Summary
A phase I study was performed to determine the safety and pharmacokinetics of XK469R in patients with refractory acute leukemia. The study aimed to determine the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of XK469R given intravenously over 30 to 60 min on days 1, 3, and 5 of a 21 day cycle. Patients were treated in successive cohorts of six until DLT was observed. Once the MTD was determined, an additional cohort of six patients was enrolled at the previous dose level and that dose was considered the recommended phase 2 dose (RPTD). Forty-six patients were treated at dose levels of 1,400, 1,750, 2,200, and 2,750 mg. The DLTs were: mucositis, colitis and hyperbilirubinemia. Reversible myelosuppression was noted at all dose levels. One (2%) of 42 patients achieved a complete remission and five patients (11%) had hematologic improvement. The half-life of the drug was long with a mean value of 48 h. The mean clearance was 206 mL/h with a coefficient of variation of 32%. No correlation was observed between the development of DLT and pharmacokinetics. The RTPD is 1,750 mg. XK469R induced hematological responses in patients with refractory leukemia at tolerable doses.
Keywords: XK469R, Phase I study, Refractory leukemia, Myelosuppression
Introduction
There is a need to identify new agents for treatment of acute leukemias [1]. XK469 (NSC 697887) and XK469R (NSC 698215), the R enantiomer of XK469, are quinoxaline phenoxypropionic acid derivatives that possess broad activity against murine and human tumors and have significant activity against multidrug-resistant tumors [2]. COMPARE analysis of cytotoxicity data from the NCI cell line screen showed that the mechanism of action is unusual and not comparable to that of any known cytotoxic agent. However, inhibition of topoisomerase II, as well as induction of DNA-protein cross links, have been reported [3].
Using murine leukemia L1210 cells in culture, Kessel et al. reported that the chiral R(+) form of XK469, XK469R, is considerably more cytotoxic than the S(−) form [4]. The cytotoxic response to these agents was accompanied by apoptosis, and was found to be correlated with drug binding to the peripheral benzodiazepine receptor in cell culture, suggesting that receptor binding may be a factor in drug-induced cytotoxicity. Lin et al. have examined the signaling pathways involved in mediating the antiproliferative activity of XK469 using human U-937 leukemia cells in culture [5]. Cell cycle analysis revealed that XK469 arrested U-937 cells at the G2/M phase [6, 7]. XK469 showed little cytotoxic or proapoptotic effect against U-937 cells; whereas, treatment of U-937 cells with vinblastine, doxorubicin and m-AMSA resulted in extensive cell death through apoptotic pathways. XK469, but not other agents, potently inhibited the phosphorylation/activation of MEK in U-937 cells cultured in serum-containing medium [5]. XK469 was also able to block the activation of MEK by serum addition in starved U-937 cells. Exposure of cells to XK469 for 1 h was sufficient to inhibit the activation of MEK and its downstream kinase, MAPK. The antiproliferative response to XK469 was correlated with a steady accumulation of cyclins B1 and A, which appeared to be a direct result of G2/M arrest [5].
XK469R has significant antitumor activity in several human tumor xenografts, including breast, colon, lung, prostate and central nervous system tumors [8]. Polin et al. have recommended split dose regimens (e.g., daily, every other day, or twice weekly) based on preclinical data [8]. Based on the preclinical toxicology of XK469R, hematological and gastrointestinal toxicities were expected in patients [8].
A recently completed phase I study of XK469R in patients with refractory solid tumors, (given on days 1, 3, and 5 of a 21 day schedule),demonstrated that neutropenia was the dose limiting toxicity (DLT). There was only a single instance of treatment-related grade 3 anemia. Thrombocytopenia was limited to grade 1 severity, except for a single instance of grade 3 at the highest dose level of 1,400 mg. Non-hematologic toxicities (at least possibly related to drug) included alopecia, anorexia, diarrhea, nausea, fatigue, stomatitis, vomiting, increased bilirubin, and elevated ALT. The single most common toxicity was neutropenia, which was seen at all dose levels above 850 mg. At the MTD of 1,400 mg, the median days of grade 3+ neutropenia were 18 (range; 10–22 days). The recommended phase II dose (RPTD) for solid tumors is 850–1,100 mg/day using this schedule [9].
