Abstract
Objective
To assess the effects of supervised and home-based aerobic exercise training, and antidepressant pharmacotherapy (sertraline) on coronary heart disease (CHD) risk factors in a sample of participants with major depressive disorder (MDD).
Methods
The SMILE-II study randomized 202 adults (153 women; 49 men) diagnosed with MDD to one of four interventions, each of 4 months duration: supervised exercise; home-based exercise; antidepressant medication (sertraline, 50–200 mg daily); or placebo pill. Patients underwent a structured clinical interview for depression and completed the Hamilton Depression Rating Scale (HAM-D). CHD risk factors included brachial artery flow-mediated dilation (FMD), carotid intima-media thickness (IMT), serum lipids, and 10-year Atherosclerotic Cardiovascular Disease (ASCVD) risk.
Results
Compared to placebo, active treatment for depression (supervised exercise, home-based exercise, sertraline therapy) was associated with an improvement in CHD risk factors (improved FMD (P=.032), reduced progression of IMT (P=.037), and a reduction in 10-year ASCVD (P=.049)). The active treatments did not differ from each other in their effects on the CHD risk outcomes.
Conclusions
Both exercise and antidepressant medication improved CHD risk factors and lowered ASCVD risk in patients with MDD. Because MDD is associated with increased risk for CHD events, treatment of depression with exercise or sertraline may reduce the risk of developing CHD in patients with MDD.
Keywords: depression, exercise, antidepressant medication, atherosclerosis, carotid intima media thickness, flow-mediated dilation
INTRODUCTION
The presence of depression in otherwise healthy individuals confers about a 1.5 to 2.0-fold adjusted relative risk for the subsequent development of coronary artery disease (1–4) and is prospectively associated with a 2- to 4-fold higher relative risk for cardiac mortality (5) and non-fatal acute myocardial infarction (3). In patients with established coronary heart disease (CHD), depression is associated with a 2 to 4-fold increased risk for adverse cardiac events, including fatal and non-fatal myocardial infarction (MI) and all-cause mortality(6–9) In a comprehensive review of the extant literature, the American Heart Association Scientific Advisory Panel recently recommended that depression be considered a risk factor for patients with CHD (10). Despite the clinical relevance of depression in healthy adults and in patients with established CHD, the value of treating depression in cardiac patients has produced mixed results. The ENRICHD trial reported modest improvements in depressive symptoms relative to usual care controls in a sample of over 2500 post-myocardial infarction patients, but there was no survival benefit (11). The SADHART trial found that CHD patients with MDD randomized to sertraline had greater reductions in depressive symptoms compared to placebo controls, but only in a subgroup of patients with more severe MDD (12). However, in a study of depressed heart failure patients, sertraline did not perform any better than placebo, and there was no survival advantage for sertraline compared to placebo (13).
Recently, exercise training has received widespread attention for successfully treating depression in otherwise healthy individuals with MDD (14–17) and in patients with stable CHD (18) and heart failure (19). Exercise also may improve clinical outcomes in patients with depression. For example, a post hoc analysis of the ENRICHD trial revealed that patients who exercised during the 6-months following their myocardial infarctions not only had reduced depression but also had a 50% improvement in all-cause mortality risk (20). Similarly, the HF-ACTION trial reported that exercise reduced depressive symptoms and improved survival (19, 21). The SMILE-II study was a randomized clinical trial comparing exercise and sertraline to placebo in a sample of 202 outpatients with MDD without documented CHD. Primary results of the trial, which showed significant and comparable improvements in depression for exercise and sertraline relative to placebo controls, have been previously reported (16).
The present report describes the effects of exercise and sertraline on CHD risk factors in this sample of adults with MDD without known CHD. Two specific risk factors related to subclinical atherosclerosis were examined: brachial artery flow-mediated dilation (FMD), and carotid artery intima-media thickness (IMT), both of which are prognostic of adverse cardiovascular outcomes (22, 23). In addition, we assessed intervention effects on the estimated 10-year Atherosclerotic Cardiovascular Disease (ASCVD) risk using the recently developed Pooled Cohort Equations (24). We hypothesized that both exercise and sertraline would result in improved CHD risk compared to placebo controls, and that exercise would produce greater reductions in subclinical atherosclerosis compared to sertraline.
