Abstract
The tumor suppressive microRNA miR-34a is transcriptionally regulated by p53 and shown to inhibit breast cancer cell proliferation as well as being a marker of increased disease free survival. Indole-3-carbinol (I3C) derived from cruciferous vegetables, artemisinin, extracted from the sweet wormwood plant, and artesunate, a semi-synthetic derivative of artemisinin, are phytochemicals with anti-tumorigenic properties however, little is known about the role of microRNAs in their mechanism of action. Human breast cancer cells expressing wild-type (MCF-7) or mutant p53 (T47D) were treated with a concentration range and time course of each phytochemical under conditions of cell cycle arrest as detected by flow cytometry to examine the potential connection between miR-34a expression and their anti-proliferative responses. Real-time PCR and western blot analysis of extracted RNA and total protein revealed artemsinin and artesunate increased miR-34a expression in a dose-dependent manner correlating with down-regulation of the miR-34a target gene, CDK4. I3C stimulation of miR-34a expression required functional p53, whereas, both artemisinin and artesunate up-regulated miR-34a expression regardless of p53 mutational status or in the presence of dominant negative p53. Phytochemical treatments inhibited the luciferase activity of a construct containing the wild-type 3′UTR of CDK4, but not those with a mutated miR-34a binding site, whereas, transfection of miR-34a inhibitors ablated the phytochemical mediated down-regulation of CDK4 and induction of cell cycle arrest. Our results suggest that miR-34a is an essential component of the anti-proliferative activities of I3C, artemisinin and artesunate and demonstrate that both wild-type p53 dependent and independent pathways are responsible for miR-34a induction.
Additional keywords: Indole-3-carbinol, artemisinin, artesunate, CDK4, anti-proliferative response
INTRODUCTION
MicroRNAs are small, non-coding RNA molecules 18–24 nucleotides in length that post-transcriptionally regulate gene expression through inhibition of mRNA translation and stability [1, 2]. Most microRNA bind imperfectly to their complementary sites within individual mRNAs, permitting each molecule to target multiple transcripts and modify complex biological processes including development, differentiation, apoptosis and cellular proliferation [3,4]. Bioinformatic and microarray analyses suggest that microRNAs regulate as much as 30–50% of the transcribed human genome through imperfect base pairing of the 6–8 nucleotide “seed region” of the microRNA to its complementary region in the 3′ un-translated region of target mRNA transcripts [3, 5, 6]. MicroRNAs are thus considered essential components of the overall cellular regulatory machinery and aberrant microRNA expression is often associated with human disorders such as cancer [7–9]. The functions of individual microRNAs within specific cancer types can be defined by their expression levels and transcriptional targets in that over-expressed microRNAs are often classified as oncogenic while down-regulated microRNAs are classified as tumor-suppressors [7, 9].
The p53 tumor suppressor protein is a transcription factor whose inactivation often occurs concomitantly with cancer progression [10, 11]. This tumor suppressor stimulates expression of specific sets of target genes including the non-coding RNA gene promoter of the miR-34a microRNA. miR-34a is a 23 nucleotide microRNA first linked to cancer regulatory mechanisms due to its observed down-regulation in neuroblastomas [8, 12]. It has been proposed that upon activation in normal cells, p53 transcriptionally up-regulates miR-34a to exert its repressive effects, and that in the mutant p53 or null p53 genotype, miR-34a levels remain low and relatively unchanged [12–14]. Recent studies have demonstrated that miR-34a mediates key steps within the p53 apoptotic and anti-proliferative response, in part, by mediating repression of Bcl-2 and other anti-apoptotic proteins, inhibition of chromatin silencers of p53 target genes that promote p53-dependent transcription, and down-regulation of cell cycle regulatory proteins including cyclin dependent kinase 4 (CDK4), CDK6, and cyclin D1 as well as the transcription factors N-Myc and E2F3 [12]. Consistent with the functions of these target genes, ectopic expression of miR-34a in human cancer cell lines can induce cell cycle arrest, senescence and/or apoptosis [12, 13].
In cancerous lesions, alterations in the p53 and the miR-34a gene loci interrupt their inhibition on cell proliferation. The p53 gene is mutated in nearly half of all human tumors while CpG methylation of the miR-34a promoter has been detected in the most commonly diagnosed carcinomas, sarcomas and associated cell lines [10, 15, 16]. Elevated levels of miR-34a in breast cancer patients are associated with decreased lymph node metastasis and a lower risk of recurrence or death from the disease, while ectopic expression of miR-34a in human breast cancer cells decreased chemotherapy resistance and inhibited cell proliferation and metastasis [17–21]. p53 and miR-34a thus have clinical value as diagnostic indicators for cancer and as potential targets of therapeutic strategies to stimulate levels of endogenous miR-34a.
One strategy to identify potential anti-cancer compounds that regulate specific microRNA is to characterize the microRNA regulatory potential of plant-based therapeutics with established anti-proliferative properties in human cancer. Two such intriguing classes of phytochemicals include indole-carbinol compounds such as indole-3-carbinol (I3C), derived from cruciferous vegetables such as broccoli, cauliflower and cabbage, as well as the anti-malarial compounds artemisinin isolated from the sweet wormwood plant Artemisia Annua and its semi-synthetic derivative artesunate formed by the carbonyl reduction of artemisinin. I3C and the artemisinin-based compounds have been shown to possess potent anti-proliferative and pro-apoptotic properties in a variety of human cancer cell lines and tumor xenografts [22–26]. Both classes of phytochemicals have also been the focus of clinical trials due to their reduced side effects in normal cells and pronounced anti-tumorigenic activities [23, 26].
Our previous work has shown that I3C arrests the proliferation of human preneoplastic mammary epithelial cells through stabilization of wild type p53, implicating a potential role for downstream targets, such as miR-34a, in this indole carbinol response [27]. We and others have also observed that artemisinin and its derivatives mediate their proliferative arrest in reproductive cancer cells and other cancer cell types expressing either wild-type or mutant p53 indicating that this class of phytochemical may stimulate miR-34a expression regardless of p53 mutational status [28–32]. However, little is known about the potential effects of artemisinin compounds or I3C on microRNA expression. We now demonstrate that artemsinin and artesunate upregulate miR-34a to direct the down-regulation of CDK4, independent of wild-type p53 while, in contrast, I3C stimulation of miR-34a expression requires the presence of wild-type p53.
