Abstract
Background
Converging evidence suggests that physical activity is an effective intervention for both clinical depression and sub-threshold depressive symptoms; however, findings are not always consistent. These mixed results might reflect heterogeneity in response to physical activity, with some subgroups of individuals responding positively, but not others.
Objectives
1) To examine the impact of genetic variation and sex on changes in depressive symptoms in older adults after a physical activity (PA) intervention, and 2) determine if PA differentially improves particular symptom dimensions of depression.
Design
Randomized controlled trial.
Setting
Four field centers (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University).
Participants
396 community-dwelling adults aged 70–89 years who participated in the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P).
Intervention
12-month PA intervention compared to an education control.
Measurements
Polymorphisms in the serotonin transporter (5-HTT), brain-derived neurotrophic factor (BDNF), and apolipoprotein E (APOE) genes; 12-month change in the Center for Epidemiologic Studies Depression Scale total score, as well as scores on the depressed affect, somatic symptoms, and lack of positive affect subscales.
Results
Men randomized to the PA arm showed the greatest decreases in somatic symptoms, with a preferential benefit in male carriers of the BDNF Met allele. Symptoms of lack of positive affect decreased more in men compared to women, particularly in those possessing the 5-HTT L allele, but the effect did not differ by intervention arm. APOE status did not affect change in depressive symptoms.
Conclusions
Results of this study suggest that the impact of PA on depressive symptoms varies by genotype and sex, and that PA may mitigate somatic symptoms of depression more than other symptoms. The results suggest that a targeted approach to recommending PA therapy for treatment of depression is viable.
Keywords: exercise intervention, depression, aging, BDNF, APOE, serotonin transporter
INTRODUCTION
Traditional treatment for late-life depression with pharmacotherapy is efficacious in many patients [1] but often is accompanied by deleterious side effects, including increased falls and hyponatraemia [2]. Consequently, recent efforts have focused on alternative non-pharmacological interventions, particularly for older adults experiencing clinically significant sub-threshold depressive symptoms. Converging evidence suggests that physical activity (PA) is an effective intervention for both clinical depression and sub-threshold depressive symptoms [3]. Clinical trials have found similar efficacy for PA and antidepressant medication in the treatment of depression [4], with PA providing greater protection against relapse compared to medication [5].
Other studies, including previous work in the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P) [6], showed no overall impact of physical activity increases on depressive symptoms. These inconsistencies may be due in part to methodological issues, but may also reflect heterogeneity in response to PA, with some subgroups of individuals responding to PA, but not others. Attempts to target traditional antidepressant treatments have examined the impact of genetic variation on treatment efficacy. These studies focus on genes that are putatively related to antidepressant mechanisms, such as the serotonin transporter gene (5-HTT) for selective serotonin reuptake inhibitors, or genes that are related to the risk of depression, including the brain-derived neurotrophic factor (BDNF) and apolipoprotein E (APOE) genes. The role of these particular genes in the antidepressant response has been documented by recent meta-analyses [7–9], which showed that the APOE ε4 allele, the BDNF Met allele, and the 5-HTT long (L) allele are associated with higher likelihood of positive response and remission after antidepressant treatment. It is unclear whether genetic differences also impact the efficacy of PA in treating depression or depressive symptoms, as the evidence is limited and results are mixed. One study reported that young adults with at least one 5-HTT L allele showed greater reductions in depressive symptoms after a 5-week exercise intervention [10]. In contrast, a recent cross-sectional study in middle-aged adults found that the BDNF Val66Met polymorphism did not moderate the relationship between self-reported physical activity and depressive symptoms [11]. This question has not been investigated in older adults.
Also unclear is whether PA impacts particular symptom dimensions of depression more than others. Depression is a clinically heterogeneous disorder that comprises a variety of different symptoms (e.g., depressed affect, reduced positive affect, and somatic symptoms). Emerging evidence suggests that specific dimensions of depressive symptoms are related to specific brain changes and domains of cognitive dysfunction [12, 13]. Corroborating the distinction of symptom dimensions of depression, there is evidence of distinct vascular, degenerative and inflammatory contributors to different depressive symptom clusters [14], and genetic work has shown significant positive familial correlations for different symptom dimensions [15]. As such, it is possible that PA would improve certain types of depressive symptoms, but not others.
Moreover, the impact of PA on depressive symptoms may vary by sex. Numerous studies have shown that men and women not only differ in their risk for depression and vulnerability to depression-related negative sequelae, but also in the associations of genotype with depression risk and response to depression treatment [16, 17]. Some studies have reported a sex difference on the relationship between PA and depressive symptoms, with the effect being found exclusively or to a greater extent in either men [e.g., 18] or women [e.g., 11]. A recent meta-analysis of randomized trials showed a stronger effect of exercise in men [19].
The goal of the present investigation was to expand upon previous work in the LIFE-P cohort [6] by examining the role of variants in the BDNF, 5-HTT, and APOE genes in the antidepressant response to a physical activity intervention, and by separately examining different symptom dimensions of depression. Based on previous studies documenting a better treatment response in depressed carriers of the APOE ε4 allele, the BDNF Met allele, and the 5-HTT L allele, we expected LIFE-P participants possessing these genetic markers to show the greatest reduction in depressive symptoms after a 12-month PA intervention compared to an educational control intervention.