Based on the above preclinical data demonstrating that XK469R appears to have novel mechanisms of action, its activity against leukemia cell lines, and its limited nonhematological toxicity in patients with solid tumors, a phase I study of XK469R was initiated in patients with refractory leukemias.
Patients and methods
Patient eligibility
Patients aged 18 years or older with refractory leukemias were eligible if they had no potentially curative options. Patients with previously untreated leukemia who were considered inappropriate candidates for standard induction chemotherapy due to advanced age or concurrent co-morbid medical conditions or those who refused such therapy were also eligible. Patients were required to have an ECOG performance status of <2, a serum bilirubin ≤1.5 mg/dL; AST and ALT levels less than five times the upper limit of normal (ULN) and serum creatinine <1.5 times the ULN. Patients with evidence of uncontrolled infection at the time of study enrollment were not eligible for enrollment. The institutional review boards at both participating institutions approved the protocol, and all patients gave signed informed consent indicating that they were aware of the investigational nature of this study.
XK469R was supplied by the Division of Cancer Treatment, Diagnosis and Centers, National Cancer Institute [NCI], Bethesda, MD in a 250 mg vial. The drug was administered without further dilution or was diluted in 0.9% sodium chloride to a concentration as low as 0.05 mg/mL. The drug was administered through a central venous catheter with an in-line filter over 30 min (until dose levels 4 and above, at which time the infusion time was increased to 60 min) on days 1, 3, and 5 of a 21-day cycle. Patients who achieved complete remission (CR), CR with incomplete platelet recovery (CRp), partial response (PR), hematological improvement (HI), or stable disease were allowed to receive additional cycles of therapy at the same or a reduced dose if there were no unacceptable toxicities or inter-current illnesses anticipated to prevent the patient from safely receiving further therapy. Intrapatient dose escalation was not allowed. Standard supportive measures, including prophylactic antibacterial, antifungal and antiviral therapy, and blood and platelet transfusions were permitted as per institutional guidelines. Erythropoietin or other hematopoietic colony stimulating factors were not given prophylactically, but were permitted in the second and subsequent cycles if deemed clinically necessary. The administration of concomitant anticancer therapies was not permitted, with the exception during cycle 1: During the first 21 days of study, patients could undergo leukapheresis or receive hydroxyurea and/or anagrelide for a maximum of 14 days to control elevated blast and/or platelet counts. Baseline tests, including bone marrow aspiration and/or biopsy, serum chemistries, complete blood count (CBC) with differential and platelet count, coagulation testing, and physical examination (including vital signs, height, weight and performance status) were done within 1 week prior to the first dose of XK469R. Patients were re-examined weekly and as indicated clinically with vital signs, serum chemistries, CBC with differential and platelet count, and coagulation testing. A bone marrow aspiration and/or biopsy was done every 3 to 6 weeks and as clinically indicated to determine response.
Response and toxicity criteria
CR was defined as normalization of the blood and bone marrow, with <5% blasts, neutrophil count >1×109/L, and platelet count >100×109/L, with no evidence of extramedullary disease. PR was defined as per CR except for the presence of 6–25% blasts in the bone marrow or >50% decrease in the bone marrow blasts. A CRp was defined as per CR with a platelet count <100×109/L. HI was described by the number of individual, positively affected cell lines: erythroid (E); neutrophil (N); and platelet (P); (e.g., HI-E; HI-E+HI-N; HI-E+HI-P+HI-N). For patients with pretreatment hemoglobin less than 11 g/dL, a greater than 2 g/dL increase in hemoglobin was considered HI; for RBC transfusion-dependent patients, transfusion independence was considered HI. For patients with a pre-treatment platelet count less than 100×109/L, an absolute increase of 30×109/L was considered as HI-P; for platelet transfusion-dependent patients, HI-P was defined as stabilization of platelet counts and transfusion independence. Neutrophil response (HI-N) was defined as an increase of more than 0.5×109/L if the absolute neutrophil count (ANC) was less than 1.5×109/L before therapy.
Adverse events were graded based on the NCI’s Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical methods
Study design
This was a two-center, open-label, phase I dose escalation study to determine the DLT, RPTD and pharmacokinetics of XK469R in patients with refractory leukemia. The starting dose level was 1,400 mg administered by intravenous infusion over 30 min through a central venous catheter on days 1, 3, and 5 of a 21-day cycle. For doses at and above 2,750 mg, the infusion time was increased to 60 min. Because of the marked pharmacokinetic variability observed in the solid tumor study, cohorts of six patients were enrolled at each dose level. Dose escalation to the next level occurred after the last patient enrolled in the prior dose level had been observed for at least 21 days without evidence of DLT. All patients who received any therapy on study were considered evaluable for toxicity.