MATERIALS & METHODS
Cell culture
Cells were grown to sub-confluency in a humidified incubator at 37°C containing 5% CO2. MCF-7 and T47D cell lines were cultured as described by the American Tissue Culture Collection (Manassas, VA). Cells were treated for the indicated time points in complete medium with indole-3-carbinol (Sigma-Aldrich, St. Louis, MO), artemisinin or artesunate (Sigma-Aldrich, St. Louis, MO) dissolved 1000X in DMSO. Pure DMSO (Sigma-Aldrich, St. Louis, MO) added at 1 μl/1 ml medium for the control treatments. The medium was changed every 24 hours for the duration of each experiment.
Flow cytometry
For cell cycle analysis, attached and non-adherent cells treated in 6-well plates were collected within the media, rinsed with PBS, fixed in 70% ethanol overnight, and hypotonically lysed in 0.5 ml of propidium iodide buffer (0.5mg/ml propidium iodide, 0.1% sodium citrate, 0.05% Triton X-100). Samples were analyzed on a Beckman-Coulter (Fullerton, CA) EPICS XL flow cytometer with laser output adjusted to deliver 15 MW at 488 nm. Ten thousand cells were counted. Cell cycle analysis was then performed using MultiCycle software WinCycle 32 (Phoenix Flow Systems, San Diego, CA).
RNA extraction
Cells were harvested in 1.0 ml TRIzol reagent (Invitrogen, Carlsbad, CA) and total RNA extracted following the manufacturer’s protocol with the phase separation procedure being performed twice to extract microRNA. Removal of contaminating DNA was performed on 10μg of extracted RNA using a DNA-free Kit (Invitrogen, Carlsbad, CA) per the manufacturer’s protocol. RNA integrity was confirmed by running a 1.5% formaldehyde (Sigma-Aldrich, St. Louis, MO) denaturing agarose gel (Invitrogen, Carlsbad, CA) using 1μg of RNA per sample and visualizing intact bands corresponding to the molecular weights of the 28S and 18S subunits of ribosomal RNA. Gels contained GelRed Nucleic Acid Gel Stain (Biotium, Hayward, CA) diluted to a 2X concentration for band visualization using short wavelength ultraviolet light.
Reverse transcription and real-time PCR analyses
Total RNA was reverse transcribed using stem loop primers for miR-34a as well as random primers for β-actin or GAPDH, housekeeping genes insensitive to artemisinins or indole-3-carbinol treatment respectively. Each reverse transcriptase reaction contained 10XRT buffer, 100mM dNTPS, 50U/μl MultiScribe reverse transcriptase, and 20U/μl RNAse inhibitor (Applied Biosystems, Foster City, CA) dissolved in nuclease-free water. The reverse transcription reaction for β-actin and GAPDH contained 100ng of purified total RNA as well as 10X random primers while the reaction for microRNA reverse transcription contained 560 ng of purified total RNA and 5X miR-34a stem-loop RT primer (Applied Biosystems, Foster City, CA). The microRNA reactions were incubated in a thermal cycler for 30 minutes at 16°C, 30 minutes at 42°C, and 5 minutes at 85°C while reactions for the control gene were incubated for 10 minutes at 25°C, 120 minutes at 37°C, and 5 minutes at 85°C.
Taqman microRNA assays (Applied Biosystems, Foster City, CA) were used to quantify the levels of mature miR-34a. Real-time PCR reactions (10 μl volume) were performed in triplicate, and each reaction mixture included 4 μl of diluted RT product (1:2 dilution), 5 μl of 2X TaqMan Universal PCR Master Mix, 0.2 μM TaqMan probe, 1.5 μM forward primer, 0.7 μM reverse primer with the probes and primers specific to mature miR-34a, human β-actin or human GAPDH (Applied Biosystems, Foster City, CA). Reactions were incubated in an Applied Biosystems 7900HT Fast Real-Time PCR system in 96-well plates at 95°C for 10 min, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 min. Changes in fluorescence levels of miR-34a were normalized to β-actin or GAPDH, and fold changes compared between the target sample (miR-34a levels in cells treated with phytochemicals) and calibrator samples (miR-34a levels in DMSO treated cells).
Immunoblotting
Cell extracts were harvested in RIPA lysis buffer containing inhibitors, and standardized to 20–30μg protein using the Bradford protein assay. Equal protein amounts were subjected to SDS-PAGE on an 8% or 10% poly-acrylamide gel, transferred onto nitrocellulose membranes, and incubated for 1 hour at room temperature with blocking solution (5% milk dissolved in TBST). Primary antibodies to p53, p53 phosphorylated at serine residue 15, HSP90 (Cell Signaling Technology, Beverly, MA) p21, actin, CDK4, CDK6 and cyclin D1 (Santa Cruz Biotechnologies, Santa Cruz, CA) were incubated overnight at 4°C. After three washes in 5% milk dissolved in TBST, membranes were incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies for 1.5 hours at room temperature. Following an additional five washes in 5% milk, chemo luminescent signals were generated by incubation with Western Lightning ECL reagents according to the manufactures instructions (Perkin Elmer, Shelton, CT) and the results transferred to ECL-sensitive film (GE Healthcare, United Kingdom).
Transfections and luciferase assay
100nM miR-34a or control locked nucleic acid inhibitors (Exiqon, Woburn, MA) were transfected into MCF-7 and T47D according to the manufacturer’s instructions with Lipofectamine 2000 reagent (Applied Biosystems, Foster City, CA) while 2μg of indicated plasmids were transfected using Superfect reagent (Qiagen, Germantown, Maryland). Upon overnight incubation, cells were treated with or without 300μM artemisinin or varying doses of artesunate for 48 hours and harvested in ice-cold PBS. The cells were then centrifuged at 14,000 rpm for 5 minutes at 4°C, combined with 1X Passive lysis buffer, and Promega Luciferase assay performed according to the manufacturer’s instructions (Promega, San Luis Obispo, CA). Relative light units were normalized to protein expression as determined by the Bradford protein assay. Dominant negative p53 was purchased from Clontech Laboratories (Mountain View, CA). CDK4-miR-34a and ΔCDK4-miR-34a luciferase constructs were generated as described previously [14].
