Abstract
Resistance to cytarabine remains a major challenge in the treatment of acute myeloid leukemia (AML). Based on previous studies implicating ABCC4/MRP4 in the transport of nucleosides, we hypothesized that cytarabine is sensitive to ABCC4‐mediated efflux, thereby decreasing its cytotoxic response against AML blasts. The uptake of cytarabine and its monophosphate metabolite was found to be facilitated in ABCC4‐expressing vesicles and intracellular retention was significantly impaired by overexpression of human ABCC4 or mouse Abcc4 (P < 0.05). ABCC4 was expressed highly in AML primary blasts and cell lines, and cytotoxicity of cytarabine in cells was increased in the presence of the ABCC4 inhibitors MK571 or sorafenib, as well as after ABCC4 siRNA. In Abcc4‐null mice, cytarabine‐induced hematological toxicity was enhanced and ex vivo colony‐forming assays showed that Abcc4‐deficiency sensitized myeloid progenitors to cytarabine. Collectively, these studies demonstrate that ABCC4 plays a protective role against cytarabine‐mediated insults in leukemic and host myeloid cells.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Resistance to cytarabine remains a major challenge in the treatment of acute myeloid leukemia and details of the underlying mechanism remain unclear.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ We hypothesized that ABCC4 (MRP4) is an important contributor to the transport of cytarabine in leukemia and affects its cytotoxic response against leukemic blasts.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ Our studies have demonstrated that ABCC4 plays a protective role against cytarabine‐mediated insults in leukemic and host myeloid cells.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS?
✓ This work may lead to the development of novel intervention strategies aimed at resensitization of resistant leukemic cells to cytarabine.
Resistance to chemotherapeutic agents remains a major obstacle to successful treatment in acute leukemias, and several members of the ABCC (MRP) efflux transporters have been implicated in this process by their ability to actively extrude structurally diverse compounds.1 Expression of ABCC4 (MRP4) in leukemia cells is of particular interest, since it has been correlated with drug resistance to several chemotherapeutic agents used in leukemia treatment. For example, CEM‐MP5 leukemia cells selected for resistance to 6‐mercaptopurine through stepwise exposure displayed dramatically increased expression of ABCC4,2 and the myeloid leukemia cell line K562/ADR showed elevated expression of ABCC4 and resistance to anthracyclines compared with the parental line.3 In addition to playing a role in drug‐resistance of leukemia cell lines, ABCC4 also appears to regulate leukemia cell proliferation and differentiation independently of drug efflux through the endogenous substrate, cyclic AMP (cAMP). In particular, genetic or pharmacologic inhibition of ABCC4 function in the acute myeloid leukemia (AML) cell line U937 resulted in enhanced intracellular accumulation of cAMP and subsequent leukemic maturation toward a more differentiated phenotype.4 These findings suggest a role for ABCC4 in cAMP‐mediated signaling in normal hematopoietic cell development, where ABCC4 expression levels decrease during differentiation toward mature leukocytes.5 However, it should be noted that constitutive absence of Abcc4 has not revealed any readily apparent hematologic defects in mice.6 Furthermore, silencing of ABCC4 through lentiviral‐mediated shRNA within the K562/ADR cell line enhanced anthracycline‐induced apoptosis without affecting drug efflux, suggesting that ABCC4 may contribute to drug resistance in AML by other means, such as removal of toxic metabolites associated with drug exposure.3 Because ABCC4 has been implicated in the transport of multiple nucleoside and nucleotide antimetabolites,7, 8 as well as their monophosphorylated forms, overexpression of this transporter is a possible mechanism for reduced efficacy of AML therapy involving deoxynucleoside analogs such as cytarabine (1‐β‐D‐arabinofuranosyl‐cytosine). In the present study we investigated this possibility by comparing the resistance profile and ABCC4‐mediated transport properties of cytarabine using an array of in vitro and in vivo model systems. Our results show that ABCC4 confers resistance to cytarabine in AML cells by restricting its intracellular retention, that this process can be reversed by the multikinase inhibitor sorafenib, and that ABCC4‐deficiency causes exacerbated hematologic toxicity.
METHODS
Chemicals and cell culture
Cytarabine was purchased from Sigma‐Aldrich (St. Louis, MO), sorafenib from Toronto Research Chemicals (Canada), and MK571 from Calbiochem (La Jolla, CA). [3H]Cytarabine (specific activity, 14.9 Ci/mmol), [14C]cytarabine‐monophosphate (MP) (specific activity, 57.7 mCi/mmol), [3H]9‐(2‐(phosphonomethoxy)ethyl)‐adenine (hereafter referred to as PMEA; specific activity, 12.3 Ci/mmol) were purchased from Moravek Biochemicals (La Brea, CA), and [3H]estradiol‐17β‐D‐glucuronide (specific activity, 41.8 Ci/mmol) from Perkin Elmer (Boston, MA).