METHODS
Experimental Design
As described in our initial report (9) participants were recruited between October 2000 and November 2005. In addition to the presence of MDD, eligibility criteria included age ≥40 years, not currently exercising, and no current psychiatric treatment. Exclusion criteria included the presence of another primary psychiatric diagnosis, such as a history of bipolar disorder or psychosis; and medical comorbidities that would preclude participation in the trial. All participants provided written informed consent and the protocol was approved by the Institutional Review Board at Duke University Medical Center. This study was a randomized, parallel group, placebo-controlled trial of exercise (group-supervised and individual home-based), and sertraline treatment for MDD. CHD biomarker data were collected before and following 4 months of treatment.
Treatment Groups
Participants were assigned randomly in equal proportions to a 4-month intervention of supervised aerobic exercise (n =51), home-based aerobic exercise (n = 53), sertraline (n = 49), or placebo (n = 49). Randomization was performed centrally by computer with conditional randomization (stratified by age, sex, and depression severity).
Exercise Conditions
Patients in the Supervised Aerobic Exercise condition attended three supervised group exercise sessions per week and exercised at 70% to 85% maximum heart rate reserve for 30 minutes of walking or jogging on a treadmill. Participants in the Home-based Exercise program received the same individualized exercise prescription but exercised at home, on their own, with minimal staff contact; participants received visits from an exercise physiologist after 1 month and 2 months, and brief weekly telephone calls for the first month and biweekly thereafter until the end of the 16-week trial.
Pill Conditions
Participants in the “pill” conditions were given sertraline, or matching placebo, provided by Pfizer, Inc. The dosage depended on the clinical response, but usually each patient received a starting dose of 50 mg (one pill) daily with increasing doses up to 200 mg (four pills) daily, based on therapeutic response and presence of side effects. The treating psychiatrist was blinded to pill condition and used supportive measures to help manage medication side effects.
Aerobic Fitness
Exercise-Treadmill Testing
Graded treadmill exercise testing was conducted before and after the 4-month treatment program to: a) establish an initial exercise prescription for participants randomized to exercise, and; b) to document changes in patients’ aerobic fitness. Patients exercised to exhaustion under continuous electrocardiographic monitoring in which workloads were increased at a rate of 1 metabolic equivalent/min (25). Expired gases were analyzed by a Parvo Medics True One measurement system (Parvo Medics, Sandy, Utah). Peak VO2 served as the primary measure of aerobic fitness.
CHD Risk Assessments
Flow Mediated Dilation (FMD) of the Brachial Artery
FMD of the brachial artery was assessed in the morning, after overnight fasting. Longitudinal B-mode ultrasound images of the brachial artery, 4 to 6 cm proximal to the antecubital crease, were obtained using an ultrasound platform (Aspen, Mountain View, CA) with a 10-MHz linear array transducer. Images were obtained after 10 minutes of supine relaxation and during reactive hyperemia, induced by 5 minutes of inflation of a pneumatic occlusion cuff placed around the forearm to a supra-systolic pressure (≥200 mm Hg). End-diastolic images were stored to a magnetic-optical disk and arterial diameters were measured as the distance between the proximal and distal arterial wall intima-media interfaces, using PC-based software (Brachial Analyzer Version 4.0, Medical Imaging Applications LLC, Iowa City, IA). Peak FMD response was assessed from 10 to 120 seconds post deflation of the cuff, with peak arterial diameter quantified using polynomial curve fitting. FMD was defined as the maximum percent change in arterial diameter relative to resting baseline
Intima Medial Thickness (IMT) in Carotid Arteries
Carotid artery IMT was assessed by a high-resolution B-mode ultrasound vascular imaging system (Acuson Aspen, Mountain View, CA) with a 10-Mhz linear array transducer. Ultrasound examinations of the far wall of the left and right common carotid arteries (CCAs) were used to acquire longitudinal images spanning 2 cm proximal to the carotid bulb. IMT of the far wall of the left and right CCAs was measured over a 1-cm segment using edge detection software (Carotid Analyzer 5.0.5, Medical Imaging Applications LLC, Iowa City, IA).
Atherosclerotic Disease Risk Scores
Cardiovascular risk scores were calculated using the Pooled Cohort Risk Assessment Equations recently developed by the American College of Cardiology/American Heart Association Risk Assessment Work Group (24). These equations quantify the 10-year risk of Atherosclerotic Cardiovascular Disease (ASCVD), defined as nonfatal myocardial infarction, coronary heart disease death, or fatal or non-fatal stroke. Scores are determined based on risk algorithms using systolic blood pressure, use of antihypertensive therapy, diabetes mellitus, cigarette smoking, total cholesterol, and high density lipoprotein (HDL) cholesterol. Blood pressure was measured by a Physician’s Assistant during a history and physical examination that also was the basis for determining medication use and presence of diabetes. The average of two seated blood pressure measurements was used in the present analyses. Blood samples were obtained following an overnight fast. Total cholesterol, HDL cholesterol and low-density lipoprotein (LDL) cholesterol were measured enzymatically (LabCorp, Burlington, NC). HDL cholesterol was determined by assay of the supernatant remaining after precipitation of serum LDL with dextran sulfate plus magnesium chloride. Risk scores are determined separately by sex and ethnicity (Caucasian or African-American). For the purposes of the present analyses, the ASCVD scores for all minority participants were determined using risk algorithms for African-Americans.