RESULTS
I3C stimulation of miR-34a expression is a wild type p53-dependent response in human breast cancer cells
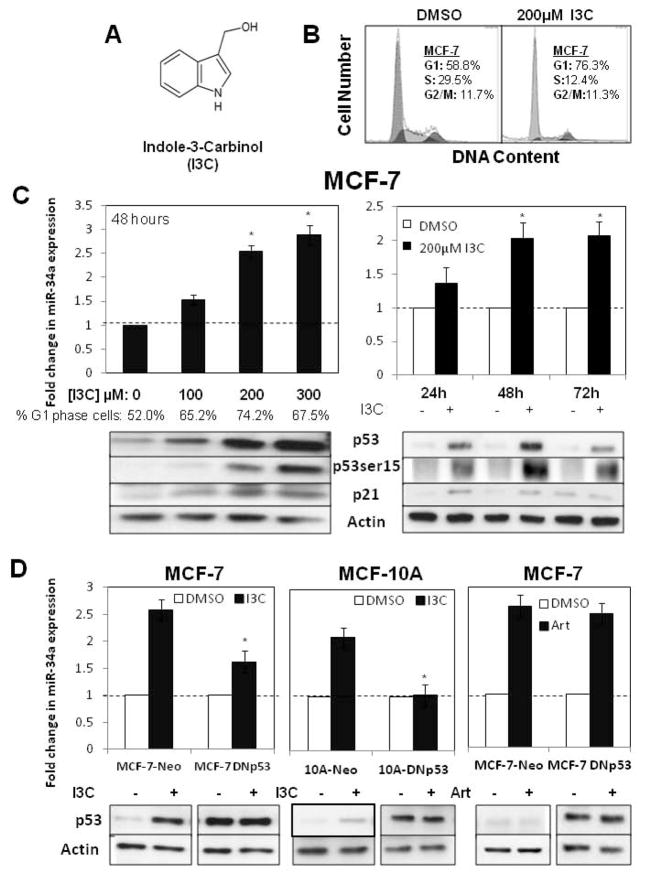
Relatively little is known about the abilities of natural anti-proliferative phytochemicals to regulate expression of miR-34a, a tumor suppressive microRNA that is highly associated with the presence of wild type p53 [12,14]. We initially tested the possibility that indole-3-carbinol (I3C) (structure in Fig 1A) can stimulate miR-34a expression concomitant with its anti-proliferative effects in MCF-7 human breast cancer cells, which display a luminal A cancer subtype that expresses relatively low levels of wild type p53 protein [33,35]. To define the proliferative context of the experiments, flow cytometry analysis of propidium iodide stained nuclei revealed that 48 hour treatment with 200 μM I3C induced a pronounced G1 cell cycle arrest compared to cells treated with the DMSO vehicle control (Fig 1B). The 200 μM concentration of I3C is the optimal amount required to observe the maximal arrest of cell proliferation in several types of cancer cells [22–30] including breast cancer cells, due in part to the low 0.3% efficiency by which I3C enters cells [34]. Under these conditions, a sub-G1 peak, which is typically indicative of apoptosis, was not detected. To assess the potential effects of I3C on miR-34-a expression, in one experiment MCF-7 cells were treated for 48 hours with several concentrations of I3C (0 μM, 100 μM, 200 μM or 300 μM) and in another experiment cells were treated with or without 200 μM I3C for up to 72 hours, and the levels of expressed miR-34a determined by real time quantitative PCR. Changes in fluorescence levels of amplified miR-34a sequences were normalized to the levels of GAPDH control transcripts. After 48 hours treatment, I3C dose dependently stimulated miR-34a expression, with near maximal induction observed at 200 μM I3C (Fig 1C, left panel). The time course analysis revealed that stimulation in miR-34a expression was first detected at 24 hours treatment with 200 μM I3C and that maximal expression of this microRNA was observed after 48 hours (Fig 1C, right panel). Western blots of total cell extracts revealed that I3C strongly induced p53 protein levels, with the accumulation of p53 dose dependently correlated with the maximal stimulation of miR-34a expression and G1 cell cycle arrest (Fig 1C). Importantly, I3C strongly up-regulated the level of serine 15 phosphorylated p53, a stabilized and active form of this tumor suppressor protein [27], in addition to increasing levels of the p21 cyclin dependant kinase inhibitor, a known p53 target gene (Fig 1C).
Figure 1.
Indole-3-carbinol stimulation of miR-34a is a wild type p53-dependent response. A) Chemical structure of indole-3-carbinol (I3C). B) MCF-7 human breast cancer cells were treated with 200 μM I3C or the DMSO vehicle control for 48 hours and the cell populations DNA content quantified by flow cytometry of propidium iodide stained nuclei. C) MCF-7 cells were either treated for 48 hours with various I3C concentrations (0 μM, 100 μM, 200 μM or 300 μM) or treated with 200 μM I3C throughout a 72 hour time course. The bar graphs display the average fold change in miR-34a expression as determined by real-time PCR. The data are normalized to GAPDH levels from triplicate samples in three independent experiments. Error bars indicate standard deviation and the dotted line represents the real-time PCR base line.* p <0.05. Western blots of electrophoretically fractionated total cell extracts were probed for p53, serine-15 phosphorylated p53, p21 and the actin gel loading control using the corresponding antibodies. The western blots are representative of three independent experiments. The percentage of G1 phase cells was determined by flow cytometry of propidium iodide stained nuclei. D) MCF-7 cells or preneoplastic human mammary epithelial MCF-10A cells were treated with a dominant negative p53 expression vector (DNp53) or with the empty vector control (Neo). Cells were treated with or without 200 μM I3C (left and middle panel) or with or without 300 μM artemisinin (right panel). The levels of expressed miR-34a was determined by real-time PCR and the levels of p53 protein and the actin gel loading control determined by western blots. Bar graphs represent average fold change in miR-34a levels normalized to expression of β-actin or GAPDH from triplicate samples as determined in three independent real-time PCR experiments. Error bars indicate standard deviation and the dotted line represents the real-time PCR base line.* p <0.05.