Cell culture reagents, including RPMI‐1640, Dulbecco's Modified Eagle Medium (DMEM), and fetal bovine serum (FBS), were purchased from Invitrogen (La Jolla, CA). The human AML cell lines HL‐60, KG‐1, ML‐2, MOLM13, MV4‐11, NB4, and U937 were purchased from the American Tissue Culture Collection (Rockville, MD), and MO7e cells were purchased from DSMZ (Germany). OCI‐AML3 cell lines were obtained from Dr Brian Sorrentino (St. Jude Children's Research Hospital), as previously described.9 CMS cell line was obtained from Dr Yubin Ge (Karmanos Cancer Center) and CHRF288‐11 cell lines were obtained from Dr Tanja Gruber (St. Jude Children's Research Hospital). Cells were cultured in RPMI‐1640 containing 10% or 20% FBS at 37°C in a humidified atmosphere with 5% CO2. MO7e cells were supplemented with interleukin‐3 (10 ng/mL). OCI‐AML3 cells were maintained in DMEM containing 10% FBS. Cytogenetic profiles, unique leukemogenic translocation and/or fusion genes, and tyrosine kinase mutations in AML cell lines were verified as described previously.10 The human osteosarcoma cell line Saos‐2 was cultured in DMEM containing 10% FBS, penicillin (100 units/mL), streptomycin (100 μg/mL), L‐glutamine (2 mM), and G418 sulfate (500 μg/mL). Saos‐2 cells transfected with human ABCC4, mouse Abcc4, or an empty pcDNA3 vector were described before.11
In vitro cellular accumulation
Saos‐2 cells were seeded at 5 × 105 cells/well in 6‐well plates. At 70% confluence, cells were washed with phosphate‐buffered saline (PBS) and replaced with fresh, serum‐free medium containing varying concentrations of radiolabeled drug. At the end of a 2–4 hour incubation period, the medium was removed and cells were washed twice with ice‐cold PBS. Intracellular drug levels were determined as described.10 Cytarabine and phosphorylated metabolites were measured in Saos‐2 and OCI‐AML3 cell extracts by high‐performance liquid chromatography (HPLC) as previously described.9 Cellular drug accumulation in OCI‐AML3 cells was also determined after transfection with an ABCC4‐siRNA construct. Briefly, 3 × 105 viable cells were seeded in 96‐well plates containing serum‐free medium with the drug of interest and incubated for 2 hours at 37°C with 5% CO2. After incubation, cells were washed three times with ice‐cold PBS, lysed with 100‐μL of 0.1 N NaOH, and agitated for 1 hour. A 25‐μL aliquot of the lysate was used to estimate protein concentration using a BCA protein assay kit (ThermoScientific). Radioactivity was measured on a Perkin Elmer Topcount, after mixing the sample with 175 μL of Scinitisafe 30% scintillation fluid (Perkin Elmer). Cellular accumulation was expressed as pmol per mg of protein and normalized to control siRNA as percent. Two independent experiments were performed using multiple replicates.
Vesicular transport
In an initial screen, inside‐out vesicles of Sf9 cells expressing ABCC1, ABCC2, ABCC3, ABCC4, and ABCC11 (GenoMembrane) (n = 3 replicates) were used to determine uptake of either [14C]cytarabine‐monophosphate (Ara‐CMP) (50 μM) or [3H]cytarabine (1.25 μM) according to the manufacturer's protocol; details are provided in the Supplementary Information.
Microarray analysis
Total RNA was extracted from AML cell lines using TRIzol (Life Technologies, Bethesda, MD), precipitated by isopropyl alcohol, washed with 70% ethanol, and resuspended in water. RNA concentration was determined using a Nanodrop Spectrophotometer (ThermoScientific, Pittsburgh, PA). Gene expression data were acquired from Affymetrix Human U133 plus 2.0 arrays in the St. Jude Children's Research Hospital (SJCRH) Hartwell Center. Gene expression in childhood AML primary blast samples was obtained from previously reported microarray data from SJCRH.12 Data for ABCC4 were extracted, log2 transformed, normalized by Z‐score, and visualized using R software package (R Studio v. 0.98.1091).
Immunoblot analysis
For protein expression of ABCC4 in AML cells, cells were lysed and crude membrane fractions were prepared using the ProteoExtract Native Membrane Protein Extraction Kit (Calbiochem) according to the manufacturer's protocol. Protein concentration of the membrane preps were determined using the Pierce BCA Protein Assay Kit (ThermoScientific). Subsequently, immunoblots were performed using 20 μg of membrane preps. The proteins were subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to PVDF membranes. The membrane was probed using α‐Mrp4 (1:500) or α‐transferrin receptor (1:1,000; Invitrogen) as the primary antibody. Either a secondary α‐rabbit or α‐mouse IgG conjugated to peroxidase (Jackson ImmunoResearch, West Grove, PA) was used at a dilution of 1:2,000. Proteins were visualized by chemiluminescence (SignalFire ECL Reagent, Cell Signaling, Beverly, MA). Immunoblots were performed a minimum of two times on samples collected from different experiments.
Cytotoxicity assays
Cytotoxicity in response to cytarabine in the presence or absence of MK571 or sorafenib, as inhibitors of ABCC4,13, 14 was determined using a (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) Cell Proliferation Kit I (Roche, Nutley, NJ), as previously described10; details are provided in the Supplemental Information.
Silencing of ABCC4 expression
The Silencer Select Validated siRNA for ABCC4 silencing (Ambion, Austin, TX) and the Silencer Select Negative Control #1 siRNA were used in all experiments. Cells were transfected with ABCC4 siRNA using Nucleofector II and nucleofector Kit T (Amaxa), according to the manufacturer's protocol. OCI‐AML3 cells (3 × 106) were treated with 100 μL reagent of solution T for each sample, using 1–3 μg of siRNA for each sample to optimize the best silencing effect. To evaluate ABCC4 suppression (see below), total RNA was extracted using TRIzol (Invitrogen) and analyzed by real‐time polymerase chain reaction (PCR) at variable times posttransfection. cDNA was synthesized by using a primer set designed to amplify exon 3, which encompasses the siRNA cleavage point. In each sample, the copy number of ABCC4 relative to glyceraldehydes‐3‐phosphate dehydrogenase (GAPDH) expression was calculated as follows: 2^dCt [dCt = Ct(GAPDH)‐Ct(ABCC4)]. Protein expression of ABCC4 was determined by western blot as described above.
ABCC4 mRNA expression analysis
Real‐time PCR was performed to examine the siRNA silencing effect according to previously described methods.15 Briefly, total RNA extracted from cells with TRIzol was transcribed to cDNA by the SuperScript First Strand Synthesis kit (Invitrogen). PCR reactions were run on an ABI 7900HT Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA) using the QuantiTect SYBER Green PCR master Mix. The expression of GAPDH was simultaneously analyzed as a quality control and was used for normalization. The following primer sequences were used for analysis: ABCC4 (forward): CAGCCCATATTTTTGGGAAA; ABCC4 (reverse): TGGCACATGGCTACTCGTAA GAPDH (forward): AAGGACTCATGACCACAGTCCAT; and GAPDH (reverse): CCATCACGCCACAGTTTCC.