Statistical Analysis
Treatment group effects for CHD risk factors were evaluated using general linear models through SAS 9.2 (PROC GLM, SAS Institute, Cary, NC). For our primary analysis of changes in CHD risk factors, a rank-based composite was created combining IMT, FMD, and CVD risk scores. The composite measure was created by ranking each individual on each biomarker at baseline and following treatment. A mean rank score was then created by averaging across all CHD risk markers at pre-treatment and at post-treatment. At each time-point the mean rank was then standardized using a midrank specification (i.e. mean rank divided by sample size plus one). The post-treatment midrank was the outcome variable in our primary analysis (26). Analysis of treatment effects followed the intention-to-treat (ITT) principle, with post-treatment missing data managed using Markov chain Monte Carlo multiple imputation methods available in SAS (PROC MI) and 150 imputations. After examining change in the composite measure, we conducted follow-up analyses of each CHD risk marker individually. The ASCVD risk score was found to be significantly skewed and was therefore rank-transformed prior to analysis, whereas no transformation was required for either IMT or FMD. Improvements in depression were examined using a logistic model in which post-treatment remission (defined as no longer meeting criteria for MDD and achieving a HAM-D rating of < 8) was modeled as the outcome of interest (PROC GENMOD). As reported in our primary paper, we eliminated individual “early responders” and considered individuals without post-treatment depression as remitted (16). Within all models we controlled baseline randomization stratification factors (age, sex, pretreatment depression severity (HAM-D≥18), and the respective pre-treatment level of each outcome variable. We also controlled for baseline arterial diameter in our analyses of FMD. Planned, orthogonal contrasts were conducted comparing all three active treatment groups vs. placebo, the two exercise groups vs. sertraline, and the supervised and home-based exercise programs. We evaluated the extent to which models met assumptions, including additivity, linearity, and distribution of residuals. We found no evidence of significant violations of these assumptions.
RESULTS
Participants
Four hundred fifty-seven participants were screened, of whom 202 were randomized. Among individuals who were excluded (n = 255), 135 did not meet inclusion criteria, 47 withdrew consent, 40 had significant psychiatric comorbidities, 13 had contraindications to exercise, 15 had poor assessment adherence, 3 individuals had contraindications to Sertraline, one participant was already exercising, and one was actively involved in psychiatric treatment. Participants were generally middle-aged and Caucasian, with relatively few CHD risk factors. Few participants were taking either antihypertensive or lipid lowering medications. Treatment groups did not differ in any background CHD risk factor characteristics at baseline (see Table 1).
Table 1.