DLT was defined as a clinically significant adverse event or abnormal laboratory value assessed as unrelated to disease progression, inter-current illness, or concomitant medications and occurring during the first 21 days on study and which met any of the following criteria: CTCAE grade 3 AST or ALT for >7 days, CTCAE grade 4 AST or ALT of any duration or other clinically significant NCI Common Terminology Criteria that was CTCAE grade 3 or 4.
Patients were treated in successive cohorts of at least six patients because of marked interindividual variability in toxicity and pharmacokinetics in the solid tumor study. If zero or one of six patients treated at a dose level experienced DLT, six more patients were entered at the next highest dose level. Two or more patients experiencing DLT in a dose level terminated the dose escalation; however, if the DLTs were inconsistent with other data observed, additional patients could be enrolled up to a maximum of 12. Dose escalation could then proceed only if less than one-third of patients had a DLT. A minimum of 12 patients were planned at the recommended phase 2 dose (RPTD). The RPTD was the highest dose level at which less than one-third of patients experienced DLT.
Pharmacokinetic analysis
Blood sampling for pharmacokinetics was performed during the first treatment cycle. On day 1 (first dose), samples were obtained before the start of XK469R (pre-dose sample); 15 and 30 min during the infusion; and 15 min, 30 min, 1, 2, 4, 6, and 8 h after the end of the infusion. On day 2, a single blood sample was taken approximately 24 h after drug administration on day 1. On day 3 (second dose), a single sample was taken before the start of the infusion. On day 5 (third dose), a single sample was taken before the start of the infusion. Blood samples were collected in 7.5 mL green top plastic Vacutainer tubes containing sodium heparin. Tubes were centrifuged (2,500 rpm, 10 min, 4°C) and the plasma immediately transferred, labeled, and stored at −20°C for a period not exceeding one week or at −80°C until analysis. On day 1 of cycle 1, a pre-dose urine sample was collected, and a 20-mL aliquot retained as a pre-dose sample. Patients were asked to void their bladder before drug administration. Patients’ complete urinary output during the 0–8 and 8–24 h intervals (“0” was time of drug administration) was collected. The urine was mixed, the volume measured and noted, and two 10 mL aliquots from each interval stored at −20°C for a period not exceeding 1 week or at −80°C until analysis. Measurement of plasma and urine levels of XK469R and metabolites were performed by HPLC using the assay method reported by Zheng et al. which detects both enantiomers [10]. Although stereoconversion of the R(+) form to the S(−) form is modest in animals (<5%), significant in vivo chiral R→S inversion in humans cannot be excluded a priori. One milliliter of plasma and urine samples were processed by solid-phase extraction, and enantiomeric separation of the R(+) and S(−) forms achieved by using Astec Chirobiotic T column (Whippany, NJ), isocratic elution (water-methanol, 65:35, v/v, containing 20 mM NH4NO3), and UV detection (255 nm). The (±) 7-fluoro-analog of XK469R was used as internal standard. This HPLC method was validated by assessment of interand intra-assay accuracy and precision determined over a period of three days. Recovery was determined at low, mid and high concentrations. Samples were analyzed in duplicate, and their levels extrapolated from a standard curve analyzed each run day.
Pharmacokinetic profiles of XK469R and its metabolites were constructed for each patient. The data employed in the pharmacokinetic analysis were data pairs consisting of a time point (from the beginning of the infusion) and a concentration. Data were analyzed by noncompartmental method using the WinNonlin software (Pharsight, Mountain View, CA). The pharmacokinetic parameters estimated included the elimination rate constant, the area under the concentration vs time curve (AUC), terminal half-life, total (nonrenal + renal) clearance, and volume of distribution at steady state. Urinary extraction was evaluated by the calculation of the fraction of XK469R dose excreted as parent drug or metabolites in the urine. Mean, standard deviation, and coefficient of variation (CV) were determined for each parameter. Plasma and urine metabolic ratios between metabolite and XK469R concentrations were used as an index of metabolic activity and phenotype for each patient. The modality of the frequency distribution of metabolic ratios was also described.