Analysis of hematopoietic toxicity
Female Abcc4‐null [Abcc4(–/–)] mice6 and female, age‐matched (8–12 weeks old) wildtype control mice, both on a C57BL/6 background, were used in all in vivo studies. Previously, we confirmed by real‐time PCR that expression of the nucleoside uptake transporters Slc28a3 and Slc29a2 in myeloid progenitor cells was unchanged, and that the expression of Slc29a1 was undetectable in the bone marrow of wildtype or Abcc4(–/–) mice.16 Mice were housed in a temperature‐controlled environment, and all experiments were approved by the Institutional Animal Care and Use Committee of SJCRH. Cytarabine was administered once daily for 5 days via i.p. injection at a dose of 12.5 mg/kg of body weight. This dose was selected based on toxicity considerations.9 Blood samples (50 μL each) were obtained from the retro‐orbital venous plexus before treatment (baseline) and after start of treatment on days 4, 8, and 11. The absolute neutrophil count was monitored in each sample and is reported as a percent change of baseline.
Pharmacokinetic analyses
Plasma concentrations of cytarabine were evaluated in wildtype mice and Abcc4(–/–) mice after a single i.p. administration of cytarabine at a dose of 12.5 mg/kg. Blood was obtained from individual mice at serial timepoints from the orbital plexus or by cardiac puncture, using three mice per group. Blood was put on ice, centrifuged at 3,000g for 5 minutes, and the plasma supernatant was removed and stored at –80°C until analysis. Concentrations of cytarabine in plasma were determined using a validated method based on liquid chromatography with tandem mass‐spectrometric detection, as previously described,17 using clofazimine as an internal standard. Modifications are described in the Supplementary Information. The linear range of cytarabine was set from 50 ng/mL to 10,000 ng/mL. Detection of cytarabine metabolites was determine by HPLC, as previously described.9
In vitro myeloid progenitor assays
Myeloid progenitor assays were performed as described previously.16 Briefly, bone marrow was harvested from the hind limbs of age‐matched wildtype mice and Abcc4(–/–) mice by flushing tibias and femurs with PBS containing 2% FBS. Cells from bone marrow were sorted to obtain c‐Kit+, Sca‐1+, and Lin– (KSL) cells by magnetic cell sorting (MACS Miltenyi Biotec, Auburn, CA). For granulocyte macrophage colony‐forming cell assays, 3,000 cells were plated in methyl cellulose culture medium (methocult M3534; StemCell Technologies, Vancouver, BC, Canada) with or without cytarabine, and number of colonies determined after 7 days.
Statistical considerations
All data are presented as mean ± SD. Statistical analyses were done using NCSS 2004 and P < 0.05 was regarded as statistically significant. A Student's t‐test (2 groups) or a one‐way analysis of variance (ANOVA) (>2 groups) was used for statistical analysis on all in vitro and in vivo data.
RESULTS
In vitro transport of cytarabine by ABCC4
Because ABCC4 has previously been implicated in the transport of various nucleoside analogs,7, 8 we determined if cytarabine is a substrate of ABCC4 using inside‐out vesicles and Saos‐2 cells transfected with human ABCC4. Vesicles expressing human ABCC4 demonstrated significantly increased uptake of cytarabine and its active metabolite cytarabine‐monophosphate by 330% (Figure 1 a) and 250% (Figure 1 b), respectively, compared with control vesicles. Uptake by vesicles expressing ABCC1, ABCC2, ABCC3, and ABCC11 was not observed, despite the functional transport of the positive control substrate estradiol‐17β‐D‐glucuronide (see Supplementary Figure S1). Likewise, the intracellular accumulation of cytarabine (Figure 1 c) and phosphorylated metabolites (Figure 1 d) were decreased in Saos‐2 cells overexpressing human ABCC4, compared with vector control cells. These data indicate that both cytarabine and cytarabine‐monophosphate are transported substrates of ABCC4.
Figure 1.
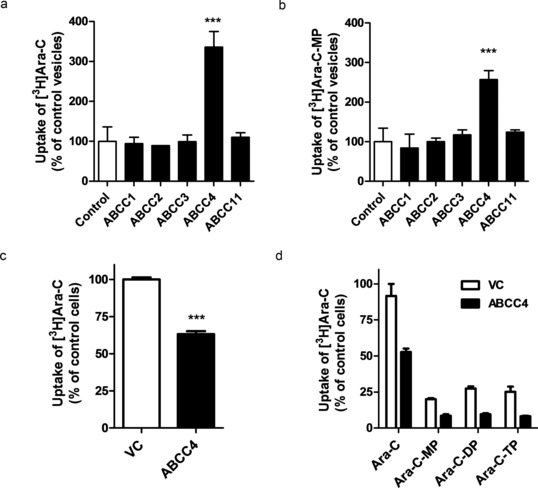
Transport of cytarabine (Ara‐C) and cytarabine‐monophosphate (Ara‐CMP) by ABCC4. Uptake of (a) Ara‐C and (b) Ara‐CMP in vesicles expressing the indicated ABCC transporters. Vesicles were incubated with 1.25 μM Ara‐C or 50 μM Ara‐CMP for 5 minutes. Results were normalized to ATP‐independent transport and shown as percentage of uptake in control vesicles. Intracellular accumulation of (c) Ara‐C and (d) Ara‐C and phosphorylated metabolites in Saos‐2 cells transfected with human ABCC4 (ABCC4). Cells were incubated with 1.25 μM Ara‐C for 2 hours (in C) or 4 hours (in D). Ara‐C, Ara‐C‐MP, Ara‐C‐diphosphate (Ara‐C‐DP), and Ara‐C triphosphate (Ara‐C‐TP) were measured in cell lysates by HPLC and radioactivity was determined by liquid scintillation counting. Results are shown as percentage of uptake in control cells transfected with empty vector (VC). Controls were set at a value of 100% and data are presented as mean (bars) and SD (error bars) of two to three independent experiments (four to six replicates). ***P < 0.001 vs. control.