Demographic, Clinical and Physiological Characteristics at Pre-Randomization Baseline
| Variable | Home-based (n = 53) | Supervised (n = 51) | Sertraline (n = 49) | Placebo (n = 49) | Cohort (n = 202) | P-value |
|---|---|---|---|---|---|---|
| Age, years | 52.8 (7.9) | 51.1 (7.0) | 51.8 (7.7) | 51.2 (7.8) | 51.7 (7.6) | .386 |
| Sex, female, n (%) | 39 (74) | 39 (76) | 37 (76) | 38 (78) | 153 (76) | .980 |
| Black, n (%) | 14 (26) | 12 (24) | 12 (25) | 14 (29) | 52 (26) | .802 |
| Caucasian, n (%) | 35 (66) | 36 (71) | 35 (71) | 31 (63) | 137 (68) | |
| Other Ethnicity, n (%) | 4 (8) | 3 (5) | 2 (4) | 4 (8) | 13 (6) | |
| Antihypertensive Medications, n (%) | 10 (19) | 11 (22) | 11 (22) | 15 (31) | 47 (32) | .487 |
| Diabetes, n (%) | 4 (8) | 3 (6) | 3 (6) | 4 (8) | 14 (7) | .895 |
| Current Tobacco Use, n (%) | 8 (15) | 7 (14) | 9 (18) | 8 (16) | 32 (16) | .756 |
| Systolic Blood Pressure, mm Hg | 122 (16) | 122 (17) | 126 (19) | 127 (18) | 124 (17) | .270 |
| Diastolic Blood Pressure, mm Hg | 78 (9) | 79 (9) | 81 (10) | 80 (9) | 79 (9) | .364 |
| Lipid-Lowering Medications, n (%) | 6 (11) | 1 (2) | 4 (8) | 7 (14) | 19 (9) | .067 |
| Total Cholesterol, ng/ml | 204 (36) | 207 (48) | 210 (40) | 209 (37) | 207 (40) | .973 |
| High Density Lipoprotein, ng/ml | 56 (13) | 55 (14) | 58 (21) | 58 (15) | 57 (16) | .820 |
| Low Density Lipoprotein, ng/ml | 123 (31) | 118 (31) | 124 (33) | 124 (37) | 122 (34) | .831 |
| Flow-Mediated Dilation, % | 5.3 (3.6) | 6.0 (4.3) | 6.1 (4.9) | 6.3 (4.5) | 5.9 (4.3) | .361 |
| Intima Medial Thickness, mm | 0.64 (0.15) | 0.60 (0.11) | 0.64 (0.13) | 0.64 (0.13) | 0.63 (0.13) | .341 |
| ASCVD Risk Score, 10-year % Risk | 5.0 (7.1) | 3.9 (5.5) | 5.4 (6.9) | 4.5 (4.7) | 4.7 (6.1) | .751 |
Treatment group differences were assessed using general linear models for continuous variables and chi-square tests for categorical variables.
Adherence and Treatment Fidelity
Thirty-four participants dropped out during the course of the trial (3 in home-based exercise, 10 in supervised exercise, 7 in sertraline, and 14 in Placebo). Dropouts did not differ from completers on any clinical or demographic characteristics, or in pre-treatment CHD biomarker levels. As reported in the primary paper, adherence to medication was evaluated by pill count and was excellent for sertraline and placebo groups, with an average of 89% (SD = 24.6) pill adherence in the sertraline group and 88% (SD = 29.6) in the placebo group. When dropouts were eliminated, adherence was 95% (SD = 8.4) in the sertraline group and 97% (SD = 8.1) in the placebo group.
Exercise adherence also was excellent, with supervised and home-based exercisers completing 70% and 79% of scheduled sessions, respectively, excluding dropouts. Individuals in the home-based exercise group reported exercising in more sessions compared with supervised exercise (36.9 [14.1] vs. 28.5 [15.5], P = .005). However, the supervised group exhibited higher average heart rates (138.6 beats per minute [SD = 12.1]) during exercise sessions and reported higher ratings of perceived exertion (13.4 [SD = 1.4]) relative to the home-based group (131.6 beats per minute [SD = 16.9], 12.8 [1.5]; P = .025, P = .091, respectively).
Cardiopulmonary Training Effects
As previously reported (16), greater improvements in peak oxygen consumption (peak VO2) were observed in the active treatment groups compared to the placebo group (P < .001) and the exercise groups exhibited larger improvements compared to the sertraline group (P < .001). A similar pattern of results was noted for exercise treadmill duration, with the supervised exercise (1.8 minutes [1.4, 2.3]) and home-based exercise (0.9 minutes [0.4, 1.3]) groups showing the greatest improvements, whereas the sertraline (−0.20 minutes [−0.6, 0.3]) and placebo (−0.32 minutes [−0.8, 0.2]) groups showed slight decreases in treadmill time.
CHD Risk Changes
Examination of treatment changes in CHD risk factors revealed that the three active treatment groups showed greater improvements on the composite measure of CHD risk compared to placebo (P = .001), whereas the exercise and sertraline groups did not differ from each other (P = .896) (Table 2). Specifically, the supervised exercise group (post-treatment midrank 0.52 [0.48, 0.55]) and sertraline group (post-treatment midrank 0.52 [0.48, 0.55]) demonstrated the greatest improvements on our composite CHD biomarker measure, followed by the home-based exercise group (post-treatment midrank 0.47 [0.44, 0.51]) and placebo group (post-treatment midrank 0.44 [0.42, 0.48]). Having established evidence of an overall treatment effect, we proceeded to examine the component measures comprising the composite CHD biomarker.
Table 2.