To functionally assess the role of active p53 in the I3C stimulation of miR-34a, MCF-7 cells were transfected with a dominant negative p53 expression vector (DNp53) or an empty neomycin control vector (Neo), forming MCF-7 DNp53 and MCF-7-Neo cells, respectively. Transfected breast cancer cells were then treated with or without 200 μM I3C for 48 hours. Western blot analysis revealed that, cells transfected with the DNp53 vector expressed similarly high levels of p53 protein, whereas endogenous p53 is fully I3C responsive in MCF-7-Neo cells (Fig 1D, left panels). Detection of miR-34a by real time quantitative PCR revealed that expression of dominant negative p53 strongly attenuated the I3C stimulation of miR-34a expression in MCF-7 cells (Fig 1D, left panels).
To determine whether the p53 dependence on I3C stimulation of miR-34a occurs in other wild type p53 expressing mammary epithelial cell lines, human MCF-10A cells were similarly transfected with either the dominant negative p53 expression plasmid or the control expression plasmid, forming 10A-DNp53 and 10A-Neo cells. As shown in Figure 1D (middle panels), the I3C stimulation of miR-34a and p53 protein in MCF-10A cells was completely ablated after expression of dominant negative p53. (The artemisinin response in dominant negative expressing MCF-7 cells, shown in the right panels of Figure 1D, will be discussed in the following section.) Thus, consistent with other studies characterizing miR-34a expression in human cancer cells [12], our results demonstrate that the I3C stimulation of miR-34a in breast cancer cells and in preneoplastic mammary epithelial cells is dependent on the presence of wild type p53.
The anti-cancer phytochemical artemisinin stimulates miR-34a expression independent of p53 mutational status
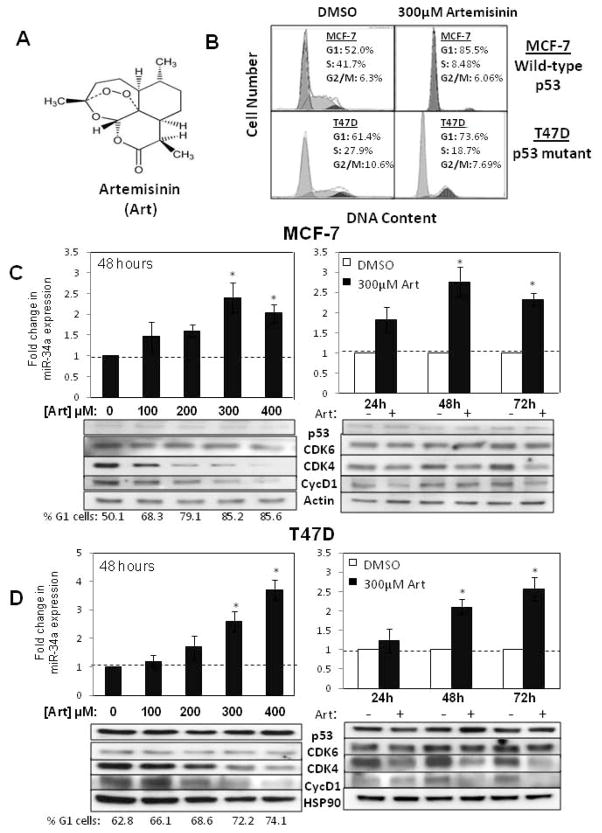
We have previously demonstrated that artemisinin, a natural sesquiterpene lactone (structure in Fig 2A), has a strong anti-proliferative effect in human breast cancer cells [28]. The wild type p53 expressing MCF-7 cells, and mutant p53 expressing T47D cells [33,35], which are both estrogen responsive human breast cancer cell lines, were used to determine whether artemisinin stimulates miR-34a expression coordinately with its anti-proliferative response, and whether p53 mutational status dictates this potential effect. To confirm the anti-proliferative effects of artemisinin, flow cytometry of propidium iodide stained nuclei revealed that 48 hour treatments with 300 μM artemisinin induced a strong G1 cell cycle arrest in both MCF-7 cells and T47D cells compared to cells treated with the DMSO vehicle control (Fig 2B). This concentration of artemisinin is needed to maximally inhibit the proliferation of human breast cancer cells [28]. It is important to point out that in these flow cytometry analyses, a sub-G1 peak, which is typically indicative of apoptosis, was undetected in either cell line at all tested time points and concentrations, and a western blot analysis revealed that the levels of the apoptotic proteins PARP and Bax remained unchanged (data not shown). Therefore, under conditions in which a strong G1 cell cycle arrest is observed, there were no obvious effects on apoptosis.
Figure 2.
Artemisinin stimulation of miR-34a expression is independent of the presence of wild type p53. A) Chemical structure of artemisinin. B) Wild type p53 expressing MCF-7 breast cancer cells or mutant p53 expressing T47D breast cancer cells were treated with 300 μM artemisinin or the DMSO vehicle control for 48 hours and the cell populations DNA content quantified by flow cytometry of propidium iodide stained nuclei. C) MCF-7 cells were either treated for 48 hours with various artemisinin concentrations (0 μM, 100 μM, 200 μM, 300 μM or 400 μM) or treated with 300 μM artemisinin throughout a 72 hour time course. Bar graphs represent the average fold change in miR-34a expression as determined by real-time PCR. The data are normalized to β-actin levels from triplicate samples in three independent experiments. Error bars indicate standard deviation and the dotted line represents the real-time PCR base line.* p <0.05. Western blots of electrophoretically fractionated total cell extracts were probed for p53, CDK6, CDK4, cyclin D1, and the actin gel loading control using the corresponding antibodies. The western blots are representative of three independent experiments. The percentage of G1 phase cells was determined by flow cytometry of propidium stained nuclei. D) T47D cells were treated with or without the indicated concentrations and time duration of artemisinin. Real time PCR expression of miR-34a levels, percentage of G1 phase cells and western blot analyses were performed as described above. * p <0.05.