METHODS
Participants
Data for the present investigation came from the Lifestyle Intervention and Independence for Elders Pilot (LIFE-P) Study, a randomized controlled trial evaluating the effect of physical activity on physical performance measures linked with mobility disability. Details of the study design for LIFE-P have been described elsewhere [20]. Briefly, community-dwelling adults aged 70–89 years were recruited from four field centers (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University). Participants were required to have a sedentary lifestyle (<20 minutes of structured physical activity per week during the previous month) and a score of <10 on the Short Physical Performance Battery [21], but be able to complete a 400-m walk unaided in less than 15 minutes. Major exclusion criteria included presence of severe heart failure, uncontrolled angina, and other severe illnesses that might interfere with physical activity. The National Institutes of Health and by the Institutional Review Boards of all participating institutions approved the study procedures. All participants provided written informed consent. A total of 424 participants were enrolled between May 2004 and February 2005. Participants were randomly assigned to either a physical activity or health education control arm, described briefly below and in more detail elsewhere [20]. At baseline and at 6 and 12 months, comprehensive standardized assessments were conducted by trained research staff masked to intervention assignment. Of the 424 participants in LIFE-P, 396 consented to DNA testing, of which 365 had available baseline and follow-up data for the variables used in the present investigation. Sample characteristics for the present investigation are provided in Table 1.
Table 1.
Demographic characteristics, genotype and baseline CES-D scores.
| Total Sample | Women | Men | |
|---|---|---|---|
| N | 365a | 249 | 116 |
| Age (yrs) | 76.79 (4.26) | 76.71 (4.12) | 76.94 (4.56) |
| Race (Caucasian/AA/Other) | 283/63/19 | 190/49/10 | 93/14/9 |
| BDNF Status (Met+/Met−) | 107/255 | 70/178 | 37/77 |
| 5-HTT Status (L+/L−) | 269/78 | 185/52 | 84/26 |
| APOE Status (ε4+/ε4−) | 93/266 | 66/177 | 27/89 |
| Baseline CES-D Scores | |||
| Total Score | 7.29 (6.75) | 7.25 (6.38) | 7.40 (7.52) |
| Depressed Affect | 1.54 (2.43) | 1.66 (2.49) | 1.28 (2.28) |
| Somatic Symptoms | 3.38 (2.93) | 3.31 (2.69) | 3.53 (3.40) |
| Lack of Positive Affect | 2.19 (2.53) | 2.10 (2.40) | 2.38 (2.78) |
| % with CES-D ≥ 16 | 11.2% | 10.4% | 12.9% |
Total sample varied across gene: BDNF = 362, 5-HTT = 347, APOE = 359
CES-D = Center for Epidemiologic Studies Depression Scale; AA = African-American; BDNF = brain-derived neurotrophic factor; APOE = apolipoprotein E
Physical Activity (PA) Intervention
Participants randomly assigned to the PA arm performed aerobic, strength, flexibility, and balance training in both center- and home-based settings. Walking was the primary mode of aerobic actvity, given its widespread popularity and ease of administration across a broad segment of the older adult population. Other forms of endurance activity (e.g., stationary cycling) were utilized when regular walking was contraindicated. Each center-based session began with a brief warm-up, followed by 40 minutes of moderate-intensity walking, and concluding with 15 minutes of flexibility and balance training exercises.
PA intensity was gradually increased over the first 2–3 weeks, with a target of reaching moderate intensity as assessed by the Borg scale [22], a numerical scale indicating a rating of perceived exertion from minimal to maximal. Participants were asked to walk at a target intensity of 13 (somewhat hard) and perform strength training at an intensity of 15–16 (hard). The proportion of center-based to home-based sessions changed during the course of treatment: The number of center-based sessions was reduced to two times per week and home-based activities were increased during weeks 9–24. In the maintenance phase (week 25 to the end of the study), participants were encouraged to perform home-based physical activity a minimum of 5 days per week, and one weekly center-based session was offered.
Control Intervention
A successful aging health education intervention served as an attention control arm. Half of participants were randomly assigned to the control arm and attended education workshops on health topics of relevance to older adults, such as nutrition, medication use, foot care, and preventive medicine. Each class was concluded with gentle seated upper extremity stretching. These classes lasted approximately 60 minutes and were given weekly for the first 26 weeks, and then monthly until the end of the study. This amount of contact time is similar to the contact time for control arms employed in other successful randomized trials of physical activity in older adults [6].
Depressive Symptom Measurement
The Center for Epidemiologic Studies Depression Scale [CES-D; 23] was administered at baseline and at 12-months. The CES-D has shown a consistent factor structure across numerous patient populations and ethnic groups, which has been confirmed by meta-analysis [24]. The four-factor structure of the CES-D includes depressed affect (e.g., sadness and fearfulness), somatic symptoms (e.g., loss of appetite, concentration difficulties), lack of positive affect (e.g., diminished capacity to experience pleasure), and interpersonal difficulties (i.e., perceived problems in social relationships) subscales. Baseline and 12-month scores were used in analyses for the current investigation.