Post-treatment values for IMT, FMD and ASCVD risk (and values related to its computation).
| Variable | Home-based (n = 53) | Supervised (n = 51) | Sertraline (n = 49) | Placebo (n = 49) | Active vs. Placebo | Exercise vs. Sertraline |
|---|---|---|---|---|---|---|
| Intima Medial Thickness, mm * | 0.65 (0.01) | 0.64 (0.01) | 0.63 (0.01) | 0.67 (0.01) | .037 | .291 |
| Flow-Mediated Dilation, % * | 5.8 (0.5) | 6.9 (0.6) | 6.2 (0.6) | 4.8 (0.6) | .032 | .798 |
| ASCVD Risk Score, 10-year % Risk (ranked) * | 4.6 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 4.3 (0.3) | .049 | .741 |
| Systolic Blood Pressure, mm Hg* | 122 (2) | 118 (2) | 117 (2) | 124 (2) | .012 | .245 |
| Total Cholesterol, ng/ml | 209 (4) | 204 (4) | 215 (4) | 203 (4) | .134 | .047 |
| High Density Lipoprotein, ng/ml | 55 (1) | 57 (1) | 55 (1) | 56 (1) | .894 | .534 |
Analyses are adjusted for age, sex, severity of depression, and the respective pretreatment level of each outcome. FMD analyses were also adjusted for baseline arterial diameter.
Active treatments differ from Placebo at P < .05. Values are derived from multiple imputation estimates based on the entire sample (n = 202). Complete data were available for 169 participants for the ASCVD risk score and its components (84%), 172 participants for flow-mediated dilation analyses (85%), and 168 for intima medial thickness analyses (83%).
Flow Mediated Dilation
Examination of treatment changes showed that the active treatment groups exhibited greater improvements in FMD over the course of the intervention compared with placebo participants FMD (P = .032). The exercise and sertraline groups did not differ from one another (P = .798). Examination of changes in FMD showed that the supervised exercise (1.02% [−0.15, 2.20]) and sertraline (0.30% [−0.80, 1.40]) groups showed improvements, whereas the home-based exercise (−0.11% [−1.12, 0.90]) and placebo (−1.08% [−2.23, 0.07]) groups showed reductions in FMD (Table 2).
Intima Media Thickness
Participants in the exercise and sertraline groups exhibited lower post-treatment IMT compared with participants in the placebo group (P = .037). Examination of IMT changes demonstrated that all groups experienced a small increase in IMT from pre- to post-treatment, although the progression of IMT was smaller in the active treatment groups. Home-based (0.02 mm [0.00, 0.05]) and supervised (0.02 mm [−0.01, 0.04]) exercise groups exhibited small increases in IMT, whereas the IMT level for the sertraline group remained essentially unchanged (0.00 mm [−0.03, 0.02]); the placebo group exhibited a relatively greater progression post-treatment (0.04 mm [0.01, 0.07]).
In addition to the main effects of treatment, we observed a treatment group by baseline IMT interaction (P = .025). Examination of this interaction showed that individuals in the active treatment groups with higher levels of IMT at baseline experienced larger reductions in IMT over the course of the intervention; this pattern was especially pronounced in the exercise groups (Figure 2). We observed a moderate association between baseline IMT and changes in IMT from pre- to post-treatment in the exercise groups (r = −0.37, P < .001), suggesting that higher baseline IMT was associated with larger reductions with treatment. In contrast, baseline IMT was unrelated to treatment changes in the placebo group (r = 0.03, P = .876).
Figure 2.
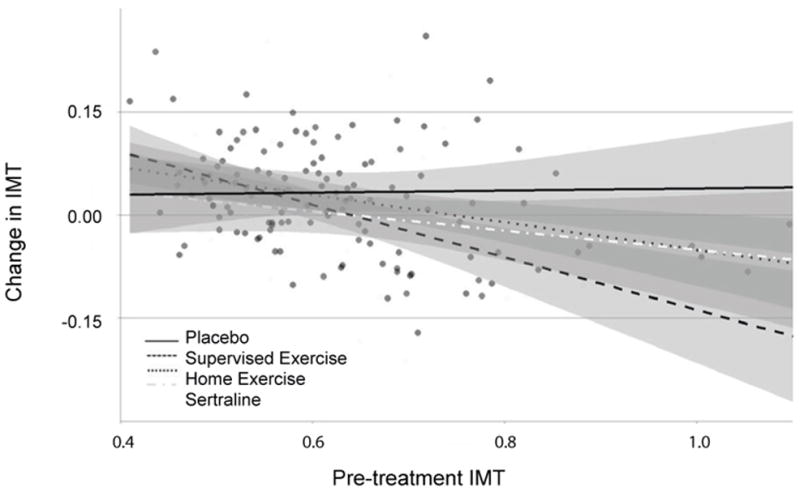
Treatment changes by treatment group in Intima-Media Thickness (IMT) in relation to baseline IMT.