Both MCF-7 and T47D breast cancer cell lines were treated for 48 hours with various doses of artemisinin (0 μM, 100 μM, 200 μM, 300 μM or 400 μM) or treated with either 300 μM artemisinin or the DMSO vehicle control over at 72 hour time course (24 hours, 48 hours or 72 hours), and then the effects on miR-34a expression examined by real-time quantitative PCR in comparison to control beta-actin transcripts. Artemisinin strongly induced miR-34a levels in breast cancer cells expressing either wild type p53 or mutant p53, with a maximal induction of 2.5 fold or above observed in MCF-7 cells treated for 48 hours with 300 μM artemisinin and in T47D cells treated for 72 hours with 300 μM artemisinin or 48 hours with 400 μM artemisinin (Fig 2C and 2D). The increased expression of miR-34a dose dependently correlated with the artemisinin mediated G1 cell cycle arrest of T47D and MCF-7 cells (Fig 2D and Fig 2C, left panels).
The p53-independent effects of artemisinin on miR-34a expression were functionally tested in MCF-7 cells in comparison to the effects of I3C in cells transfected with either the dominant negative p53 expression plasmid (MCF-7-DNp53) or an empty neomycin control vector (MCF-7-Neo). Cells were treated with or without 300 μM artemisinin for 48 hours and miR-34a expression analyzed by real-time quantitative PCR. As shown in figure 1D (right panels), artemisinin strongly induced miR-34a levels in the presence or absence of a dominant negative p53. In contrast to artemisinin response, as described in a previous section, in these same transfected MCF-7 cells, expression of the dominant negative p53 strongly attenuated the I3C induction of miR-34a transcripts (Fig 1D, left panels). Furthermore, in contrast to I3C, western blots revealed artemisinin treatment had no effect on p53 protein levels in MCF-7 cells (Fig 1D right panels and Fig 2C).
To determine if artemisinin regulates miR-34a cell cycle target genes [12] in cells expressing wild type p53 or mutant p53, the levels of the cell cycle genes CDK4, CDK6 and cyclin D1 were examined over a 0–400 μM concentration range of artemisinin and throughout a 72 hour time course of cells treated with 300 μM artemsinin. Western blot analyses revealed that in both MCF-7 cells and T47D cells, artemisinin down regulated the production of CDK4 and cyclin D1 while CDK6 levels remained unchanged. The dose dependent loss of CDK4 and cyclin D1 correlated with miR-34a stimulation (Fig 2C and Fig 2D). The western blots also revealed that after artemisinin treatment, the level of wild type p53 in MCF-7 cells remained at low levels whereas the level of mutant p53 protein in T47D cells remained consistently high (Fig 2C and Fig 2D). The p53 mutation within T47D cells is located within the DNA binding domain, rendering the protein non-functional while increasing its cellular stability [10, 35,36]. Taken together, our results indicate that artemisinin upregulated miR-34a expression in human breast cancer cells independent of p53 mutational status. Because relatively little is known about the regulated expression of miR-34a in cells displaying a mutant p53 genotype, the artemisinin regulation of miR-34a and its downstream CDK4 target gene was explored in more detail.
Loss of functional miR-34a reverses artemisinin inhibition of CDK4 and attenuates artemisinin mediated cell cycle arrest
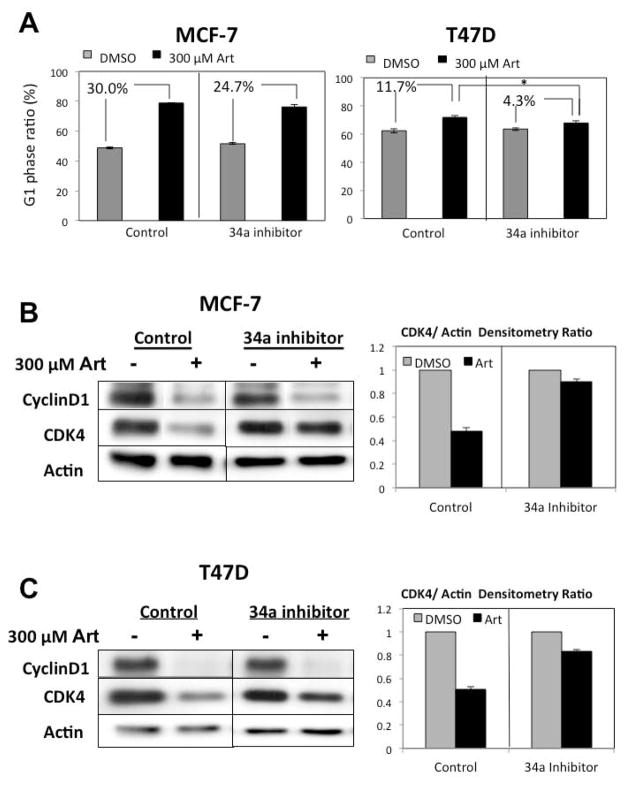
To functionally examine the mechanistic link between the artemisinin stimulation of miR-34a expression and the effects on cell cycle control, wild type p53 expressing MCF-7 cells and mutant p53 expressing T47D cells were transfected with a miR-34a locked nucleic acid inhibitor or a non-specific control sequence. The transfected cells were treated for 48 hours with or without 300 μM artemisinin, the optimal concentration that induces miR-34a expression, and the percentage of cells in the populations arrested in the G1 cell cycle phase was determined by flow cytometry of propidium iodide stained nuclei. As shown in Figure 3A, the inhibition of miR-34a function diminished, but did not completely reverse, the artemsinin mediated G1 cell cycle arrest in both breast cancer cell lines. In another set of transfected breast cancer cells, the levels of CDK4 and cyclin D1 determined by western blots of total cellular extracts. As show in Figure 3B and Figure 3C, transfection of the miR-34a inhibitor ablated the artemisinin down regulation of CDK4 in both MCF-7 cells and T47D cells. In contrast, the disruption of miR-34a function had no effect on the artemisinin down regulation of cyclin D1. These results demonstrate that in breast cancer cells, functional miR-34a is required for the artemisinin mediated down-regulation of CDK4 regardless of p53 mutational status.