Genotyping
DNA was isolated from whole blood using a commercially available DNA isolation kit, and the concentration and purity (260/280) were determined by a UV spectrophotometer. The 5-HTT gene short (484 bp) and long (528 bp) variant alleles (insertion/deletion polymorphism) were genotyped by PCR amplified fragment length polymorphism. The following forward 5’-GCGTTGCCGCTCTGAATGC-3’ and reverse 5’-GAGGGACTGAGCTGGACAACCAC-3’ PCR primers were used to amplify the short (484 bp) and long (528 bp) variant alleles. The amplified PCR products from DNA samples along with positive and negative controls were run on 1% agarose gel and the results were interpreted by two different trained personnel. The BDNF Val66Met rs6265 and APOE rs4412 polymorphisms were genotyped by Taqman® genotyping method on ABI 7900 HT platform using the following genotyping probes (IDs C__11592758_10 and C___2230322_20) respectively. The assays were performed and analyzed according to the manufacture's recommendations (Life Technologies, CA, USA). The APOE rs420358 polymorphism was genotyped by Pyrosequencing genotyping method [25], using the following PCR and sequencing primers; forward biotinylated PCR primer 5’-GCGGACATGGAGGACGTG-3’, reverse PCR primer 5’-TACACTGCCAGGCGCTTCT-3’, and reverse sequencing primer 5’-ACTGCACCAGGCGGC-3’. The Pyrosequencing reactions were carried out using the Pyrosequencing HS 96 platform according to the manufacturer’s recommendations and the genotypes were automatically called by PSQ HS 96 SNP software (Qiagen, Valencia, CA, USA).
Statistical Analyses
Analyses of variance compared baseline continuous demographic characteristics and CES-D scores for the full sample, as well as CES-D scores by sex and genotype. Chi-square tests compared genotype frequency and categorical demographic characteristics across sex.
Our primary hypotheses were tested with analyses of covariance. Dominant models rather than additive models were used for analysis of 5-HTT (presence vs. absence of the L allele) and APOE (presence vs. absence of the ε4 allele) due to small sample sizes for the interaction terms of genotype by sex in the additive models. The 12-month changes in total CES-D score and its subscales were treated as dependent variables in separate models. The interpersonal problems subscale was not included in the analyses due to the restricted range of scores on the subscale, which comprises only 2 items. Intervention arm, genotype, and sex were included as predictors. The 3-way interaction among intervention arm, genotype, and sex and their pairwise two-way interactions were also tested. Stratified analyses were explored for interactions with p <.10. Non-significant interaction terms were removed in stages until a final, parsimonious model was reached. All models included age, race/ethnicity, testing site, and baseline outcome as covariates. A significance threshold of p <.05 was used.
RESULTS
Demographic characteristics, genotype distribution and baseline CES-D scores are summarized for the total sample and for women and men separately in Table 1. No significant sex differences were observed in demographic characteristics or baseline CES-D scores (ps ≥ .085). The percentage of participants who possessed the BDNF Met allele, 5-HTT L allele, and APOE ε4 allele was 29.6%, 77.5%, and 25.9%, respectively. All frequencies were in Hardy-Weinberg equilibrium. Genotype distribution was similar for men and women (ps ≥ .41). Baseline scores on the CES-D, including the depressed affect, somatic symptoms, and lack of positive affect subscales are presented by sex and genotype in Table 2. The mean CES-D total score at baseline was 7.29±6.75; scores were similar across genotype and sex × genotype (ps ≥ .15).
Table 2.
Baseline CES-D scores by sex and genotype.
| Total Sample | Women | Men | ||||
|---|---|---|---|---|---|---|
| 5-HTT | L+ | L− | L+ | L− | L+ | L− |
| Total CES-D | 6.97 (6.36) | 8.14 (6.54) | 6.88 (6.40) | 8.71 (6.70) | 7.15 (6.32) | 7.00 (6.16) |
| Depressed Affect | 1.38 (2.27) | 1.90 (2.47) | 1.48 (2.46) | 2.31 (2.70) | 1.15 (1.76) | 1.08 (1.69) |
| Somatic Symptoms | 3.26 (2.86) | 3.58 (2.67) | 3.19 (2.82) | 3.62 (2.39) | 3.40 (2.95) | 3.50 (3.20) |
| Lack of Positive Affect | 2.14 (2.50) | 2.47 (2.66) | 2.02 (2.32) | 2.58 (2.75) | 2.43 (2.83) | 2.27 (2.52) |
| BDNF | Met+ | Met− | Met+ | Met− | Met+ | Met− |
| Total CES-D | 7.68 (6.64) | 7.07 (6.77) | 7.53 (6.33) | 7.16 (6.43) | 7.97 (7.28) | 6.86 (7.56) |
| Depressed Affect | 1.57 (2.10) | 1.51 (2.57) | 1.71 (2.18) | 1.63 (2.62) | 1.30 (1.92) | 1.21 (2.43) |
| Somatic Symptoms | 3.59 (2.94) | 3.28 (2.94) | 3.33 (2.56) | 3.31 (2.75) | 4.08 (3.54) | 3.23 (3.34) |
| Lack of Positive Affect | 2.33 (2.71) | 2.08 (2.38) | 2.27 (2.62) | 2.04 (2.32) | 2.43 (2.92) | 2.18 (2.54) |
| APOE | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− |
| Total CES-D | 7.90 (7.66) | 7.09 (6.46) | 7.94 (8.28) | 7.00 (5.59) | 7.82 (6.03) | 7.27 (7.95) |
| Depressed Affect | 1.89 (2.84) | 1.42 (2.29) | 2.15 (3.17) | 1.48 (2.21) | 1.26 (1.70) | 1.28 (2.44) |
| Somatic Symptoms | 3.68 (3.10) | 3.29 (2.89) | 3.50 (3.12) | 3.25 (2.54) | 4.11 (3.04) | 3.36 (3.50) |
| Lack of Positive Affect | 2.14 (2.71) | 2.19 (2.46) | 2.09 (2.81) | 2.08 (2.22) | 2.26 (2.50) | 2.42 (2.87) |
CES-D = Center for Epidemiologic Studies Depression Scale; BDNF = brain-derived neurotrophic factor; APOE = apolipoprotein E
The main effect of intervention arm was not significant in any of the models (ps ≥ .11). ANCOVAs examining the impact of PA, BDNF status and sex on 12-month change in depressive symptoms revealed an intervention arm × sex effect, F1, 347 = 4.13, p = .043, for somatic symptoms (Figure 1; Table 3). Men in the PA arm showed the greatest decrease in somatic symptoms over 12 months, with minimal change in the control arm. Symptoms decreased to a lesser extent in women, and were similar for the PA and control arms. Based on a marginal 3-way interaction between intervention, BDNF status, and sex, F1, 347 = 3.09, p = .079, we performed analyses stratified by intervention arm. In the PA arm, men who possessed the Met allele showed greater decreases in somatic symptoms compared to Met negative men and to women, F1, 172 = 4.54, p = .043 (Figure 2). No significant effects were observed in the control arm.