Exploratory analyses of changes in IMT among individuals with higher IMT at baseline (the highest quartile) revealed small IMT reductions in the home-based (−0.04 mm [−0.07, 0.03]) and supervised (−0.06 mm [−0.13, 0.01]) exercise groups, as well as the sertraline group (−0.02 mm [−0.07, 0.04]), whereas the placebo group exhibited a small increase in IMT (0.04 mm [−0.01, 0.10]).
Atherosclerotic Cardiovascular Disease (ASCVD) Risk Scores
Examination of changes in 10-year ASCVD risk revealed that individuals in the active treatment groups experienced greater reductions in ASCVD risk compared with placebo participants (P = .049). Although the exercise and sertraline groups did not differ (P = .741), the supervised exercise group exhibited larger reductions in ASCVD risk compared to the other treatment groups, including home-based exercise (P = .010). Individuals in the supervised exercise group (0.57% [−0.01, 1.15]) and the sertraline (0.61% [0.01, 1.2]) groups showed larger reductions in CVD risk compared to the home-based (0.06% [−0.46, 0.59]) and the placebo (0.35% [−0.21, 0.92]) groups. The impact of treatment on CHD risk factors reported above corresponded to a small to moderate effect size difference between treatment groups and controls (d = 0.29).
Analysis of individual ASCVD components demonstrated that all treatment groups showed improvements in SBP relative to the placebo group (P = .033). Individuals in the supervised exercise (−6.0 mmHg [−10.1, −1.8]), home-base exercise (−2.4 mmHg [−6.4, 1.6]), and Sertraline group (−7.3 mmHg [−11.6, −2.2]) all exhibited reductions in SBP, whereas the placebo group exhibited a minimal increase in SBP (0.3 mmHg [−4.7, 4.1]). In contrast, the active treatment groups did not demonstrate improvements in HDL cholesterol (P = .545) or total cholesterol (P = .159). However, examination of pre-planned contrasts demonstrated that the Sertraline group exhibited a reduction in total cholesterol (P = .026) relative to the exercise groups, although the groups did not differ in post-treatment HDL (P = .772). The supervised and home-based exercise groups did not differ on any of the individual ASCVD components (Ps > .175).
DISCUSSION
Our findings show that both exercise and sertraline are not only effective interventions for ameliorating symptoms of depression in patients with MDD (16), but also result in notable improvements in CHD risk factors. Overall, compared to placebo, both sertraline and exercise resulted in improved vascular endothelial function (brachial artery FMD), reduced progression of atherosclerosis (carotid IMT), and lowered the 10-year risk of developing atherosclerotic cardiovascular disease (AVSCD risk). Additionally, aerobic fitness improved significantly following treatment for participants randomized to both exercise conditions relative to the pill conditions, with supervised exercise enhancing aerobic fitness to a greater extent than home-based exercise.
Vascular endothelial dysfunction plays a vital role in the development, progression, and clinical manifestations of atherosclerosis (27–31). Therefore, improvements in FMD accompanying the successful treatment of depression could reduce patients’ CHD risk burden. However, the SADHART-CHF trial of patients with heart failure and MDD (13) did not demonstrate improved survival or reduced cardiovascular events in patients randomized to sertraline. The SADHART trial of patients with CHD and MDD (7) was not powered to evaluate the effects of treatment on clinical outcomes. Neither of the SADHART studies assessed FMD. Pooled data from clinical trials enrolling CHD patients participating in cardiac rehabilitation programs show that exercise reduces the risk of all-cause mortality and fatal CHD events by up to 25%. (32–35). To our knowledge, no studies have examined the extent to which improvements in FMD following exercise-based cardiac rehabilitation may contribute to more favorable clinical outcomes. The antidepressant effects of exercise training may also account for the survival benefits of cardiac rehabilitation for some patients. In a study of 522 consecutive coronary patients enrolled in cardiac rehabilitation, patients exhibited a 63% reduction in depressive symptoms following completion of the program, and depressed patients who completed the program had a 73% lower mortality than depressed patients who failed to complete the program. In a secondary analysis of the HF-ACTION trial (21), exercise training resulted in a reduction in depressive symptoms and improved clinical outcomes compared to usual care (19). It is possible that improved outcomes following exercise training are due, in part, to improvements in both depression and vascular function, as well as to other physiological and psychosocial benefits that may result from participation in cardiac rehabilitation.