Figure 3.
Loss of functional miR-34a reverses artemisinin inhibition of CDK4 and attenuates the artemisinin mediated cell cycle arrest of cells expressing either wild type or mutant p53. A) Wild-type p53 expressing MCF-7 cells (left panels) or mutant p53 expressing T47D cells (right panels) were transfected with 100 nM control or locked nucleic acid inhibitors against miR-34a as described in the “Materials and Methods” section and then treated with or without 300 μM artemisinin for 48 hours. The G1 phase ratio of the total cell population was determined by flow cytometry of propidium iodide stained nuclei from three independent experiments. Error bars indicate standard deviation. * p<0.05. B & C) MCF-7 cells or T47D cells were transfected with 100 nM control or locked nucleic acid inhibitors against miR-34a and then treated with or without 300 μM artemisinin for 48 hours. Western blot analysis was performed on total cell extracts using antibodies specific to cyclin D1, CDK4 or actin as a gel loading control. The bar graphs display the quantification of CDK4/actin protein levels from a densitometric analysis of three independent experiments.
miR-34a directly inhibits CDK4 expression upon artemisinin treatment
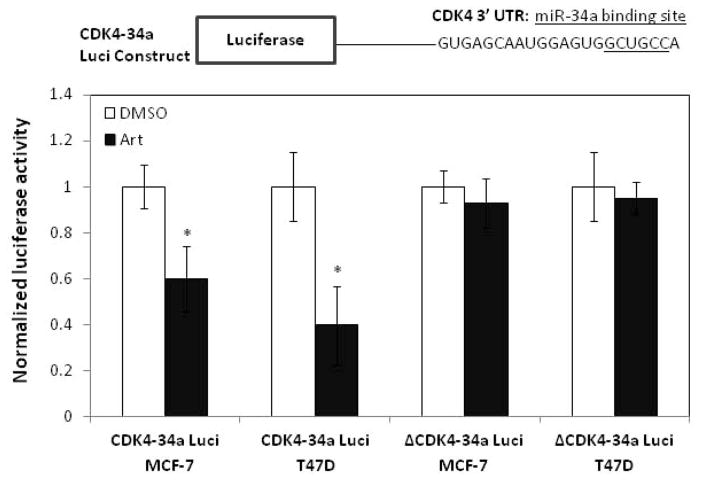
To determine if the artemisinin stimulation of miR-34a is directly responsible for the loss of CDK4 expression, MCF-7 and T47D cells were transfected with either a luciferase construct containing the miR-34a binding site in CDK4 mRNA attached to the firefly luciferase gene (CDK4-34a Luci), or a luciferase construct containing a mutated miR-34a binding site (ΔCDK4-34a Luci). Transfected cells were treated with or without 300 μM artemisinin for 48 hours, and luciferase activity assayed and normalized to total protein. As shown in Figure 4, artemisinin significantly decreased luciferase activity in MCF-7 and T47D cells transfected with the CDK4-34a Luci construct while luciferase levels remained unchanged in artemisinin treated cells transfected with the ΔCDK4-34a Luci construct. These results demonstrate that miR-34a induction by artemsinin is directly responsible for the down-regulation of CDK4 transcripts in both wild-type p53 and mutant p53 human breast cancer cells.
Figure 4.
miR-34a directly inhibits CDK4 expression upon artemisinin treatment.
Wild type p53 expressing MCF-7 cells and mutant p53 expressing T47D cells were transfected with a luciferase construct containing the miR-34a binding site in CDK4 mRNA attached to the firefly luciferase gene (CDK4-34a Luci). A parallel set of cells was transfected with a control CDK4 construct containing a mutated miR-34a binding site (ΔCDK4-34a Luci). Transfected cells were treated with or without 300 μM artemisinin for 48 hours, and a luciferase assay performed on harvested cells as described in the “Materials and Methods” section. Bar graphs indicate average light units normalized to protein expression and represent the average of triplicate samples from three independent experiments. The error bars indicate standard deviation.* p <0.01.
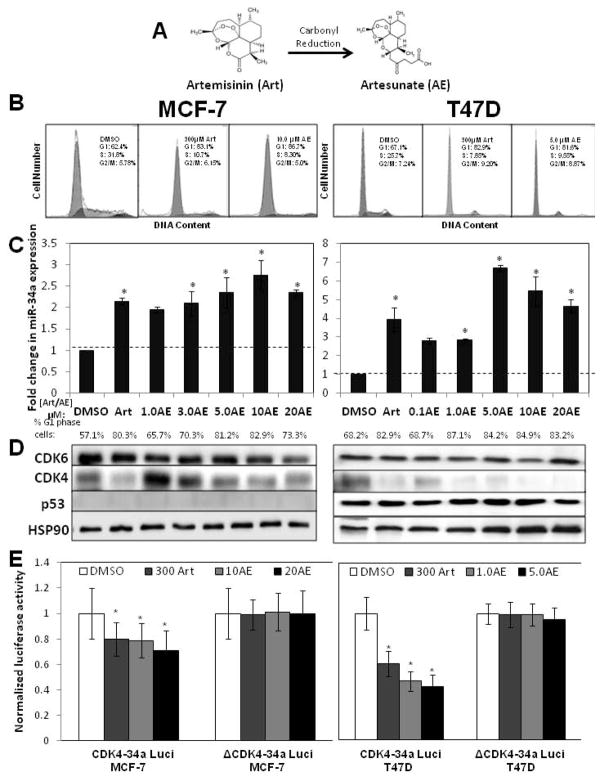
The highly potent artemisinin derivative artesunate stimulates miR-34a expression and down regulates CDK4 expression with enhanced efficiency to artemisinin in both wild type p53 and mutant p53 expressing human breast cancer cells
Artesunate is a semi-synthetic derivative of artemisinin with increased water solubility and enhanced activity against malaria [26, 37] (See Figure 5A for chemical structure). Recent studies suggest that artesunate mediated proliferative arrest can be observed at significantly lower concentrations than that needed with artemisinin in p53 mutant and p53 null cancer cell lines along with decreased expression of CDK4 [25, 31]. To determine if artesunate regulates miR-34a expression concomitant with its proliferative arrest in human breast cancer cells, MCF-7 and T47D cells were treated for 48 hours with or without various concentrations (0 μM, 1 μM, 3 μM, 5 μM, 10 μM and 20 μM) of artesunate (AE) or 300 μM artemisinin, and miR-34a expression determined by real-time quantitative PCR standardized to control beta-actin transcripts. As shown in Figure 5C, artesunate induced miR-34a expression at concentrations well below that of the optimal concentration required for the parental compound artemisinin, with maximal miR-34a expression observed at approximately 5 μM to 10 μM artesunate.