Figure 1.
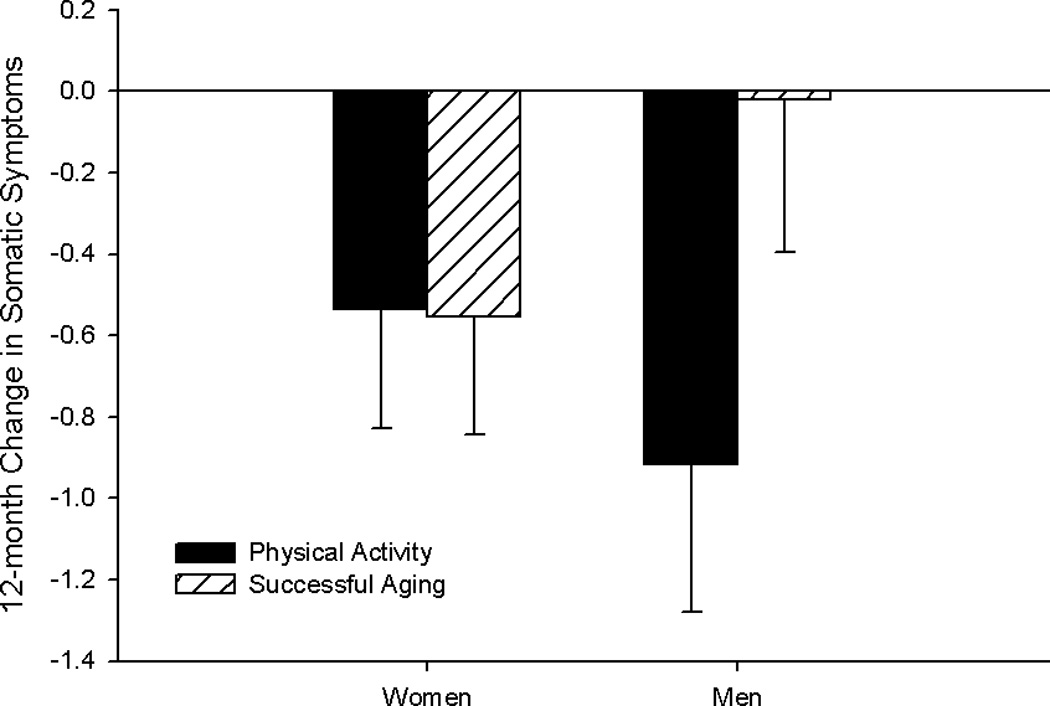
Least squares means for 12-month change in somatic symptoms by sex and intervention group.
Table 3.
Least squares means for change in CES-D scores after adjusting for intervention by sex, intervention group, and genotype.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| PA | SA | PA | SA | |||||
| 5-HTT | L+ | L− | L+ | L− | L+ | L− | L+ | L− |
| Total CES-D | −0.56 | −1.11 | −1.02 | −1.33 | −1.93 | −1.45 | −0.17 | −1.91 |
| Depressed Affect | 0.17 | −0.48 | −0.16 | −0.53 | −0.26 | −0.49 | 0.14 | 0.02 |
| Somatic Symptoms | −0.65 | −0.38 | −0.49 | −0.96 | −1.25 | −0.19 | −0.09 | −0.05 |
| Lack of Positive Affect | −0.11 | 0.15 | −0.29 | 0.05 | −0.30 | −0.67 | −0.19 | −1.77 |
| BDNF | Met+ | Met− | Met+ | Met− | Met+ | Met− | Met+ | Met− |
| Total CES-D | −0.34 | −0.84 | −1.02 | −0.93 | −2.61 | −1.24 | −0.30 | −0.91 |
| Depressed Affect | −0.05 | 0.02 | −0.51 | −0.08 | −0.58 | −0.22 | 0.13 | 0.11 |
| Somatic Symptoms | −0.09 | −0.74 | −0.62 | −0.48 | −1.73 | −0.54 | −0.08 | −0.12 |
| Lack of Positive Affect | −0.05 | −0.06 | 0.13 | −0.32 | −0.38 | −0.33 | −0.16 | −0.64 |
| APOE | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− |
| Total CES-D | −0.58 | −0.77 | −0.49 | −1.15 | −0.98 | −2.01 | −0.66 | −0.74 |
| Depressed Affect | −0.13 | 0.04 | −0.19 | −0.27 | −0.27 | −0.33 | 0.08 | 0.12 |
| Somatic Symptoms | −0.37 | −0.69 | −0.24 | −0.65 | −0.28 | −1.19 | −0.68 | −0.02 |
| Lack of Positive Affect | 0.00 | −0.07 | −0.07 | −0.21 | −0.35 | −0.37 | −0.07 | −0.57 |
PA = physical activity intervention group; SA = successful aging education control group; CES-D = Center for Epidemiologic Studies Depression Scale; BDNF = brain-derived neurotrophic factor; APOE = apolipoprotein E
Figure 2.