Results
Forty-six patients were treated between November 2004 and June 2006. Patient characteristics are summarized in Table 1. The following dose levels were explored: 1,400 (n = 6), 1,750 (n = 12), 2,200 (n = 14), and 2,750 mg (n = 14). The median number of cycles received was one; 11 patients received two cycles, three patients received three cycles, and one patient treated at the 2,200 mg cohort received four cycles of XK469R.
Table 1.
Characteristics of 46 patients treated on study
| Characteristics | Number | Percent |
|---|---|---|
| Median age = 53 years (range, 20–85) | ||
| Male | 26 | 57 |
| ECOG PS | ||
| 0, 1 | 40 | 87 |
| 2 | 6 | 13 |
| Diagnosis | ||
| Acute myeloid leukemia | 41 | 89 |
| Acute lymphoblastic leukemia | 4 | 9 |
| Chronic myeloid leukemia—blast phase | 1 | 2 |
| Relapse status | ||
| First | 5 | 11 |
| Second | 15 | 33 |
| Third | 17 | 37 |
| Fourth or more | 9 | 20 |
| Cytogenetics | ||
| Normal | 13 | 28 |
| t(9;22) | 1 | 2 |
| Miscellaneous | 12 | 26 |
| 11q abnormality | 1 | 2 |
| +8 | 5 | 11 |
| −5, −7, or complex | 10 | 22 |
| t(8;21) | 2 | 4 |
| inv (16) | 1 | 2 |
Non-hematological toxicities attributable to XK469R were generally mild and are summarized in Table 2. The most common non-hematologic toxicities were grade 1 and 2 gastrointestinal events including nausea, anorexia, vomiting, and diarrhea (Table 2). DLTs occurred at the 2,200 and 2,750 mg dose levels (Table 3). At 2,200 mg, an episode of mucositis was considered a DLT in one of six patients. An episode of grade 3 colitis was initially considered to be related to infection; therefore, the dose was escalated to 2,750 mg. Six patients were initially treated at this dose level with one DLT of elevated bilirubin. This patient also experienced grade 3 mucositis, which along with the hyperbilirubinemia, was considered to be possibly related to ongoing infection; therefore, the 2,750 mg cohort was expanded further to explore safety. Of eight patients treated in this expanded cohort, two experienced mucositis as a DLT. Therefore, as three of 14 patients experienced DLT at the 2,750 mg dose level, dose escalation was terminated. The 2,200 mg cohort was expanded and, after further review of all the patients in this cohort, it was determined that colitis noted in the first patient treated at 2,200 mg was probably XK469R-related, suggesting that a lower dose level should be expanded. Therefore, the 1,750 mg cohort was expanded to include a total of 12 patients. No DLTs were observed; thus, 1,750 mg is the RPTD of XK469R.
Table 2.
Non-hematologic adverse events by dose level
| Dose level/no. of patients | Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| 1,400 mg/6 pts | Alopecia | 1 | 2 | ||
| Anorexia | 1 | ||||
| Hyperuricemia | 1 | ||||
| 1,750 mg/12 pts | Alopecia | 2 | 1 | ||
| Diarrhea | 3 | ||||
| Nausea | 5 | 1 | |||
| Vomiting | 4 | ||||
| Indigestion | 1 | ||||
| Dysgeusia | 1 | ||||
| AST or ALT elevation | 1 | ||||
| Anorexia | 2 | ||||
| Mucositis | 2 | ||||
| Rash | 1 | ||||
| 2,200 mg/14 pts | AST or ALT elevation | 1 | |||
| Nausea | 7 | 1 | 1 | ||
| Vomiting | 4 | 2 | |||
| Diarrhea | 2 | ||||
| Mucositis | 2 | 1 | |||
| Colitis | 1 | ||||
| Rash | 1 | 1 | |||
| Anorexia | 1 | 1 | |||
| Alopecia | 1 | 1 | |||
| 2,750 mg/14pts | Nausea | 6 | 1 | ||
| Vomiting | 4 | 1 | |||
| Diarrhea | 4 | 1 | |||
| Anorexia | 2 | 1 | 1 | ||
| Alopecia | 1 | ||||
| Hepatic (Elevated Bilirubin) | 1 | ||||
| Rash | 1 | ||||
| Mucositis | 1 | 2 |
Table 3.