Figure 5.
Enhanced potency of the artemisinin-derivative artesunate on stimulated expression of miR-34a, cell cycle arrest and inhibition of CDK4 expression in human breast cancer cells. A) Chemical synthesis of artesunate (AE) from artemisinin (Art). B) Wild type p53 expressing MCF-7 breast cancer cells or mutant p53 expressing T47D breast cancer cells were treated with or without 300 μM artemisinin, the vehicle control DMSO, or with the indicated concentration (5 μM or 10 μM) of artesunate for 48 hours and the cell populations DNA content quantified by flow cytometry of propidium iodide stained nuclei. C) MCF-7 cells (left panels) or T47D cells (right panels) were treated for 48 hours with various artesunate (AE) concentrations (0 μM, 1 μM, 3 μM, 5 μM, 10 μM or 20 μM) or with 300 μM artemisinin (Art). Bar graphs represent the average fold change in miR-34a expression levels as determined by real-time PCR. The data are normalized to β-actin levels from triplicate samples in three independent experiments. Error bars indicate standard deviation and the dotted line represents the real-time PCR base line.* p <0.05. The percentage of G1 phase cells was determined by flow cytometry of propidium stained nuclei. D) Total cell extracts from cells treated with or without the indicated concentrations of artemisinin or artesunate as described above were electrophoretically fractionated and western blots probed for CDK6, CDK4, p53 and the HSP90 gel loading control using the corresponding antibodies. The western blots are representative of three independent experiments. E) MCF-7 (left panels) and T47D cells (right panels) were transfected with CDK4 luciferase constructs containing either the wild-type (CDK4-34a Luci) or mutated miR-34a binding site (ΔCDK4-34a Luci). Cell were treated with or without 300μM artemisinin or the indicated concentrations of artesunate for 48 hours and a luciferase assay performed on harvested cells as described in the “Materials and Methods” section. Bar graphs indicate average light units normalized to protein expression and represent the average of triplicate samples from three independent experiments. Error bars indicate standard deviation. * p <0.01.
Consistent with the effects of the parental compound artemisinin, the stimulation in miR-34a expression by artesunate was observed in both wild type p53 expressing MCF-7 cells and in mutant p53 expressing T47D cells. Treatment of either breast cancer cell line with artesunate concentrations that maximally stimulated miR-34a expression induced a strong G1 cell cycle arrest that is approximately equivalent to that observed with 300 μM artemisinin (Fig 5B and Fig 5C). Western blot analysis of total cell extracts revealed that artesunate strongly down regulated the miR-34a target gene CDK4 in both MCF-7 and T47D cells at concentrations well below that needed with artemisinin (Fig 5D). The loss of CDK4 production generally dose dependently correlated with the stimulation in miR-34a expression. Also, similar to the parental compound artemisinin, artesunate did not alter either the low levels of wild type p53 expressed in MCF-7 cells or the higher levels of mutant p53 produced in T47D cells.
To determine whether the artesunate stimulation of miR-34a directly down regulates CDK4 expression, MCF-7 and T47D cells were transfected with luciferase constructs containing either the wild-type (CDK4-34a Luci) or mutant (ΔCDK4-34a Luci) miR-34a binding site in the CDK4 mRNA attached to the firefly luciferase gene. Transfected MCF-7 and T47D cells were treated with or without concentrations of artesunate that significantly stimulate miR-34a expression, or 300 μM artemisinin as a positive control, for 48 hours and the cell extracts assayed for luciferase activity. As shown in Figure 5E, artesunate strongly decreased luciferase activity in MCF-7 and T47D cells transfected with the CDK4-34a Luci construct while levels of luciferase activity in the ΔCDK4-34a Luci transfected cells remained resistant to artesunate treatment (Figure 5E). Furthermore, the effects of artesunate were quantitatively similar or even greater than those observed with cells treated with 300 μM artemisinin. Taken together, our results show that miR-34a expression can be strongly stimulated by artemisinin-based compounds independent of p53 mutational status and in a manner that corresponds to the proliferative arrest of human breast cancer cells.
DISCUSSION
MicroRNAs have been linked to proliferative, cell survival, apoptotic and tumorigenic processes in several tissue types [3, 4], thus manipulation of individual microRNA levels represents intriguing targets for anti-cancer therapeutics. One strategy to identify compounds that could target specific microRNA is the use of natural phytochemicals in which their anti-cancer activities, enhanced efficacy over conventional chemotherapy and reversal of the epithelial-to-mesenchymal transition is associated with changes in expression of microRNAs such as miR-34a in cultured cancer cells as well as in vivo tumors [38, 39]. miR-34a represents a well-established tumor suppressive microRNA transcriptionally stimulated by wild type p53 and shown to play a role in p53 dependent regulation of apoptosis, cell cycle control and senescence in cancer cells [8, 12, 13]. Several clinical studies have shown a strong reverse correlation between expression of miR-34a and breast cancer. For example, miR-34a levels are significantly down regulated in nearly 30–50% of breast tumor samples, whereas, elevated levels of miR-34a in breast cancer patients are associated with lower risk of recurrence or death from the disease [18, 21]. Our results show that wild type p53-dependent and independent mechanisms regulate miR-34a expression in human breast cancer cells and direct the anti-proliferative responses of the phytochemicals indole-3-carbinol (I3C), artemisinin and artesunate.