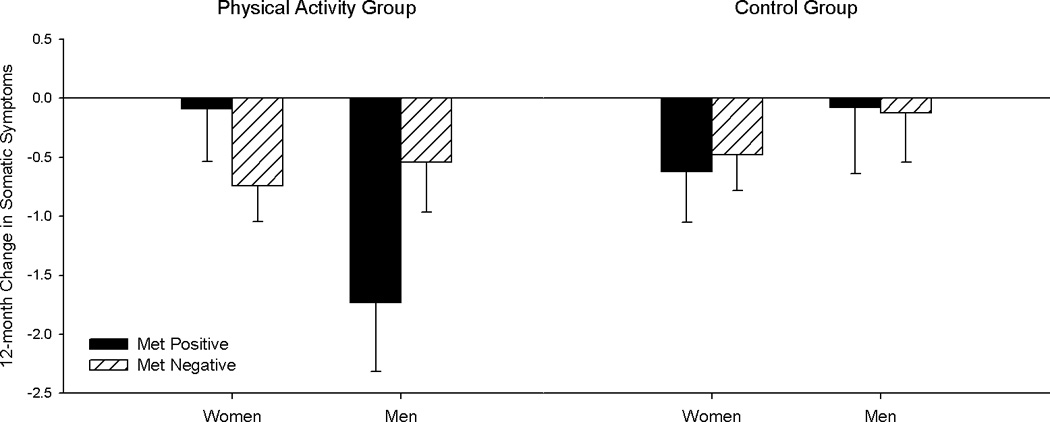
Least squares means for 12-month change in somatic symptoms by BDNF genotype, sex and intervention group.
In the analysis of 5-HTT status, significant sex, F1, 335 = 4.99, p = .026, and genotype × sex, F1, 335 = 4.27, p = .035, effects were observed for the lack of positive affect subscale, such that those depressive symptoms decreased most after 12 months in men compared to women, and more so in 5-HTT L-negative compared L-positive men (Figure 3). Results did not differ by intervention arm.
Figure 3.
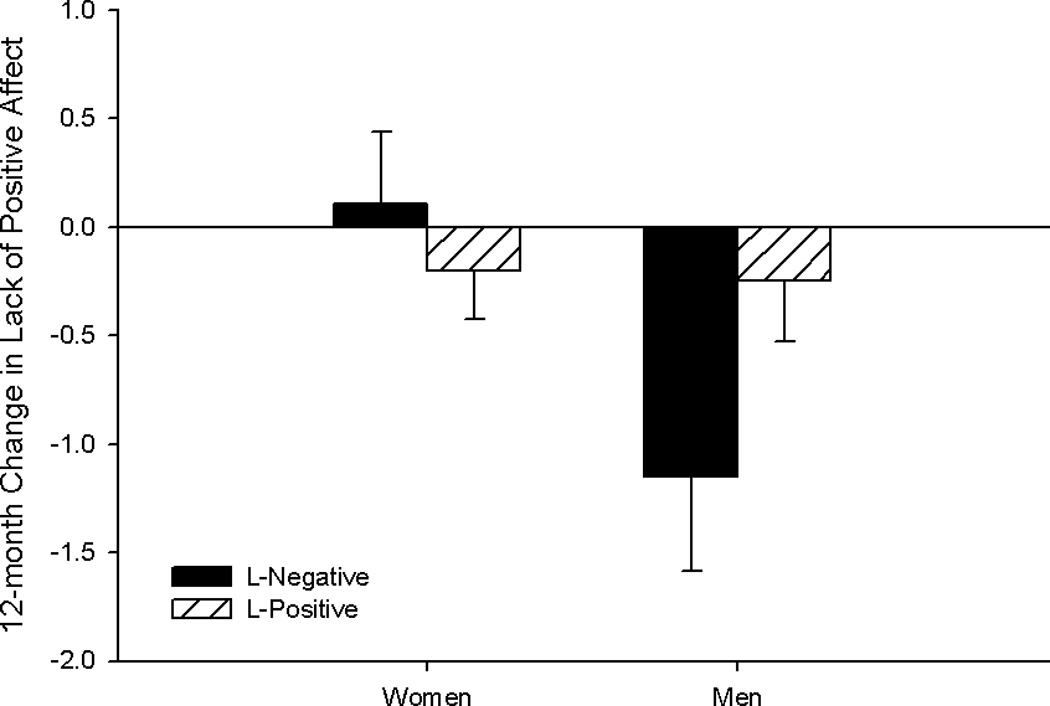
Least squares means for 12-month change in symptoms of lack of positive affect by 5-HTT genotype and sex.
No significant effects were found in the APOE analyses (ps ≥ .20).
DISCUSSION
Converging evidence suggests that PA improves mood, leads to depression remission, and protects against recurrence of depressive symptoms, and previous work has identified genetic variations that predict treatment response to antidepressant medication [3–5, 7–9]. It is less clear whether or not factors such as genetic variation and sex moderate the antidepressant effect of PA, or if PA differentially affects distinct symptom dimensions of depression in a nonclinical older population. The current study addressed these questions. We found a three-way relationship between intervention, BDNF genotype and sex for somatic symptoms of depression, suggesting that the impact of PA on depressive symptoms indeed varies by genotype, sex, and symptom dimension.
