Abstract
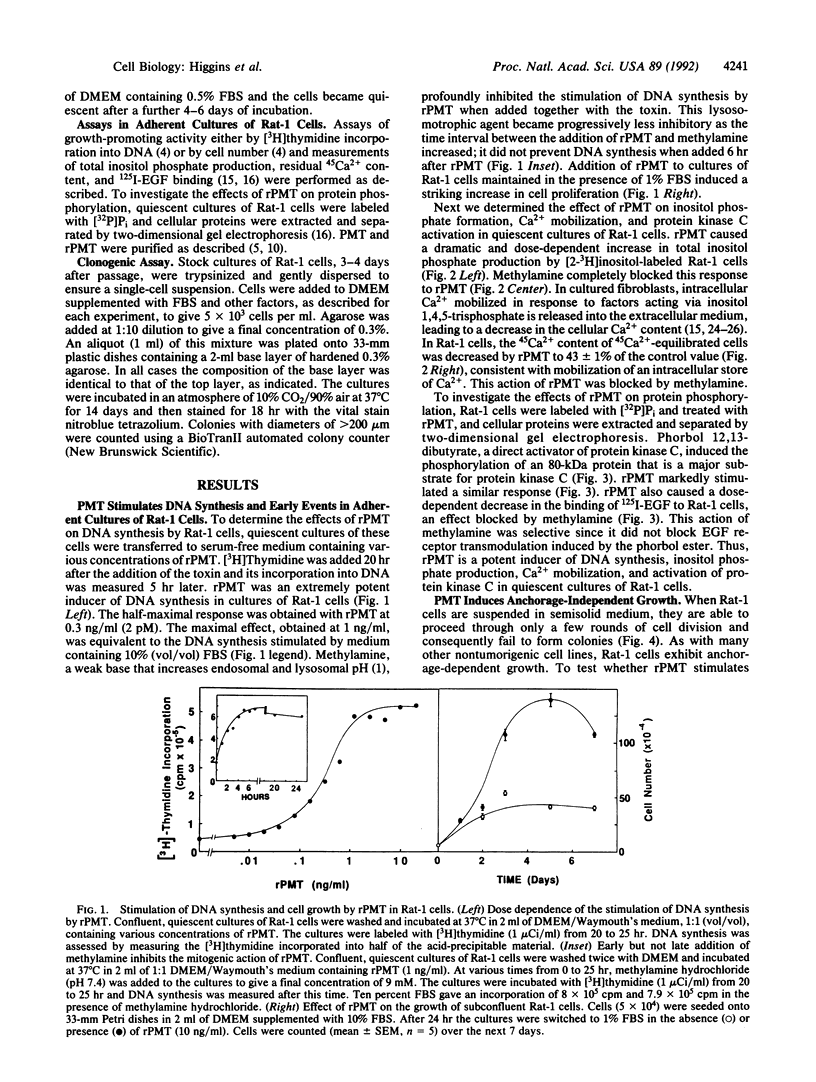
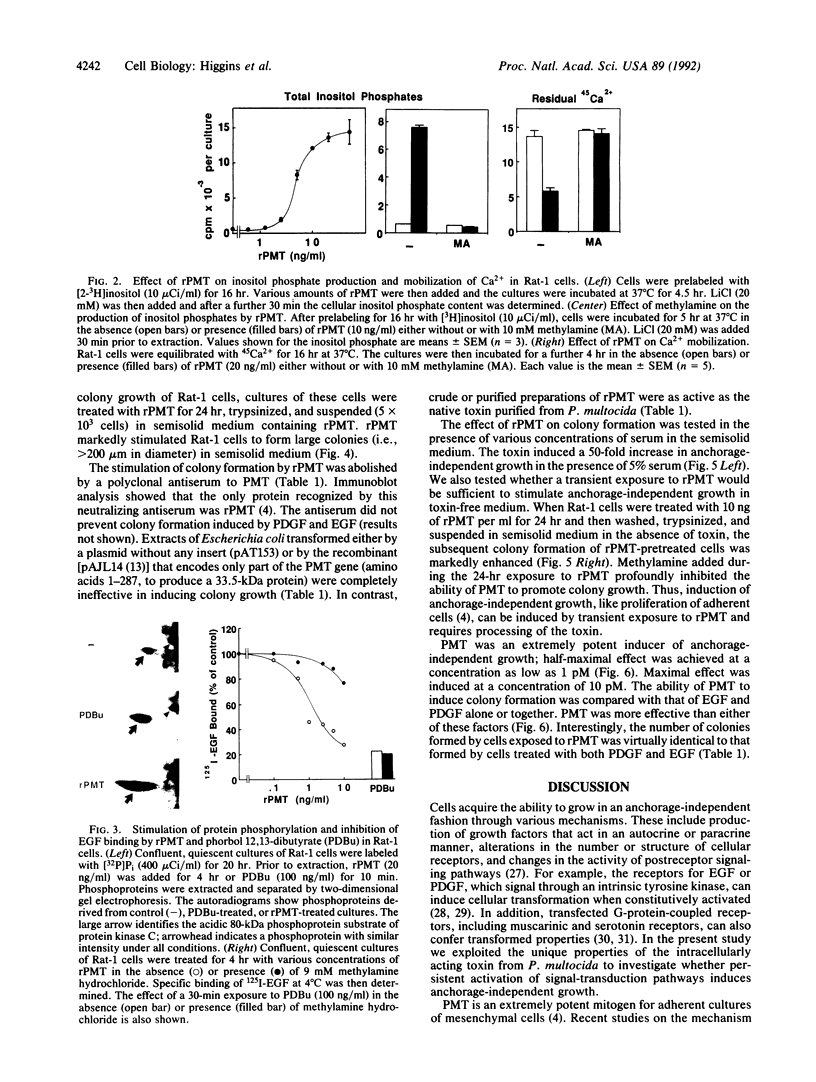
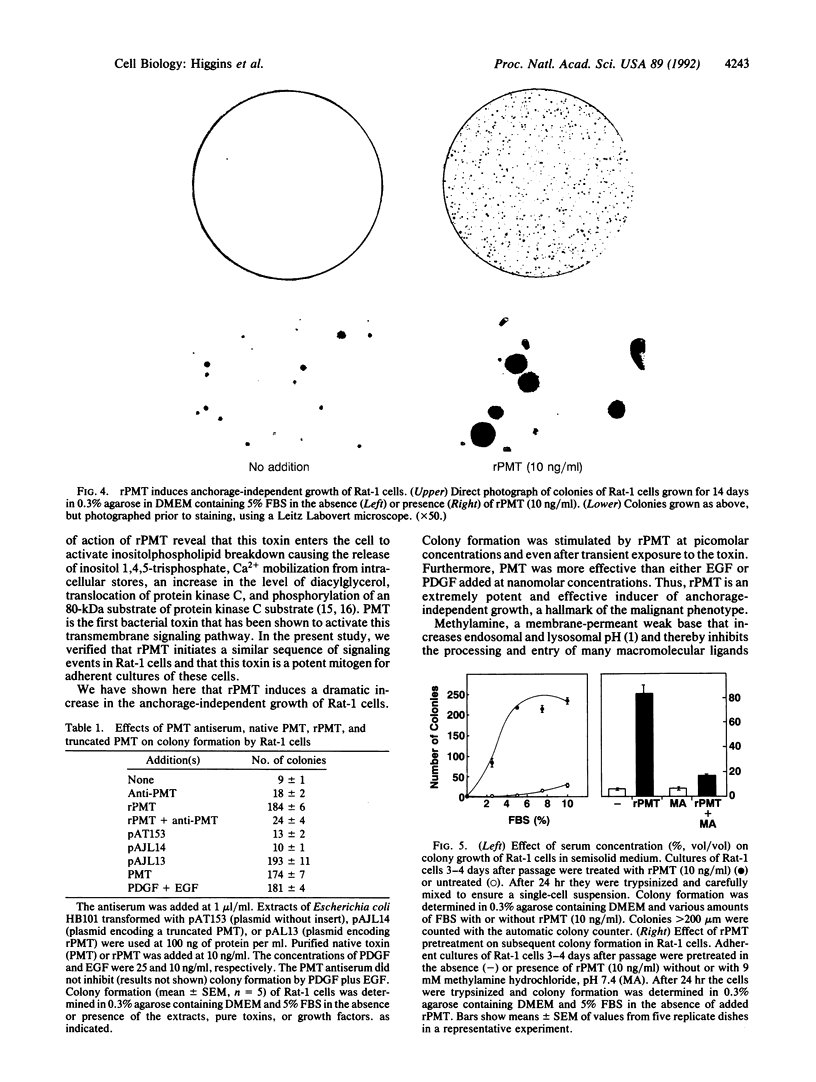
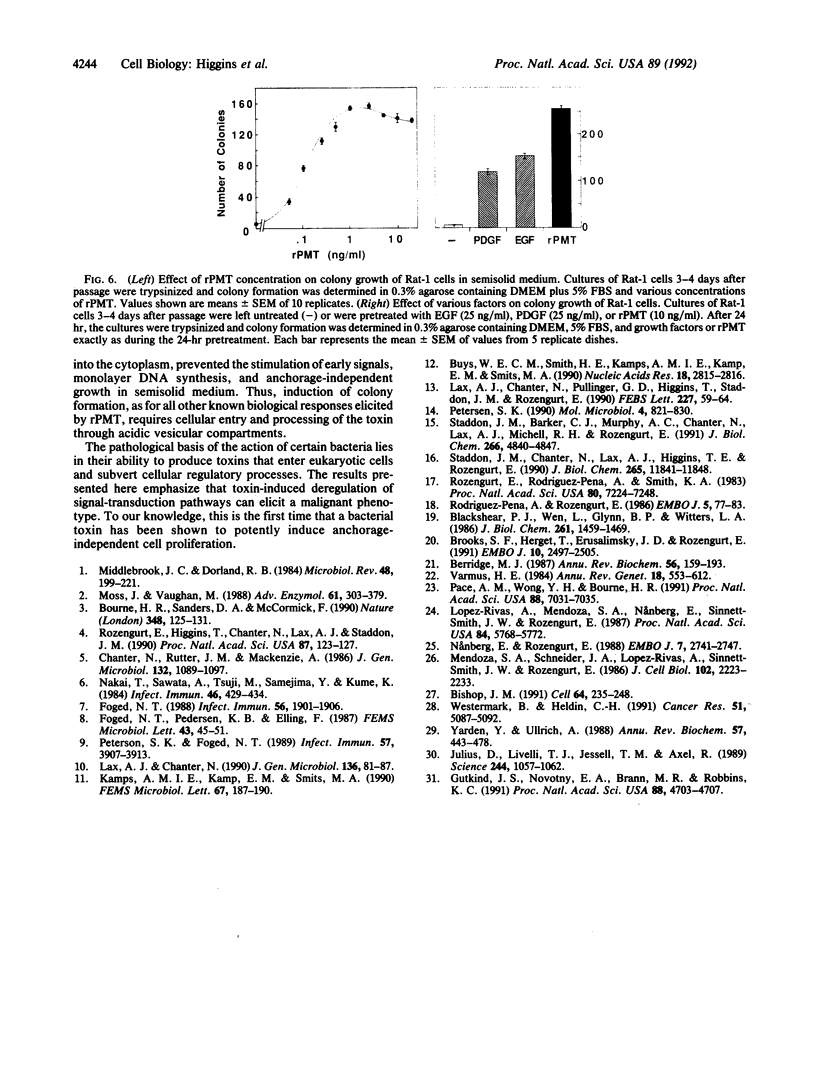
The growth of many normal cells requires contact with an adhesive substratum, a requirement that is frequently abrogated in the transformed phenotype. We have explored pathways that can lead to the anchorage-independent growth of cultured Rat-1 fibroblasts. Pasteurella multocida toxin (PMT), a 146-kDa mitogenic protein, caused a striking increase in the formation of colonies (greater than 200 microns) from single cells in soft agar. The magnitude of the effect of PMT was greater than that achieved by epidermal growth factor or platelet-derived growth factor. The toxin was extremely potent, with half-maximal and maximal effects observed at 1 and 10 pM PMT, respectively. This concentration dependence of the action of the toxin is similar to that for the stimulation of DNA synthesis in adherent cultures of the cells. Stimulation of colony formation could be achieved by a transient exposure of the cells to PMT and it was blocked by methylamine, indicating that the toxin enters the cells to act. Colony formation was stimulated equally by native and recombinant PMT, but a truncated version (33.5 kDa) of the recombinant toxin was ineffective. PMT antiserum blocked colony formation in response to PMT. In the