ABCC4 expression in AML cells and contribution to cytarabine cytotoxicity
In order to evaluate the contribution of ABCC4 to cytarabine transport in AML cells, we first evaluated ABCC4 expression in AML primary blast samples and cell lines. ABCC4 gene expression was present and variable between patients and among different AML subtypes, as well as between different cell lines (Figure 2 a). We next evaluated ABCC4 protein expression in membrane fractions of AML cell lines using a purified antiserum to human ABCC4. As shown in Figure 2 b, ABCC4 protein was found to be highly expressed in about half of the cell lines tested. Based on high basal expression of ABCC4, OCI‐AML3 cells were selected to further investigate the possible association of ABCC4 expression with cellular accumulation and cytotoxicity of cytarabine. Cotreatment of OCI‐AML3 cells with cytarabine (1.25 μM) with or without sorafenib (5 μM), resulted in an increase of cytarabine and phosphorylated metabolites (Figure 2 c). Recently, cytarabine has been shown to be a substrate for ABCC10 (MRP7) and ABCC11 (MRP8).18, 19, 20 We previously demonstrated that ABCC10 protein was highly expressed in only one AML cell line (ML‐2) and ABCC11 was not expressed in the cell lines tested.9 Therefore, the extent of accumulation of cytarabine in OCI‐AML3 cells is not likely due to these transporters. To mimic a clinically relevant time course of exposure to cytarabine, OCI‐AML3 cells were treated with cytarabine for 4 hours in the absence and presence of sorafenib or MK‐571, followed by continuous 68 hours of incubation with drug‐free medium. Cytarabine demonstrated concentration‐dependent cytotoxicity in OCI‐AML3 cells (IC50, 61 μM), and this effect was enhanced in the presence of MK‐571 (IC50, 20 μM) (Figure 2 d). Similarly, enhanced cytarabine‐induced cytotoxicity was observed in the presence of sorafenib (IC50, 12 μM) (Figure 2 d), suggesting that ABCC4 is a resistance factor for cytarabine in AML cells. These combination drug effects were determined to be additive (see Supplementary Figures S2 and S3).
Figure 2.
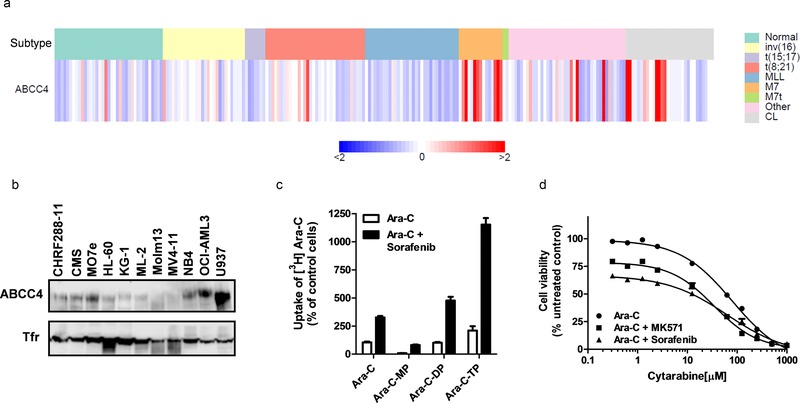
ABCC4 is expressed in childhood primary AML blast samples and human AML cell lines and contributes to cytarabine (Ara‐C)‐induced cytotoxicity. (a) ABCC4 gene expression in primary samples and cell lines assessed by microarray analysis. Each column represents normal bone marrow (normal), an individual primary sample, or cell line (CL); columns are categorized by cytogenetic AML subtypes of prognostic relevance (data from Ref. 12). (b) Protein expression of ABCC4 in AML cell lines. Cellular membrane protein (20 μg) was loaded in each lane and protein expression was assessed by western blotting using an antibody to ABCC4 and transferrin receptor (Tfr) as a loading control. (c) Accumulation of Ara‐C and phosphorylated metabolites in OCI‐AML3 cells in the absence or presence of sorafenib. Cells were treated with 1.25 μM Ara‐C alone or in combination with 5 μM sorafenib for 2 hours. Ara‐C, Ara‐C monophosphate (Ara‐C‐MP), Ara‐C‐diphosphate (Ara‐C‐DP), and Ara‐C triphosphate (Ara‐C‐TP) were measured in cell lysates by HPLC and radioactivity was determined by liquid scintillation counting. Accumulation of Ara‐C in the absence of sorafenib was set at a value of 100% (control) and data are shown as a percentage of uptake in controls and presented as mean (bars) and SD (error bars) of two independent experiments (four replicates). (d) Cytarabine‐induced cytotoxicity in the absence or presence of MK571 or sorafenib in OCI‐AML3 cells. Cells were incubated with increasing concentrations of Ara‐C alone and with 100 μM MK571 or 10 μM sorafenib. Data are presented as mean (bars) and SD (error bars) of three independent experiments (24 replicates).
Effect of ABCC4 silencing on cytarabine cytotoxicity
Given that inhibition of ABCC4 enhanced cytarabine‐induced cytotoxicity, we next used a siRNA construct to knockdown ABCC4 expression and determined if this alters cellular sensitivity to cytarabine. Transient transfection of OCI‐AML3 cells with an siRNA targeting ABCC4 resulted in suppression of its mRNA by more than 65% at 24 hours posttransfection (Figure 3 a). We next compared the intracellular accumulation of cytarabine in OCI‐AML3 cells with endogenous or reduced expression of ABCC4 after siRNA. Compared with cells transfected with a nontargeting control siRNA, ABCC4 knockdown resulted in a significant 150% increase in intracellular accumulation of cytarabine (Figure 3 b) and a similar increase in retention of the known ABCC4 substrate, PMEA (Figure 3 c). Furthermore, siRNA‐mediated knockdown of ABCC4 was associated with a 100% increase (IC50, 22 vs. 11 μM) in cytarabine‐induced cytotoxicity in OCI‐AML3 cells (Figure 3 d). These results are consistent with the involvement of ABCC4 as an efflux transporter of cytarabine and its monophosphate that can confer drug resistance in AML cells.
Figure 3.