Our primary finding was a preferential benefit of PA on somatic depressive symptoms in male BDNF Met allele carriers. The impact of BDNF genotype on change in depressive symptoms after PA is consistent with our hypothesis and parallels the antidepressant literature. Although there are reports of no effect of BDNF on antidepressant response [26], recent meta-analyses have concluded that Met carriers have a more positive response to pharmacotherapy for depression [7]. Additionally, a recent study reported that Met-positive individuals had a more positive acute mood response to a bout of moderate intensity exercise relative to those without a Met allele [27]. Our finding is in contrast to a recent study that reported no moderating effect of BDNF on the relationship between physical activity and depressive symptoms [11]; however, the former study was based on self-report of PA in middle aged participants, unlike our randomized PA trial in older adults.
Taken together, it appears that for some individuals, PA, similar to antidepressant medication, may impact depressive symptoms through its effect on BDNF. This is not surprising given the functions of the BDNF protein in the nervous system. BDNF is a member of the neurotrophin family that has a critical role in central nervous system functions including cell survival and differentiation, axonal growth, and the function and plasticity of synapses [28]. BDNF is a neurotransmitter modulator that is highly expressed in the central nervous system, particularly in the hippocampus and other brain regions related to mood, such as the frontal lobes and striatum [29]. In depression, BDNF expression is reduced in areas such as the hippocampus and prefrontal cortex and increased in the nucleus acumbens and amygdala, but antidepressant treatment normalizes BDNF levels [30]. PA has also been shown to increase levels of BDNF [31], which may explain the antidepressant effect of PA. In light of the association between the BDNF Met allele and reduced BDNF secretion [32], it is plausible that Met carriers preferentially benefit from PA due to their inherent physiological disadvantage.
In the present study, the benefits of PA on depressive symptoms were specific to somatic symptoms; there was no impact of PA on total symptoms or on symptoms of depressed affect or lack of positive affect. A growing body of evidence supports the importance of examining symptom dimensions of depression separately rather than treating symptoms as homogeneous. Different symptom dimensions of depression appear to have distinct cognitive and neural correlates, etiological contributors, and genetic associations [12–15], and at least one study found that the impact of genotype on antidepressant treatment response is specific to particular symptom dimensions [33]. Given the physical nature of PA interventions, the specificity of our finding to somatic symptoms could be seen as an indication that PA simply improves physical functioning. However, the somatic subscale of the CES-D includes items such as “I had trouble keeping my mind on what I was doing” and “…everything I did was an effort,” which do not directly reflect physical functioning. Thus, the nature of the items comprising the subscale suggests that the improvements are not solely due to changes in physical functioning. Future work relating changes in depressive symptoms after PA to depression biomarkers may clarify the mechanism underlying the effect. For example, depressive symptoms, particularly in older adults, are associated with white matter changes in the brain. We recently showed that this may be driven by somatic symptoms and depressed affect, as only these factors were associated with greater white matter lesion volume in older men, and with increases in white matter lesion volume over time in both men and women [12]. PA improves white matter integrity [34], thus, it is plausible that PA -related changes in white matter results in reduced somatic symptoms of depression after PA.
We found that men, but not women, showed improvements in somatic symptoms with increases in PA. This is in line with previous findings of sex differences in depression risk and correlates, as well as response to depression treatment [12, 16, 17]. For example, there is evidence that BDNF genotype is associated with higher depression risk only in men or to a greater extent in men [11]. Previous work has also shown sex effects on the association of BDNF with antidepressant treatment response, but these studies found an effect only in women [35]. The reasons for our sex difference effect are unclear. The result is not explained by greater regression to the mean in men, since men and women did not differ in somatic symptom scores at baseline. A recent meta-analysis [36] concluded that the BDNF Val66Met polymorphism may play a larger role in the neurobiological underpinnings of depression in men than in women. This conclusion was based on evidence of sexual dimorphisms in brain structures involved in the neurobiology of depression, particularly the hippocampus [37]. The possibility that the biological underpinnings of depressive symptoms differ in men and women is supported by studies showing stronger and more consistent neural correlates of depression in men compared to women [e.g., 38]. Thus, men may benefit more from PA interventions if PA leads to change in underlying neurobiological mechanisms that are affected more in depressed men than in depressed women. Future work with larger samples will be important for replicating our finding, particularly given the fairly small number of men in our pilot study.
We did not find the expected effect of 5-HTT or APOE on the antidepressant effect of PA. Our hypothesis was based on evidence linking the 5-HTT L allele and APOE ε4 genotype with better response to treatment with antidepressant medication [7–9]. Additionally, 5-HTT has recently been shown to impact reductions in depressive symptoms after a 5-week exercise intervention in young adults [10]. However, the relationship between these genotypes and response to PA is not fully established, as the literature is limited and some studies failed to find these associations [39, 40]. Our ability to detect possible relations may have been affected by insufficient power or the low severity of depressive symptoms. There is also evidence that gene-gene interactions should be considered, particularly the interaction between 5-HTT and BDNF polymorphisms [41, 42]. We were unable to test possible gene-gene interactions in this pilot study due to the sample size. Future extensions of the present work in the full LIFE trial (n = 1635) will allow us to address this issue in a larger sample.