DLT by XK489R dose level
| Dose (mg) | No. of patients |
Gastrointestinal | Mucositis | Hepatic |
|---|---|---|---|---|
| 1,400 | 6 | |||
| 1,750 | 12 | |||
| 2,200 | 14 | (1) | (1) | |
| 2,750 | 14 | (2) | (1) |
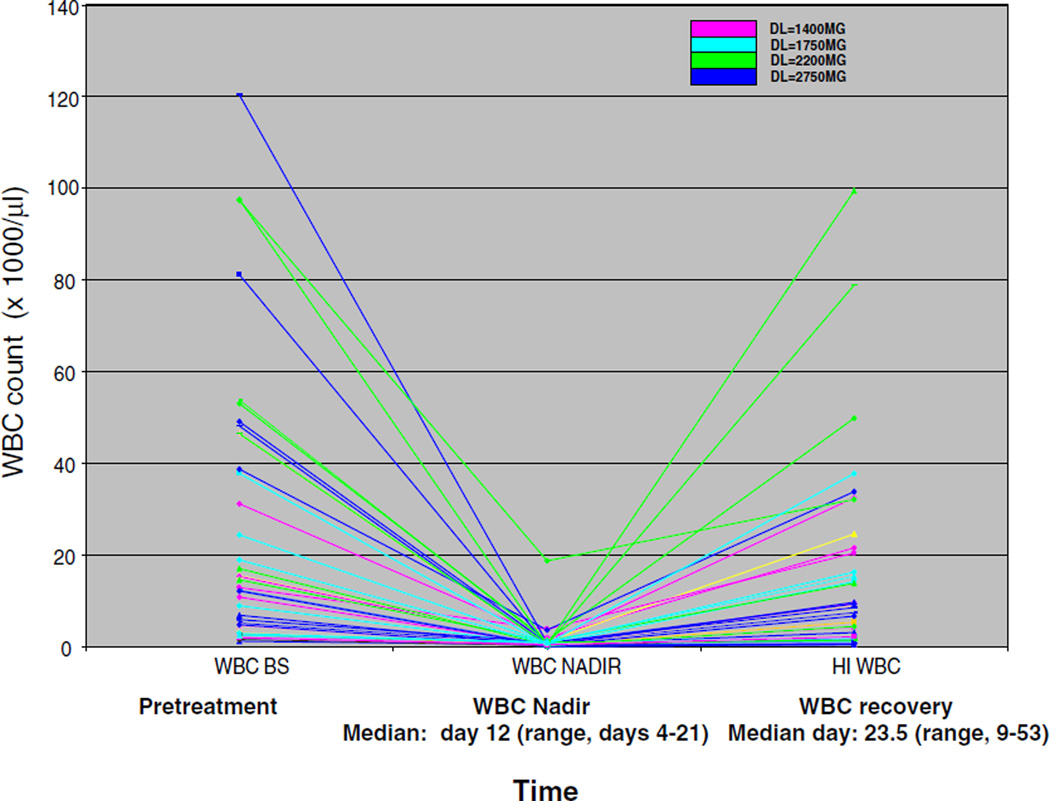
Myelosuppression occurred at all dose levels, with a median WBC count nadir occurring on day 12 of cycle one. Myelosuppression was reversible with a return to improved or baseline counts by day 23–24 of cycle one (median 23.5; range: days 9–53; Fig. 1). Fifteen patients received two or more cycles of treatment. The median time to WBC count recovery during cycle one for these patients was 12 days (range: 7–34 days) and the median WBC count at the time of beginning course 2 (or subsequent cycles) was 3.5 (range: 0.4 –86.7). During treatment, infectious complications occurred frequently. Of the 46 patients, 37 had no active infection at study entry, seven had a controlled infection, and two had fever of unknown origin. Only three patients did not experience any febrile episodes during treatment. Five patients developed gram negative bacteremia, 6 g positive infections were reported, and eight patients had infections with both gram positive and negative organisms. Sites of documented infections were as follows: one patient experienced skin cellulitis at the site of an intravenous catheter with no positive cultures, ten had pneumonia (four were present at baseline), one patient had a positive culture for fungus from pleural fluid, one patient had a positive fungal culture from a brochoalvelolar lavage, and one patient experienced neutropenic colitis. Eight patients experienced fever of unknown origin.