Rat-1 cells, PMT stimulated the phospholipase C-mediated hydrolysis of inositolphospholipids, as indicated by the stimulation of inositol phosphate release, Ca2+ mobilization, and phosphorylation of a protein kinase C substrate. The results indicate that the deregulation of signal-transduction pathways as elicited by an intracellularly acting bacterial toxin can induce a malignant phenotype.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Brooks S. F., Herget T., Erusalimsky J. D., Rozengurt E. Protein kinase C activation potently down-regulates the expression of its major substrate, 80K, in Swiss 3T3 cells. EMBO J. 1991 Sep;10(9):2497–2505. doi: 10.1002/j.1460-2075.1991.tb07789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys W. E., Smith H. E., Kamps A. M., Kamp E. M., Smits M. A. Sequence of the dermonecrotic toxin of Pasteurella multocida ssp. multocida. Nucleic Acids Res. 1990 May 11;18(9):2815–2816. doi: 10.1093/nar/18.9.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanter N., Rutter J. M., Mackenzie A. Partial purification of an osteolytic toxin from Pasteurella multocida. J Gen Microbiol. 1986 Apr;132(4):1089–1097. doi: 10.1099/00221287-132-4-1089. [DOI] [PubMed] [Google Scholar]
- Foged N. T. Quantitation and purification of the Pasteurella multocida toxin by using monoclonal antibodies. Infect Immun. 1988 Aug;56(8):1901–1906. doi: 10.1128/iai.56.8.1901-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Novotny E. A., Brann M. R., Robbins K. C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4703–4707. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Kamps A. M., Kamp E. M., Smits M. A. Cloning and expression of the dermonecrotic toxin gene of Pasteurella multocida ssp. multocida in Escherichia coli. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):187–190. doi: 10.1016/0378-1097(90)90192-s. [DOI] [PubMed] [Google Scholar]
- Lax A. J., Chanter N. Cloning of the toxin gene from Pasteurella multocida and its role in atrophic rhinitis. J Gen Microbiol. 1990 Jan;136(1):81–87. doi: 10.1099/00221287-136-1-81. [DOI] [PubMed] [Google Scholar]
- Lax A. J., Chanter N., Pullinger G. D., Higgins T., Staddon J. M., Rozengurt E. Sequence analysis of the potent mitogenic toxin of Pasteurella multocida. FEBS Lett. 1990 Dec 17;277(1-2):59–64. doi: 10.1016/0014-5793(90)80809-w. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Samejima Y., Kume K. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect Immun. 1984 Nov;46(2):429–434. doi: 10.1128/iai.46.2.429-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nånberg E., Rozengurt E. Temporal relationship between inositol polyphosphate formation and increases in cytosolic Ca2+ in quiescent 3T3 cells stimulated by platelet-derived growth factor, bombesin and vasopressin. EMBO J. 1988 Sep;7(9):2741–2747. doi: 10.1002/j.1460-2075.1988.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace A. M., Wong Y. H., Bourne H. R. A mutant alpha subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. K., Foged N. T. Cloning and expression of the Pasteurella multocida toxin gene, toxA, in Escherichia coli. Infect Immun. 1989 Dec;57(12):3907–3913. doi: 10.1128/iai.57.12.3907-3913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. K. The complete nucleotide sequence of the Pasteurella multocida toxin gene and evidence for a transcriptional repressor, TxaR. Mol Microbiol. 1990 May;4(5):821–830. doi: 10.1111/j.1365-2958.1990.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Higgins T., Chanter N., Lax A. J., Staddon J. M. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J. M., Barker C. J., Murphy A. C., Chanter N., Lax A. J., Michell R. H., Rozengurt E. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-trisphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J Biol Chem. 1991 Mar 15;266(8):4840–4847. [PubMed] [Google Scholar]
- Staddon J. M., Chanter N., Lax A. J., Higgins T. E., Rozengurt E. Pasteurella multocida toxin, a potent mitogen, stimulates protein kinase C-dependent and -independent protein phosphorylation in Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11841–11848. [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]