait speed and self-reported physical function assessments, directing high-risk patients to receive physical therapy or rehabilitation focusing on preventing falls, persistent disability, and death. Algorithms currently exist for the screening and management of mobility limitations in patients with kidney disease18 and in the geriatric population.24,25 Implementation of an interdisciplinary approach to evaluating and treating mobility limitation and function decline has the potential to substantially improve patient-centered outcomes in this population burdened by disability. The nephrology community and patients with kidney disease potentially can benefit from the insight gained by observing and measuring a patient’s walk from the weight scale to the examination room or dialysis machine.
Acknowledgments
I thank Drs Bryan Kestenbaum, Ann O’Hare, and Kushang Patel for contributions to the original draft of this editorial.
Support: Dr Roshanravan is supported by 1K23DK099442-01 from the National Institute of Diabetes and Digestive Kidney Diseases, National Institutes of Health.
Footnotes
Financial Disclosure: The author declares that he has no relevant financial interests.
References
- 1.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26(1):52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 2.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70(4):487–494. doi: 10.1093/gerona/glu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodilsen AC, Juul-Larsen HG, Petersen J, Beyer N, Andersen O, Bandholm T. Feasibility and inter-rater reliability of physical performance measures in acutely admitted older medical patients. PLoS One. 2015;10(2):e0118248. doi: 10.1371/journal.pone.0118248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LF, Boudreau R, Lee JS, et al. Kidney function as a predictor of loss of lean mass in older adults: Health, Aging and Body Composition Study. J Am Geriatr Soc. 2007;55(10):1578–1584. doi: 10.1111/j.1532-5415.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 10.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: Health, Aging and Body Composition Study. J Am Geriatr Soc. 2006;54(5):750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 11.Odden M, Chertow G, Fried L. Cystatin C and measures of physical function in elderly adults. The Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–1189. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki M, Miyashita K, Wakino S, Mitsuishi M, Hayashi K, Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014;85(6):1330–1339. doi: 10.1038/ki.2013.473. [DOI] [PubMed] [Google Scholar]
- 13.Roshanravan B, Patel KV, Robinson-Cohen C, et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis. 2015;65(5):737–747. doi: 10.1053/j.ajkd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutner NG, Zhang R, Huang Y, Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US Renal Data System special study. Am J Kidney Dis. 2015;66(2):297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311(20):2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Painter P, Marcus RL. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):861–872. doi: 10.2215/CJN.06590712. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 20.Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35(3):482–492. doi: 10.1016/s0272-6386(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 21.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 22.FDA. Clinical Outcome Assessment Qualification Program. Definition of clinical outcomes assessment (COA) and COA qualification. 2014 http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284077.htm. Accessed March 12, 2015, 2015.
- 23.Centers for Medicare & Medicaid Services. Phase III ESRD clinical performance measures in effect April, 1, 2008. http://www.cms.gov/Medicare/End-Stage-Renal-Disease/CPMProject/Downloads/ESRDPhaseIIICPM04012008Final.pdf. Accessed March 13, 2015.
- 24.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310(11):1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 25.Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]