Fig. 1.
XK469R induced myelosuppression at all dose levels. Reliable and reversible myelosuppression was induced by XK469R at all dose levels (color coded) with a median time to WBC count nadir (Y-axis) of 12 days (range, 4–21). The median time to WBC count recovery (to baseline or normalization) was 23.5 days (range, 9–53)
Forty-two (91%) patients were evaluable for response. One (2%) patient treated in the 1,750 mg cohort achieved a CR, and five patients (9%) had hematologic improvement (HI). HI-E was noted in two patients at 1,400 and 1,750 mg, respectively; HI-N in two patients at 1,750 mg; and HI-N and HI-P in one patient in the 2,750 mg cohort. Twenty-one patients had stable disease and 15 patients experienced disease progression during treatment. The patient achieving a CR was an 84 year old woman with a normal karyotype AML (FLT3 ITD negative) who had achieved a 15 month remission duration after standard idarubicin and cytarabine induction, and one consolidation cycle of chemotherapy with idarubicin and cytarabine. At the time of first relapse, she had entered a clinical trial of deferolimus, an mTOR inhibitor, but failed to have any response. Following cycle one of XK469R, stable disease was noted. She started a second cycle on day 28 which resulted in prolonged myelosuppression. Tri-lineage count recovery gradually occurred over a 3 month period and a CR was documented on day 100 following cycle 2. CR duration was 3 months.
Pharmacokinetic evaluation
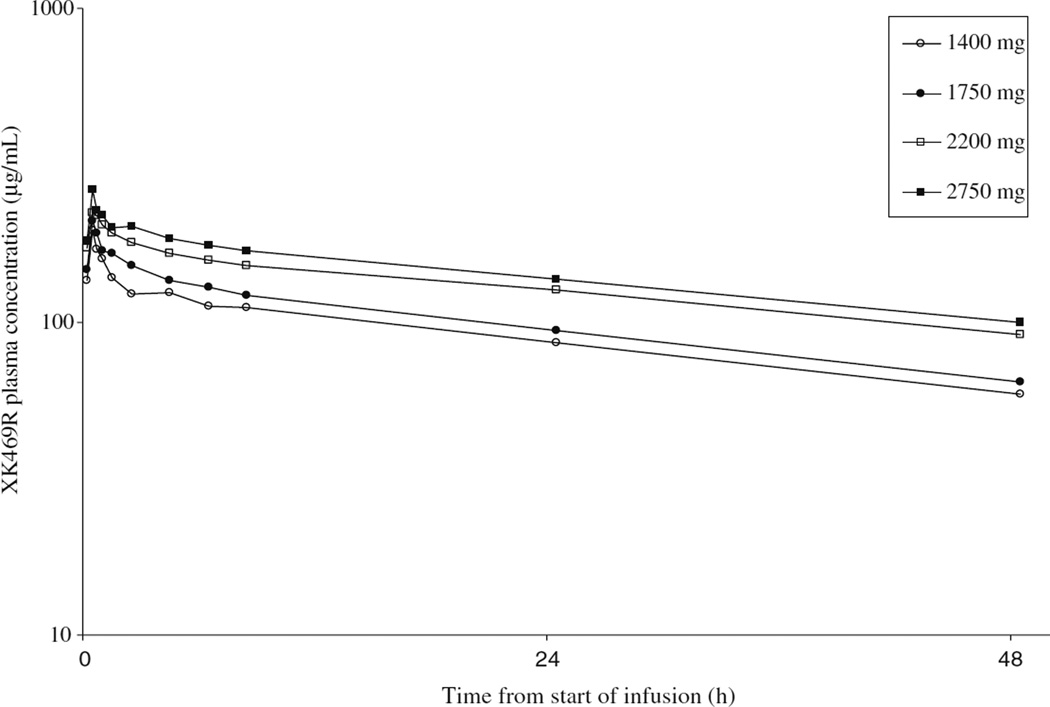
Plasma samples were obtained from all 46 patients enrolled during the first cycle of treatment. The log mean concentrations of plasma XK469R versus time for each dose level are shown in Fig. 2. The mean pharmacokinetic parameters for the first cycle are displayed in Table 4. The results of two patients treated at the 2,200 mg dose level are excluded from the table and discussed below. Half-life was long with a mean value over all dose levels of 48 h (coefficient of variation [CV]: 0.3). Mean clearance and volume of distribution at steady state across all dose levels were 206 mL/h (CV: 0.32) and 13 L (CV: 0.27), respectively. The coefficient of variation (CV) in clearance of 32% was less than observed in the phase I trial in solid tumor patients. There was evidence of drug accumulation from dose to dose on this day 1, 3, and 5 schedule as demonstrated by measurable drug concentration in the pre-infusion samples on days 3 and 5.