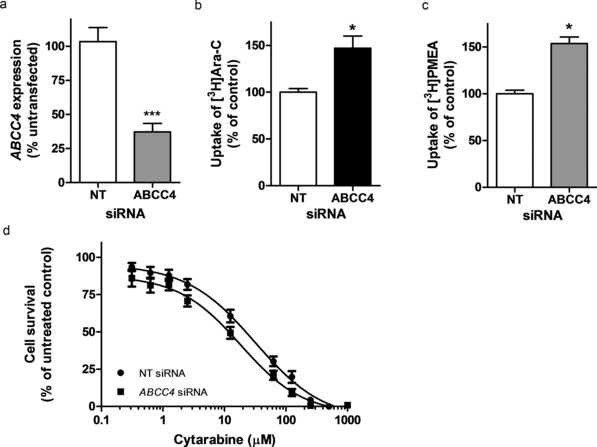
Knockdown of ABCC4 in OCI‐AML3 cells increases cellular accumulation of cytarabine, and enhances cytotoxicity to cytarabine. (a) ABCC4 mRNA expression in OCI‐AML3 cells transfected with 3 μg of ABCC4 siRNA or a nontargeting (NT) control siRNA. ABCC4 expression was determined by real‐time PCR. Results are shown as a percentage of untransfected normal cells, with values in cells transfected with control NT siRNA set at 100%. Data are representative of one of two independent experiments performed in triplicate. Cellular accumulation of (b) cytarabine (Ara‐C) and (c) PMEA were increased after ABCC4 knockdown by siRNA. The results are accumulation at 2 hours, with cells transfected with control NT siRNA set at 100%. PMEA was used as a positive control substrate. Results represent an average of two to three independent experiments with two to six replicates. (d) Cytarabine cytotoxicity in OCI‐AML3 cells after ABCC4 siRNA knockdown. Data are presented as mean of two to three independent experiments (eight replicates). *P < 0.05 vs. control; ***P < 0.001 vs. control.
Role of Abcc4 in cytarabine disposition and host toxicity
The in vivo relevance of the interaction of cytarabine with ABCC4 was determined in Abcc4‐null mice and compared with wildtype mice. Abcc4‐deficiency was not associated with significantly altered concentrations of cytarabine in plasma after i.p. injection of the drug (Figure 4 a). However, cytarabine induced significant hematopoietic toxicity in Abcc4‐null mice that was not observed in wildtype controls. In particular, neutrophil counts were significantly decreased to 30% compared with baseline of pretreatment in knockout mice, whereas neutrophil counts did not change in wildtype mice (Figure 4 b).
Figure 4.
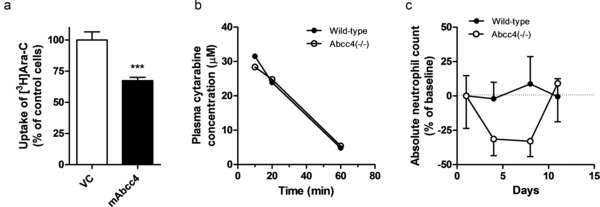
Cytarabine‐induced myelosuppression is enhanced in Abcc4(–/–) mice. (a) Intracellular accumulation of cytarabine (Ara‐C) in Saos‐2 cells transfected with mouse Abcc4 (mAbcc4). Cells were incubated with 5 μM Ara‐C for 2 hours and the results are shown as the percentage of control cells transfected with empty vector (VC) set at a value of 100%. Data are presented as mean (bars) and SD (error bars) of three independent experiments (nine replicates). ***P < 0.001 vs. control. (b) Plasma concentrations of cytarabine are unaffected by Abcc4‐deficiency in mice after i.p. injection of 12.5 mg/kg of cytarabine. (c) Mice were treated with 12.5 mg/kg of cytarabine once daily for 5 days, and neutrophil counts were monitored and shown as percentage change from baseline.
Abcc4 absence sensitizes myeloid progenitors to cytarabine
Since Abcc4 is highly expressed in monocyte and macrophage progenitors,16 we next determined the formation of myeloid colonies in methylcellulose culture from hematopoietic progenitor cells of Abcc4‐null and wildtype mice cultured ex vivo in the presence or absence of cytarabine. Exposure to cytarabine resulted in ∼twofold reduction in IC50 for a decrease in myeloid colony formation of hematopoietic cultures derived from Abcc4‐null mice compared with wildtype control (Figure 5).
Figure 5.
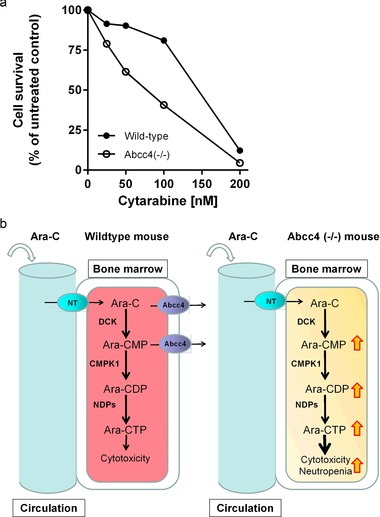
Abcc4‐deficiency sensitizes myeloid progenitor cells to cytarabine. (a) c‐Kit+, Sca‐1+, and Lin– (KSL) cells from bone marrow of wildtype and Abcc4(–/–) mice were cultured in methyl cellulose culture medium with increasing concentrations of cytarabine. Granulocyte macrophage colony‐forming cell assay was determined after 7 days of culture. Duplicate plates were cultured at each drug concentration for two independent experiments. Data represent the percentage of viable colonies at different concentrations. (b) Theoretical influence of Abcc4 knockout on cytarabine‐induced cytotoxicity and myelosuppression. A nucleotide transporter facilitates cytarabine entry into the cell, where it is rapidly metabolized into its active triphosphate form through a series of enzymatic reactions. In wildtype cells, Abcc4 can exert a protective function by facilitating export of parent compound and the Ara‐CMP metabolite. Knockout of Abcc4 in mice promotes intracellular accumulation of cytarabine and its metabolites, resulting in enhanced cytotoxicity and myelosuppression. Alternatively, Abcc4 function may be inhibited chemically (sorafenib, MK571) or through genetic variation. The black arrows indicate the direction of transport. The red/yellow arrows indicate the accumulation of cytarabine and metabolites that occurs when Abcc4 is inhibited. Ara‐C, cytarabine; Ara‐CMP, cytarabine monophosphate; Ara‐CDP, cytarabine diphosphate; Ara‐CTP, cytarabine triphosphate; CMPK1, cytidine monophosphate kinase 1; DCK, deoxycytidine kinase; NDPs, nucleotide diphosphate kinases; NT, nucleotide transporter.