Carotid IMT is a marker of atherosclerosis that is a strong predictor of CVD events, and is being used increasingly for CVD risk stratification; with a 0.1 mm increase in IMT the future risk of MI increases by 10% to 15%, and stroke risk increases by 13% to 18% (36). Our observations suggest that both exercise and sertraline may slow the progression of atherosclerosis, as indexed by changes in carotid artery IMT. To our knowledge, this is the first study to have reported beneficial effects of sertraline treatment on IMT, while the evidence of an association between exercise and IMT has been equivocal. Most cross-sectional studies indicate increased IMT in regular exercisers (37), but interventional studies of exercise and IMT have been mixed (38). Interestingly, there is growing evidence that a reduction in arterial wall thickness may be more likely to result from exercise in individuals with pre-existing arterial wall thickening (39). Our finding that exercise resulted in improvements in carotid IMT, especially in MDD patients with higher baseline IMT, is consistent with this more recent evidence. These data suggest that exercise may attenuate the progression of atherosclerosis especially among individuals with higher levels of subclinical atherosclerosis or with established CHD. In summary, these results suggest that SSRI antidepressant medication and exercise interventions are beneficial not only for reducing depressive symptoms, but also for reducing CHD risk factors. Moreover, our findings underscore the potential value of supervised exercise as a useful alternative approach to pharmacotherapy for treating patients with MDD, with both approaches potentially resulting in broader health benefits that may be especially helpful for patients at high ASCVD risk, or with documented CHD. Further research is needed to elucidate the mechanisms by which treatments for depression may yield both physical and mental health benefits, as well as improve clinical outcomes in cardiac patients.
Figure 1.
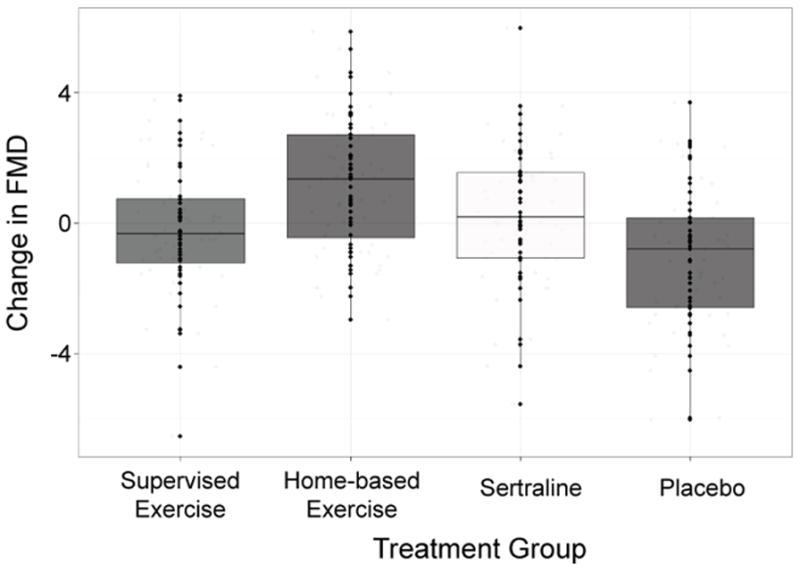
Pre-to-post-treatment changes in flow-mediated dilation (FMD) values by treatment group, adjusted for age, gender, severity of depression, baseline arterial diameter, and baseline FMD.
Acknowledgments
Source of Funding: This study was supported by Grants MH49679, HL093374, HL101383 from the National Institutes of Health and National Institutes of Health Grant MO1-RR-30 from the National Center for Research Resources, Clinical Research Centers Program.
We thank Dr. Mark Appelbaum for providing the randomization scheme and statistical consultation, and Drs. Charles Emery, Diane Catellier and David Sheps for serving on our Data and Safety Monitoring Board. We also thank Michael Ellis, RDMS, RVT, Erin Sheets, and Chevala Harris for their technical assistance, and Marcus Taylor, Jessica Tucker, and Sandra Kennedy for their assistance with exercise testing and training.
This research was supported by Grant MH 49679 from the National Institutes of Health and National Institutes of Health Grant MO1-RR-30 from the National Center for Research Resources, Clinical Research Centers Program. Medication and matched placebo pills were provided by a grant from Pfizer Pharmaceuticals, Inc. Dr. Blumenthal previously received an investigator initiated research grant from Pfizer/Eisai for an unrelated study.