Fig. 2.
Mean XK469R plasma concentrations by dose level for cycle 1. The mean log plasma concentration of XK469R is shown for each of the dose levels (Y axis) at serial time-points following drug infusion (X axis) during cycle 1 of therapy. Mean clearance and volume of distribution at steady state across all dose levels were 206 mL/h and 13 L, respectively
Table 4.
Mean (coefficient of variation) pharmacokinetic parameters
| Dose level (mg) | N | Cmax (µg/mL) | AUC0→T (h·µg/mL) | AUC0→∞ (h·µg/mL) | t1/2 (h) | Cl (mL/h) | Vss (L) |
|---|---|---|---|---|---|---|---|
| 1,400 | 6 | 196 (0.23) | 4,299 (0.21) | 8,064 (0.28) | 44 (0.22) | 184 (0.25) | 11 (0.25) |
| 1,750 | 12 | 211 (0.23) | 4,443 (0.26) | 9,150 (0.34) | 45 (0.28) | 211 (0.31) | 13 (0.25) |
| 2,200 | 12a | 239 (0.09) | 5,267 (0.25) | 11,177 (0.19) | 45 (0.23) | 205 (0.25) | 13 (0.10) |
| 2,750 | 14 | 264 (0.29) | 6,694 (0.28) | 14,863 (0.37) | 55 (0.32) | 212 (0.41) | 15 (0.32) |
Two outlier patients are excluded from the results
There was no apparent relationship between drug clearance and the risk of DLT. Only one patient, who experienced DLT had a clearance value that was greater than one standard deviation below the mean. The remainder had values that were either within one standard deviation of the mean or more than one standard deviation above the mean (and, therefore, experienced lower than average drug exposure as measured by AUC). The two patients whose results are excluded from Table 4 had clearance values of 59 and 70 mL/h. These values are greater than two standard deviations below the mean clearance of the remaining 44 patients. These patients, however, did not have excessive toxicity as compared to other patients.
Discussion
In this phase I study of XK469R in patients with advanced leukemia, the drug was generally well-tolerated and induced reproducible and reversible myelosuppression at all dose levels evaluated. It was possible to administer repeated cycles of the agent using this 21-day cycle. DLTs of mucositis and hyperbilirubinemia were noted at the highest dose level evaluated, 2,750 mg. Dose-limiting mucositis and colitis also occurred during cohort expansion at the 2,200 mg level. Thus, the RPTD for XK469R is 1,750 mg for patients with refractory leukemia. Using this dose and schedule, there were no DLTs and clinical responses occurred; therefore, it would be reasonable to explore this dose in combination with other active agents in future studies. Interestingly, the MTD and RPTDs of XK469R for patients with solid tumors and leukemias are similar due to the observation that the mucosa and marrow toxic doses are similar. In contrast to the solid tumor phase I study, clinical activity was noted in these heavily pretreated leukemia patients and included a CR of 3 months duration in an older patient with refractory AML treated at the 1,750 mg dose level. Reversible myelosuppression occurred at all dose levels and was associated with a return to baseline (or normalization of WBC count) at a median of 23.5 days, allowing for timely administration of subsequent cycles of XK469R for responding patients or those with stable disease. Infectious complications during treatment were common but did not differ from, or occur more frequently than, what is typically seen during treatment of patients with relapsed/refractory acute leukemia.
Pharmacokinetic analysis demonstrated that the drug accumulated between doses on the day 1, 3, and 5 schedule with a long half-life of approximately 48 h, which was similar to findings in the phase I solid tumor study. While significant inter-patient variability in drug clearance occurred at each dose level examined, there was no obvious relationship between development of DLTs and pharmacokinetics. In the phase I solid tumor study, decreased clearance was associated with a higher risk of prolonged severe neutropenia. This was not possible for us to assess since relative or absolute neutropenia was present in almost all of our leukemia patients at the time of study entry. Nevertheless, the duration of myelosuppression in our patients did not appear to correlate with decreased drug clearance.