DISCUSSION
Resistance to cytarabine remains a major challenge in the treatment of AML, and therefore, elucidating additional mechanisms leading to this phenomenon is of potential clinical relevance. Growing evidence continues to implicate drug efflux mediated by ATP‐binding cassette transporters in the development of resistance to nucleoside‐based agents.21 Among members of the ABCC drug transporter family, ABCC1 (MRP1) has been linked with treatment outcome in patients with de novo AML receiving cytarabine‐based chemotherapy,22, 23 and high expression of ABCC3 (MRP3) has been reported to correlate with poor prognosis in childhood AML.24 These studies did not demonstrate direct transport of cytarabine by ABCC1 and ABCC3, and our current studies did not confirm cytarabine transport by ABCC1 and ABCC3. Moreover, cytarabine was recently identified as a transported substrate of both ABCC10 (MRP7)18, 19, 20 and ABCC11 (MRP8), and in a preliminary study, high ABCC11 expression has been associated with a low probability of overall survival in adult patients with AML.25 Because ABCC4 has the ability to transport a wide range of cyclic nucleosides as well as certain nucleoside monophosphate analogs, we hypothesized that ABCC4 may affect cytarabine levels in leukemic cells. In support of this possibility, we found that heterologous expression of ABCC4 in Saos‐2 cells resulted in decreased accumulation of cytarabine and phosphorylated metabolites. Similar to other nucleoside analogs, cytarabine undergoes phosphorylation in cells to form the active metabolite cytarabine‐triphosphate through sequential action of the enzymes deoxycytidine kinase, deoxycytidine monophosphate kinase, and nucleoside diphosphate kinase.26 The first activating enzyme metabolizes cytarabine into cytarabine‐monophosphate, and this step is generally considered to be rate‐limiting in cytarabine‐triphosphate formation.27 In the current study, we found that cytarabine‐monophosphate is also effectively transported by ABCC4, thus removing an essential precursor of the cytotoxic species that ultimately affects DNA synthesis and causes leukemic cell death. This process represents a potentially important secondary mechanism by which ABCC4 induces resistance to cytarabine in addition to preventing excessive accumulation of the parent drug itself (Figure 5 b).
Previously reported studies have found that ABCC4 is highly expressed, albeit at variable levels, in blast samples from patients with childhood AML24, 28 and adult AML.25 We confirmed ABCC4 gene expression in childhood AML primary blast samples and a panel of AML cell lines. Based on these findings, we evaluated ABCC4 protein expression in AML cell lines and confirmed high and variable levels of ABCC4. In order to determine the functionality and contribution of ABCC4 to cytarabine cytotoxicity, we carried out cell viability assays with increasing concentrations of cytarabine alone or in combination with either a known ABCC4 inhibitor (MK571) or sorafenib in the OCI‐AML3 cell line. Inhibition of ABCC4 with MK571 enhanced cytarabine‐induced cytotoxicity by threefold, as indicated by a shift in IC50. OCI‐AML3 cells exposed to sorafenib showed a similar enhancement of sensitivity to cytarabine, and comparable findings were obtained with an siRNA construct directed against ABCC4. Therefore, pharmacologic or genetic inhibition of ABCC4 function in AML cells sensitizes to cytarabine‐induced cytotoxicity. These results support the possibility that our previous observation demonstrating in vitro and in vivo synergy of cytarabine and sorafenib9 is associated with inhibition of ABCC4‐mediated cytarabine transport by sorafenib. Although additional investigation is required to confirm direct involvement of sorafenib‐mediated inhibition of ABCC4 as the primary mechanistic basis for the observed in vitro and in vivo effects, it is of note that similar drug–drug interactions have been observed with other tyrosine kinase inhibitors and the related transporter ABCG2.29 It can thus be postulated that, beyond interference with signaling pathways promoting leukemic survival, sorafenib may influence the response to treatment in AML by an effect on ABCC4 function and possibly other ABC transporters that modulate cytarabine cellular retention. This recognition is particularly relevant in the context of resistant disease and regarding the future development of combination regimens involving sorafenib and possibly other tyrosine kinase inhibitors administered with deoxycytidine nucleosides.30 Our finding that cytarabine is a transported substrate of ABCC4 is consistent with a recently reported study,31 where overexpression of ABCC4 was found to confer resistance to a number of nucleoside anticancer drugs, including cytarabine and troxacitabine. It is interesting to note that an earlier study demonstrated that mammalian cells engineered to overexpress ABCC4 displayed only a rather modest 1.3‐fold decrease in cytarabine‐mediated cytotoxicity,32 although direct transport of cytarabine or cytarabine‐monophosphate by ABCC4 was not evaluated in that study. The reasons underlying the apparent differences in outcome of this previous study and our current results are not entirely clear. It is possible that the cell lines onto which these respective transporter models were developed (HEK293 cells vs. inside‐out vesicles and Saos‐2 cells, respectively) differentially impact any resulting phenotype for the same compound, for example, as a result of variable expression levels of nucleoside uptake transporters and/or enzymes of relevance to cytarabine activation or deactivation.33 Regardless of the exact mechanism, our current findings combined with those recently presented by Adema et al.31 provide evidence that ABCC4 can affect the intracellular accumulation of a remarkably broad range of substrates.34 The in vitro studies presented here suggest that cytarabine is also a transported substrate of mouse Abcc4. The mouse Abcc4 transporter shares more than 82% amino acid sequence homology to human ABCC4,35 and on the basis of their shared localization and overlapping substrate specificity,7 it is likely that in rodents Abcc4 fulfills the same function as ABCC4 in humans. Based on this premise, we evaluated the pharmacokinetic and myelosuppressive properties of cytarabine in a mouse model with a genetic deletion of Abcc4. One possible limitation of this model is that potential differences in the kinetic properties of the human ABCC4 and mouse Abcc4 transporters have been reported for some substrates such as the cyclic nucleotide, cGMP.36 Although a detailed comparison of transporter kinetics was not performed here for cytarabine, preliminary evidence suggests that mouse Abcc4 may efflux the drug more efficiently out of Saos‐2 cells compared with human ABCC4. Future studies should compare ABCC4 expression in freshly isolated blast samples with the Saos‐2 cells to further validate this model.