Glossary
- ASCVD
Atherosclerotic Cardiovascular Disease
- CHD
Coronary Heart Disease
- CI
Confidence Interval
- FMD
Flow Mediated Dilation
- HAM-D
Hamilton Depression Rating Scale
- IMT
Intima Media Thickness
- ITT
Intention to Treat
- MDD
Major Depressive Disorder
Footnotes
Conflict of Interest: The authors declare there are no conflicts of interest
Trial Registration: Clinical Trials Government Identifier: NCT-00331305.
References
- 1.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–94. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample.[see comment] Circulation. 1996;93:1976–80. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 3.Ford DE, Mead LA, Chang PP, Cooper-Patrick L, Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in men: the precursors study. Archives of Internal Medicine. 1998;158:1422–6. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- 4.Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–9. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 5.Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–7. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosomatic Medicine. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
- 8.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JGP, Ormel J, van Veldhuisen DJ, van den Brink RHS, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosomatic medicine. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 9.Meijer A, Conradi HJ, Bos EH, Anselmino M, Carney RM, Denollet J, Doyle F, Freedland KE, Grace SL, Hosseini SH, Lane DA, Pilote L, Parakh K, Rafanelli C, Sato H, Steeds RP, Welin C, de Jonge P. Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis. The British journal of psychiatry : the journal of mental science. 2013;203:90–102. doi: 10.1192/bjp.bp.112.111195. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L American Heart Association Statistics Committee of the Council on E, Prevention, the Council on C, Stroke N. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–69. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 11.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA: The Journal of the American Medical Association. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 12.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, Van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M Sertraline Antidepressant Heart Attack Randomized Trial G. Sertraline treatment of major depression in patients with acute MI or unstable angina.[see comment][erratum appears in JAMA 2002 Oct 9;288(14):1720] JAMA. 2002;288:701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–9. doi: 10.1016/j.jacc.2010.03.068. Randomized Controlled Trial Research Support, N.I.H Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. British Medical Journal. 2001;322:763–7. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2009:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic Medicine. 2007;69:587–96. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scandinavian journal of medicine & science in sports. 2014;24:259–72. doi: 10.1111/sms.12050. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, O’Hayer CV, Mabe S, Johnson J, Doraiswamy PM, Jiang W, Schocken DD, Hinderliter AL. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 2012;60:1053–63. doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Babyak M, O’Connor C, Keteyian S, Landzberg J, Howlett J, Kraus W, Gottlieb S, Blackburn G, Swank AM, Whellan D. Effects of Exercise Training on Depressive Symptoms in Patients with Chronic Heart Failure: Results from the HF-ACTION Trial. JAMA. 2012:307. doi: 10.1001/jama.2012.8720. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenthal JA, Babyak MA, Carney RM, Huber M, Saab PG, Burg MM, Sheps D, Powell L, Taylor CB, Kauffman PG. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Medicine and Science in Sports and Exercise. 2004;36:746–55. doi: 10.1249/01.mss.0000125997.63493.13. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. Multicenter Study Randomized Controlled Trial Research Support, N.I.H Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovascular ultrasound. 2014;12:34. doi: 10.1186/1476-7120-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal JA, Rejeski WJ, Walsh-Riddle M, Emery CF, Miller H, Roark S, Ribisl PM, Morris PB, Brubaker P, Williams RS. Comparison of high- and low-intensity exercise training early after acute myocardial infarction. AmJCardiol. 1988;61:26–30. doi: 10.1016/0002-9149(88)91298-2. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 27.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. American Journal of Cardiology. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 28.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 29.Chan SY, Mancini GBJ, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. Journal of the American College of Cardiology. 2003;42:1037–43. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 30.Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of non-invasively determined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 31.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–2. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 32.Wenger NK, Froelicher ES, Smith LK, Expert P. Cardiac Rehabilitation: Clinical Practice Guideline No. 17. Rockville, MD: 1995. AHCPR Publication No. 96-0673. [Google Scholar]
- 33.O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, Jr, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 34.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA: The Journal of the American Medical Association. 1988;260:945–50. [PubMed] [Google Scholar]
- 35.Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. American Journal of Medicine. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 37.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 2008;105:1323–32. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadoglou NP, Iliadis F, Liapis CD. Exercise and carotid atherosclerosis. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2008;35:264–72. doi: 10.1016/j.ejvs.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond) 2012;122:311–22. doi: 10.1042/CS20110469. [DOI] [PMC free article] [PubMed] [Google Scholar]