While clinical activity was noted in this phase I trial, including one complete remission in an older patient with refractory AML, it is unlikely that XK469R will have significant anti-leukemia efficacy as a single agent. However, due to its novel mechanism of action, reasonable toxicity profile at the RPTD, and reversible marrow suppression in these high-risk patients, further exploration of XK469R, possibly in combination with other established agents, is warranted in patients with refractory AML.
Acknowledgments
The authors wish to thank Dawn Spearmon for her administrative assistance in the preparation of this manuscript. We would also like to acknowledge the hard work of Margaret Green, RN, whose research nursing expertise was invaluable.
This work was supported by CA069852 and CA062461 and by the University of Chicago Cancer Research Center, P30 CA014599-32 S2.
Contributor Information
Wendy Stock, Email: wstock@medicine.bsd.uchicago.edu, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
Samir D. Undevia, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA
Carol Bivins, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Farhad Ravandi, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Olatoyosi Odenike, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
Stefan Faderl, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Elizabeth Rich, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
Gautam Borthakur, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Lucy Godley, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
Srdan Verstovsek, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Andrew Artz, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
William Wierda, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Richard A. Larson, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA
Yanming Zhang, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA.
Jorge Cortes, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
Mark J. Ratain, Section of Hematology/Oncology, University of Chicago Cancer Research Center, 5841 S. Maryland, M/C 2115, Chicago, IL 60637, USA
References
- 1.Aribi A, Ravandi F, Giles F. Novel agents in acute myeloid leukemia. Cancer J. 2006;12:77–91. [PubMed] [Google Scholar]
- 2.LoRusso PM, Parchment R, Demchik L, et al. Preclinical antitumor activity of XK469 (NSC 656889) Invest New Drugs. 1998;16:287–296. doi: 10.1023/a:1006206814025. [DOI] [PubMed] [Google Scholar]
- 3.Gao H, Huang KC, Yamasaki EF, et al. XK469, a selective topoisomerase IIbeta poison. Proc Natl Acad Sci U S A. 1999;96:12168–12173. doi: 10.1073/pnas.96.21.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessel D, Horwitz JP. Pro-apoptotic interactions between XK469 and the peripheral benzodiazepine receptor. Cancer Lett. 2001;168:141–144. doi: 10.1016/s0304-3835(01)00518-3. [DOI] [PubMed] [Google Scholar]
- 5.Lin H, Subramanian B, Nakeff A, et al. XK469, a novel antitumor agent, inhibits signaling by the MEK/MAPK signaling pathway. Cancer Chemother Pharmacol. 2002;49:281–286. doi: 10.1007/s00280-002-0425-7. [DOI] [PubMed] [Google Scholar]
- 6.Ding Z, Parchment RE, LoRusso PM, et al. The investigational new drug XK469 induces G(2)-M cell cycle arrest by p53-dependent and -independent pathways. Clin Cancer Res. 2001;7:3336–3342. [PubMed] [Google Scholar]
- 7.Ding Z, Zhou JY, Wei WZ, et al. Induction of apoptosis by the new anticancer drug XK469 in human ovarian cancer cell lines. Oncogene. 2002;21:4530–4538. doi: 10.1038/sj.onc.1205545. [DOI] [PubMed] [Google Scholar]
- 8.Polin L, White K, Kushner J, et al. Preclinical efficacy evaluations of XK-469: dose schedule, route and cross-resistance behavior in tumor bearing mice. Invest New Drugs. 2002;20:13–22. doi: 10.1023/a:1014469828729. [DOI] [PubMed] [Google Scholar]
- 9.Sprague E, Undevia SD, Innocenti F, et al. A dose escalation study of the quinolzaline antitumor agent R(+)XK469 (XK) in patients with refractory solid tumors. Proc Am Soc Clin Oncol. 2004;22:2021. [Google Scholar]
- 10.Zheng H, Covey JM, Tosca PJ, et al. Chiral high-performance liquid chromatographic analysis of the enantiomers of XK469, a new antitumor agent, in plasma and urine. J Pharm Biomed Anal. 2002;28:287–294. doi: 10.1016/s0731-7085(01)00566-0. [DOI] [PubMed] [Google Scholar]