Our results should be considered in the context of the characteristics of the study sample. Participants in LIFE-P were sedentary older adults at risk for disability who ranged in age from 70–89 years and who were not recruited based on a depression diagnosis. The impact of genetic variation on changes in depressive symptoms after PA may differ in clinical samples of individuals diagnosed with major depression or in a different age cohort. Given the variety of genes that have been linked to depression risk and antidepressant treatment response, future studies should also examine the association of additional genotypes on changes in depressive symptoms after PA in subthreshold depression as well as major depression. Nonetheless, this pilot study provides initial evidence that the type of comprehensive PA intervention used in the LIFE-P trial may potentially impact the somatic domain of depressive symptoms in older men, even without a diagnosis of clinical depression. In addition, the results suggest that PA may be particularly impactful in mitigating somatic depressive symptomatology in those men with specific genetic markers, paving the way for future research aimed at targeting such subgroups of older adults for further investigation. This line of work will be important for establishing the clinical significance of changes in depressive symptoms after PA. Based on evidence that the presence of even one depressive symptom increases the risk for negative functional outcomes [43, 44], the 1- to 2-point changes in depressive symptoms in the current study are likely to be clinically meaningful. Thus, this work has the potential to inform targeted non-pharmacological interventions for subthreshold depression in older adults.
Acknowledgments
FUNDING
The LIFE-P study was supported by the National Institutes of Health/National Institute of Aging Cooperative Agreement (U01AG22376) and sponsored in part by the Intramural Research Program, National Institute for Aging, and National Institutes of Health. Dr. Dotson is partially supported by the UF Claude D. Pepper Center (NIA P30 AG028740-01). The Pittsburgh Field Center was partially supported by the Pittsburgh Claude D. Pepper Center P30 AG024827. The Wake Forest University Field Center is, in part, supported by the Claude D. Older American Independence Pepper Center #1 P30 AG21332. Yale University Thomas M. Gill, M.D. Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
The LIFE Study: Lifestyle Interventions and Independence for Elders, Pilot http://clinicaltrials.gov/show/NCT00116194
Research Investigators for Pilot Phase of LIFE: Cooper Institute, Dallas, TX: Steven N. Blair, P.E.D. – Field Center Principal Investigator, Timothy Church, M.D., Ph.D., M.P.H. – Field Center Co-Principal Investigator, Jamile A. Ashmore, Ph.D. Judy Dubreuil, M.S. Georita Frierson, Ph.D. Alexander N. Jordan, M.S., Gina Morss, M.A. Ruben Q. Rodarte, M.S. Jason M. Wallace, M.P.H. National Institute on Aging: Jack M. Guralnik, M.D., Ph.D. – Co-Principal Investigator of the Study: Evan C. Hadley, M.D. Sergei Romashkan, M.D., Ph.D. Stanford University, Palo Alto, CA Abby C. King, Ph.D. – Field Center Principal Investigator: William L. Haskell, Ph.D. – Field Center Co-Principal Investigator: Leslie A. Pruitt, Ph.D. Kari Abbott-Pilolla, M.S. Karen Bolen, M.S. Stephen Fortmann, M.D. Ami Laws, M.D. Carolyn Prosak, R.D. Kristin Wallace, M.P.H. Tufts University: Roger Fielding, Ph.D. Miriam Nelson, Ph.D. Dr. Fielding's contribution is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-4-401. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture. University of California, Los Angeles, Los Angeles, CA Robert M. Kaplan, Ph.D., M.A. VA San Diego Healthcare System and University of California, San Diego, San Diego, CA Erik J. Groessl, Ph.D. University of Florida, Gainesville, FL Marco Pahor, M.D. – Principal Investigator of the Study Michael Perri, Ph.D. Connie Caudle Lauren Crump, M.P.H Sarah Hayden Latonia Holmes Cinzia Maraldi, M.D. Crystal Quirin University of Pittsburgh, Pittsburgh, PA Anne B. Newman, M.D., M.P.H. – Field Center Principal Investigator Stephanie Studenski, M.D., M.P.H. – Field Center Co-Principal Investigator Bret H. Goodpaster, Ph.D., M.S. Nancy W. Glynn, Ph.D. Erin K. Aiken, B.S. Steve Anthony, M.S. Sarah Beck (for recruitment papers only) Judith Kadosh, B.S.N., R.N. Piera Kost, B.A. Mark Newman, M.S. Jennifer Rush, M.P.H. (for recruitment papers only) Roberta Spanos (for recruitment papers only) Christopher A. Taylor, B.S. Pam Vincent, C.M.A. Diane Ives, M.P.H Wake Forest University, Winston-Salem, NC Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator Peter Brubaker, Ph.D. Jamehl Demons, M.D. Curt Furberg, M.D., Ph.D. Jeffrey A. Katula, Ph.D., M.A. Anthony Marsh, Ph.D. Barbara J. Nicklas, Ph.D. Jeff D. Williamson, M.D., M.P.H. Rose Fries, L.P.M. Kimberly Kennedy Karin M. Murphy, B.S., M.T. (ASCP) Shruti Nagaria, M.S. Katie Wickley-Krupel, M.S. Data Management, Analysis and Quality Control Center (DMAQC) Michael E. Miller, Ph.D. – DMAQC Principal Investigator Mark Espeland, Ph.D. – DMAQC Co-Principal Investigator Fang-Chi Hsu, Ph.D. Walter J. Rejeski, Ph.D. Don P. Babcock, Jr., P.E. Lorraine Costanza Lea N. Harvin Lisa Kaltenbach, M.S. Wei Lang, Ph.D. Wesley A. Roberson Julia Rushing, M.S. Scott Rushing Michael P. Walkup, M.S.