In the Abcc4‐null mice, we found that, compared with wildtype mice, the systemic exposure to cytarabine was unaffected after i.p. drug administration, which is consistent with previous studies employing other parenterally administered substrates, including PMEA37 and dasatinib.38 In contrast, cytarabine‐related myelosuppression was substantially increased in the Abcc4‐null mice, and this was subsequently found to be linked with a direct cytotoxic effect of the drug on myeloid progenitor cells from the murine bone marrow. This finding is consistent with the notion that Abcc4 is highly expressed in monocyte and macrophage progenitors,16 and with a previous study indicating that the nucleoside analog PMEA causes intense aplasia in the bone marrow of Abcc4‐deficient mice as well as increased lethality attributable to neutropenia.37 These findings, in association with the absence of changes in the systemic concentration of cytarabine, suggest that Abcc4‐mediated transport is a critically important host factor that protects the hematopoietic sites from insults mediated by cytotoxic substrates. This is potentially clinically relevant in that cytarabine has been shown to induce a severe yet transient myelosuppression that can lead to serious complications.39 Moreover, ABCC4 is known to be highly polymorphic, with multiple genetic variants resulting in altered function.16, 40 These findings have been confirmed in multiple clinical reports that show a significant association of ABCC4 genetic variants with risk of hematopoietic toxicity in patients treated with nucleoside analogs.41, 42 Of note, the rs3742106 (4131T>C) genetic variant has been associated with increased concentrations of tenofovir diphosphate in human peripheral blood mononuclear cells.43 Interestingly, we found that the same ABCC4 variant, rs3742106, significantly associated with febrile neutropenia in a pharmacogenomic study evaluating the association of single nucleotide polymorphisms in drug metabolizing enzymes and drug transporters with clinical outcomes and toxicity in pediatric AML patients.44
Collectively, our results indicate that ABCC4 is abundantly expressed in AML, where it facilitates the efflux of cytarabine and its monophosphorylated metabolite. Moreover, Abcc4‐deficiency in mice was associated with increased cytarabine‐mediated toxicity of myeloid progenitor cells and neutropenia. These results identified ABCC4 as an important, previously unrecognized, contributor to the toxicity of cytarabine, and this process may be relevant for other related nucleoside analogs used in the treatment of AML, such as clofarabine45 and fludarabine.46
Author Contributions
C.D.D. wrote manuscript and analyzed data. S.H. wrote manuscript, performed research, and analyzed data. L.L. designed research, performed research, and analyzed data. D.R.B., S.J.O., and A.A.G. performed research. J.D.S. wrote manuscript and contributed new reagents. A.S. and S.D.B. wrote manuscript, designed research, and analyzed data.
Conflict of Interest
The authors declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Supporting information
Additional supporting information may/can be found online in the supporting information tab for this article.
Supporting Information
Figure 1
Figure 2
Figure 3
Data
Acknowledgments
We thank Deepa Nachagari for assistance with experimental procedures. This study was supported in part by ALSAC and the National Institutes of Health grants R01 CA138744 (to S.D.B.), R01 GM060904 (to J.D.S.), and 3P30CA021765.
References
- 1. Borst, P. , de Wolf, C. & van de Wetering, K. Multidrug resistance‐associated proteins 3, 4, and 5. Pflugers Arch. 453, 661–673 (2007). [DOI] [PubMed] [Google Scholar]
- 2. Peng, X.X. et al Up‐regulation of MRP4 and down‐regulation of influx transporters in human leukemic cells with acquired resistance to 6‐mercaptopurine. Leuk. Res. 32, 799–809 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Liu, B. , Zhao, L. , Ma, H. , Zhang, W. & Jin, Y. Knockdown of MRP4 by lentivirus‐mediated siRNA improves sensitivity to adriamycin in adriamycin‐resistant acute myeloid leukemia cells. Chin. Sci. Bull. 57, 90–97 (2012). [Google Scholar]
- 4. Copsel, S. et al Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 286, 6979–6988 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oevermann, L. et al Hematopoietic stem cell differentiation affects expression and function of MRP4 (ABCC4), a transport protein for signaling molecules and drugs. Int. J. Cancer 124, 2303–2311 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Leggas, M. et al Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol. Cell Biol. 24, 7612–7621 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russel, F.G. , Koenderink, J.B. & Masereeuw, R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol. Sci. 29, 200–207 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Schuetz, J.D. et al MRP4: A previously unidentified factor in resistance to nucleoside‐based antiviral drugs. Nat. Med. 5, 1048–1051 (1999). [DOI] [PubMed] [Google Scholar]
- 9. Hu, S. et al Activity of the multikinase inhibitor sorafenib in combination with cytarabine in acute myeloid leukemia. J. Natl. Cancer Inst. 103, 893–905 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu, S. et al Comparison of antitumor effects of multitargeted tyrosine kinase inhibitors in acute myelogenous leukemia. Mol. Cancer Ther. 7, 1110–1120 (2008). [DOI] [PubMed] [Google Scholar]
- 11. Adachi, M. et al Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J. Biol. Chem. 277, 38998–39004 (2002). [DOI] [PubMed] [Google Scholar]
- 12. Ross, M.E. et al Gene expression profiling of pediatric acute myelogenous leukemia. Blood 104, 3679–3687 (2004). [DOI] [PubMed] [Google Scholar]
- 13. Hu, S. et al Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP‐binding cassette transporters. Clin. Cancer Res. 15, 6062–6069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu, C.P. , Klokouzas, A. , Hladky, S.B. , Ambudkar, S.V. & Barrand, M.A. Interactions of mefloquine with ABC proteins, MRP1 (ABCC1) and MRP4 (ABCC4) that are present in human red cell membranes. Biochem. Pharmacol. 70, 500–510 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu, S. et al Interaction of imatinib with human organic ion carriers. Clin. Cancer Res. 14, 3141–3148 (2008). [DOI] [PubMed] [Google Scholar]