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- 1.Tedeschini E, Levkovitz Y, Iovieno N, et al. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(12):1660–1668. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- 2.Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. Exercise Interventions for Mental Health: A Quantitative and Qualitative Review. Clinical Psychology: Science and Practice. 2006;13(2):179–193. [Google Scholar]
- 4.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Matthews MM, Hsu FC, Walkup MP, et al. Depressive symptoms and physical performance in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2011;59(3):495–500. doi: 10.1111/j.1532-5415.2011.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15(5):473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GM, Kremer C, Rodrigues H, Schatzberg AF G Mitrazapine versus paroxetine Study. The apolipoprotein E epsilon4 allele and antidepressant efficacy in cognitively intact elderly depressed patients. Biol Psychiatry. 2003;54(7):665–673. doi: 10.1016/s0006-3223(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 9.Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Rethorst CD, Landers DM, Nagoshi CT, Ross JT. Efficacy of exercise in reducing depressive symptoms across 5-HTTLPR genotypes. Med Sci Sports Exerc. 2010;42(11):2141–2147. doi: 10.1249/MSS.0b013e3181de7d51. [DOI] [PubMed] [Google Scholar]
- 11.Gujral S, Manuck SB, Ferrell RE, Flory JD, Erickson KI. The BDNF Val66Met polymorphism does not moderate the effect of self-reported physical activity on depressive symptoms in midlife. Psychiatry Res. 2014;218(1–2):93–97. doi: 10.1016/j.psychres.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirton JW, Resnick SM, Davatzikos C, Kraut MA, Dotson VM. Depressive Symptoms, Symptom Dimensions, and White Matter Lesion Volume in Older Adults: A Longitudinal Study. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korten NC, Penninx BW, Kok RM, et al. Heterogeneity of late-life depression: relationship with cognitive functioning. Int Psychogeriatr. 2014;26(6):953–963. doi: 10.1017/S1041610214000155. [DOI] [PubMed] [Google Scholar]
- 14.Naarding P, Schoevers RA, Janzing JG, et al. A study on symptom profiles of late-life depression: the influence of vascular, degenerative and inflammatory risk-indicators. J Affect Disord. 2005;88(2):155–162. doi: 10.1016/j.jad.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Korszun A, Moskvina V, Brewster S, et al. Familiality of symptom dimensions in depression. Arch Gen Psychiatry. 2004;61(5):468–474. doi: 10.1001/archpsyc.61.5.468. [DOI] [PubMed] [Google Scholar]
- 16.Brummett BH, Boyle SH, Siegler IC, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38(1):34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan CO, Kearney PM, Savva GM, Cronin H, Kenny RA. Age and sex differences in prevalence and clinical correlates of depression: first results from the Irish Longitudinal Study on Ageing. Int J Geriatr Psychiatry. 2013;28(12):1280–1287. doi: 10.1002/gps.3955. [DOI] [PubMed] [Google Scholar]
- 18.Bhui K, Fletcher A. Common mood and anxiety states: gender differences in the protective effect of physical activity. Soc Psychiatry Psychiatr Epidemiol. 2000;35(1):28–35. doi: 10.1007/s001270050005. [DOI] [PubMed] [Google Scholar]
- 19.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 20.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26(2):141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. pp. viii–**104. [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 24.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 25.Langaee T, Ronaghi M. Genetic variation analyses by Pyrosequencing. Mutat Res. 2005;573(1–2):96–102. doi: 10.1016/j.mrfmmm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Illi A, Viikki M, Poutanen O, et al. No support for a role for BDNF gene polymorphisms rs11030101 and rs61888800 in major depressive disorder or antidepressant response in patients of Finnish origin. Psychiatr Genet. 2013;23(1):33–35. doi: 10.1097/YPG.0b013e3283586308. [DOI] [PubMed] [Google Scholar]
- 27.Bryan A, Hutchison KE, Seals DR, Allen DL. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychol. 2007;26(1):30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26(4):511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Rybakowski JK. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9(11):1589–1593. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- 33.Huezo-Diaz P, Uher R, Smith R, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195(1):30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- 34.Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34(11):2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domschke K, Lawford B, Laje G, et al. Brain-derived neurotrophic factor (BDNF) gene: no major impact on antidepressant treatment response. Int J Neuropsychopharmacol. 2010;13(1):93–101. doi: 10.1017/S1461145709000030. [DOI] [PubMed] [Google Scholar]
- 36.Verhagen M, van der Meij A, van Deurzen PA, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 37.Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45(4):275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 38.Lavretsky H, Kurbanyan K, Ballmaier M, et al. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12(6):653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- 39.Poland RE, Lesser IM, Wan YJ, et al. Response to citalopram is not associated with SLC6A4 genotype in African-Americans and Caucasians with major depression. Life Sci. 2013;92(20–21):967–970. doi: 10.1016/j.lfs.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor MJ, Sen S, Bhagwagar Z. Antidepressant response and the serotonin transporter gene-linked polymorphic region. Biol Psychiatry. 2010;68(6):536–543. doi: 10.1016/j.biopsych.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33(1):73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 42.Lenze E, Rawsom K, Dixon D, et al. An interaction between polymorphisms in brain-derived neurotrophic factor and serotonin transporter genes predicts depressive symptoms in older adults after hip fracture. The American Journal of Geriatric Psychiatry. 22(3):S108–S109. [Google Scholar]
- 43.Judd LL, Akiskal HS, Paulus MP. The role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorder. J Affect Disord. 1997;45(1–2):5–17. doi: 10.1016/s0165-0327(97)00055-4. discussion 17–8. [DOI] [PubMed] [Google Scholar]
- 44.Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med. 2012;42(4):683–693. doi: 10.1017/S0033291711001784. [DOI] [PubMed] [Google Scholar]