- 16. Krishnamurthy, P. et al Transporter‐mediated protection against thiopurine‐induced hematopoietic toxicity. Cancer Res. 68, 4983–4989 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsieh, Y. , Duncan, C.J. & Liu, M. A mixed‐mode liquid chromatography‐tandem mass spectrometric method for the determination of cytarabine in mouse plasma. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 854, 8–12 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Hopper‐Borge, E. , Xu, X. , Shen, T. , Shi, Z. , Chen, Z.S. & Kruh, G.D. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 69, 178–184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopper‐Borge, E.A. et al Contribution of Abcc10 (Mrp7) to in vivo paclitaxel resistance as assessed in Abcc10(–/–) mice. Cancer Res. 71, 3649–3657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malofeeva, E.V. , Domanitskaya, N. , Gudima, M. & Hopper‐Borge, E.A. Modulation of the ATPase and transport activities of broad‐acting multidrug resistance factor ABCC10 (MRP7). Cancer Res. 72, 6457–6467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Kolk, D.M. , de Vries, E.G. , Muller, M. & Vellenga, E. The role of drug efflux pumps in acute myeloid leukemia. Leuk. Lymphoma 43, 685–701 (2002). [DOI] [PubMed] [Google Scholar]
- 22. Schaich, M. , Soucek, S. , Thiede, C. , Ehninger, G. & Illmer, T. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br. J. Haematol. 128, 324–332 (2005). [DOI] [PubMed] [Google Scholar]
- 23. van der Kolk, D.M. et al Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood 99, 3763–3770 (2002). [DOI] [PubMed] [Google Scholar]
- 24. Steinbach, D. , Lengemann, J. , Voigt, A. , Hermann, J. , Zintl, F. & Sauerbrey, A. Response to chemotherapy and expression of the genes encoding the multidrug resistance‐associated proteins MRP2, MRP3, MRP4, MRP5, and SMRP in childhood acute myeloid leukemia. Clin. Cancer Res. 9, 1083–1086 (2003). [PubMed] [Google Scholar]
- 25. Guo, Y. et al Expression of ABCC‐type nucleotide exporters in blasts of adult acute myeloid leukemia: Relation to long‐term survival. Clin. Cancer Res. 15, 1762–1769 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grant, S. Ara‐C: Cellular and molecular pharmacology. Adv. Cancer Res. 72, 197–233 (1998). [DOI] [PubMed] [Google Scholar]
- 27. Gandhi, V. & Plunkett, W. Cellular and clinical pharmacology of fludarabine. Clin. Pharmacokinet. 41, 93–103 (2002). [DOI] [PubMed] [Google Scholar]
- 28. Patel, C. et al Multidrug resistance in relapsed acute myeloid leukemia: Evidence of biological heterogeneity. Cancer 119, 3076–3083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozvegy‐Laczka, C. , Cserepes, J. , Elkind, N.B. & Sarkadi, B. Tyrosine kinase inhibitor resistance in cancer: Role of ABC multidrug transporters. Drug Resist. Updat. 8, 15–26 (2005). [DOI] [PubMed] [Google Scholar]
- 30. Burnett, A. , Wetzler, M. & Lowenberg, B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 29, 487–494 (2011). [DOI] [PubMed] [Google Scholar]
- 31. Adema, A. D. , et al Overexpression of MRP4 (ABCC4) or MRP5 (ABCC5) confer resistance to the nucleoside analogs cytarabine and troxacitabine, but not gemcitabine. Springerplus 3, 732–742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reid, G. et al Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 63, 1094–1103 (2003). [DOI] [PubMed] [Google Scholar]
- 33. Clarke, M.L. , Mackey, J.R. , Baldwin, S.A. , Young, J.D. & Cass, C.E. The role of membrane transporters in cellular resistance to anticancer nucleoside drugs. Cancer Treat. Res. 112, 27–47 (2002). [DOI] [PubMed] [Google Scholar]
- 34. Zhou, S.F. et al Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 15, 1981–2039 (2008). [DOI] [PubMed] [Google Scholar]
- 35. Lamba, J.K. et al Nonsense mediated decay downregulates conserved alternatively spliced ABCC4 transcripts bearing nonsense codons. Hum. Mol. Genet. 12, 99–109 (2003). [DOI] [PubMed] [Google Scholar]
- 36. de Wolf, C.J. et al cGMP transport by vesicles from human and mouse erythrocytes. FEBS 274, 439–450 (2007). [DOI] [PubMed] [Google Scholar]
- 37. Belinsky, M.G. et al Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue‐induced damage. Cancer Res. 67, 262–268 (2007). [DOI] [PubMed] [Google Scholar]
- 38. Furmanski, B.D. et al Contribution of ABCC4‐mediated gastric transport to the absorption and efficacy of dasatinib. Clin. Cancer Res. 19, 4359–4370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stentoft, J. The toxicity of cytarabine. Drug Saf. 5, 7–27 (1990). [DOI] [PubMed] [Google Scholar]
- 40. Abla, N. et al The human multidrug resistance protein 4 (MRP4, ABCC4): Functional analysis of a highly polymorphic gene. J. Pharmacol. Exp. Ther. 325, 859–868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ban, H. et al The multidrug‐resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 45, 1014–1021 (2010). [DOI] [PubMed] [Google Scholar]
- 42. Tanaka, Y. et al Multidrug resistance protein 4 (MRP4) polymorphisms impact the 6‐mercaptopurine dose tolerance during maintenance therapy in Japanese childhood acute lymphoblastic leukemia. Pharmacogenomics J. 15, 380–384 (2015). [DOI] [PubMed] [Google Scholar]
- 43. Kiser, J.J. , Aquilante, C.L. , Anderson, P.L. , King, T.M. , Carten, M.L. & Fletcher, C.V. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV‐infected patients. J. Acquir. Immune Defic. Syndr. 47, 298–303 (2008). [DOI] [PubMed] [Google Scholar]
- 44. Drenberg, C.D. et al Inherited variation in OATP1B1 is associated with treatment outcome in acute myeloid leukemia. Clin. Pharmacol. Ther. (In press; DOI:10.1002/cpt.315). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghanem, H. , Kantarjian, H. , Ohanian, M. & Jabbour, E. The role of clofarabine in acute myeloid leukemia. Leuk. Lymphoma 54, 688–698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lukenbill, J. & Kalaycio, M. Fludarabine: A review of the clear benefits and potential harms. Leuk. Res. 37, 986–994 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may/can be found online in the supporting information tab for this article.
Supporting Information
Figure 1
Figure 2
Figure 3
Data