I3C treatment of wild-type p53 expressing human breast cancer and mammary epithelial cells stimulated miR-34a levels in a p53-dependant manner. Transfection of dominant negative p53 in either cell line disrupted the I3C induction of miR-34a, demonstrating that indole-carbinol stimulation of miR-34a requires functional p53. I3C also strongly up-regulated total levels of wild type p53 as well as the activated serine-15 phosphorylated form of the protein. Our observation is the first to show I3C can regulate expression of a tumor suppressive microRNA. Previous studies associated I3C treatment with reversal of carcinogen induced alterations in microRNA levels in rat and mouse lung tumors as well as decreased levels of the oncogenic microRNA, miR-21, in pancreatic cancer cells [40–42]. Phytochemical enhancement of miR-34a levels may be particularly relevant to target breast cancer cell proliferation as miR-34a has been shown to control breast cancer cell tumorigenesis. In vitro studies have determined that miR-34a decreases chemotherapy resistance, cell proliferation and metastasis in human breast cancer cells through processes that disrupt Akt signaling as well as inhibition of target genes such as the receptor tyrosine kinase Axl and transmembrane receptor Notch-1 [17, 19, 20,43]. In addition, reduced miR-34a levels were associated with the progression of rat mammary carcinogenesis [44], while exogenous expression of miR-34a inhibited the estrogen-mediated formation of breast cancer cell-derived tumor xenografts in a murine model [45].
In contrast to I3C, the anti-malarial compounds artemisinin and its highly potent semi-synthetic derivative artesunate strongly stimulated miR-34a expression in human breast cancer cell lines regardless of p53 mutational status. Each compound upregulated miR-34a levels in wild-type p53 expressing (MCF-7) or mutant p53 (T47D) expressing cells, while exogenous expression of dominant negative p53 had no effect on artemisinin induction of miR-34a in MCF-7 cells. Functional miR-34a appears critical for the anti-proliferative effects of artemisinin in human breast cancer as transfection of non-translatable miR-34a inhibitors attenuated artemisinin mediated cell cycle arrest in either cell line, and reversed the artemisinin mediated down-regulation of CDK4, a known miR-34a target [12, 14]. While a recent study correlated the artemisinin derivative dihydroartemisinin with microRNA regulation in gastric cancer [46], our work is the first to demonstrate that artemisinin and artesunate regulated microRNA expression as part of their anti-proliferative responses. Transfection of the miR-34a inhibitors attenuated, but did not completely ablate, the artemisinin cell cycle arrest. This effect is likely due to artemisinin triggering anti-proliferative signaling events beyond those that stimulate miR-34a expression. In this regard, in the presence of miR-34a inhibitor sequences, artemisinin remained capable of down-regulating cyclin D1 levels, whereas, under these conditions, the artemisinin inhibition of the CDK4 miR-34a target is almost completely disrupted. From a technical perspective, it is also likely that the transfection efficiency of the miR-34a inhibitor sequences is not 100% and the actual effect on miR-34a function may vary somewhat from cell to cell.
The observation of a p53-independent mechanism by which miR-34a expression can be elevated suggests the intriguing possibility that miR-34a levels can be enhanced in human cancers expressing a mutant or null p53 phenotype. We are currently attempting to determine artemisinin-regulated transcription factor(s) that could act on miR-34a independent of p53. In this regard, a recent study showed that the ETS family transcription factor, Elk1, can up-regulate miR-34a expression to mediate senescence of human primary fibroblasts independent of p53 status [47]. Constitutive activation of B-RAF increased Elk1 interaction with identified binding sites in the miR-34a promoter, resulting in an up-regulation in miR-34a levels and enhanced miR-34a inhibition of c-Myc expression. The induction of miR-34a occurred in the presence of both dominant-negative p53 and p53 siRNA, whereas, siRNA mediated ablation of Elk-1 expression significantly impaired B-RAF induction of miR-34a [47]. Artemisinin and artesunate have also been shown to regulate E2F1, Sp1 or NFκB transcriptional activities in reproductive cancer cells [28, 29, 48, 49], which conceivably may drive the wild type p53-independent stimulation of miR-34a.
Artemisinin and artesunate have previously been shown to down regulate CDK4 in human breast cancer cells [25, 28]. Our observation that both compounds require the miR-34a hybridization site in the CDK4 3′ UTR to decrease CDK4 expression levels demonstrates that miR-34a stimulation mechanistically accounts for the loss of CDK4 in human breast cancer cells treated with artemisinin compounds. The overall effects of artemisinin and artesunate on the stimulation of miR-34a expression and their anti-proliferative responses were qualitatively similar, however, artesunate was significantly more potent than artemisinin, exerting its responses at approximately 30-fold to 100-fold lower concentrations. This finding is consistent with the observation that artesunate is more effective than artemisinin in reducing the viability of lung and colon cancer cell lines [50]. Artesunate was originally designed to overcome the short half-life and poor bioavailability of artemisinin in order to treat malaria with increased clinical efficiency [26, 37].
Consistent with many other reports [22–30], our study shows that breast cancer cells require relatively high concentrations of I3C and artemisinin in order to achieve the maximal cell cycle arrest and stimulated expression of miR34a. It is important to note that the functional intracellular concentration of these phytochemicals are likely to be significantly lower because of the low efficiency by which these compounds enter cells. For example only 0.3% of I3C has been shown to enter cultured breast cancer cells [34]. Pharmacokinetic studies show that after ingestion, the tissue levels of indolecarbinol compounds (and likely artemisinin-based compounds) exceed that of the plasma levels because of accumulation in specific tissues, suggesting that organ-attainable levels of these compounds in the micromolar range can potentially account for their anti-cancer effects [51, 52]. High concentrations of artemisinin or I3C are well tolerated in humans, for example cytotoxicity studies show that individuals can receive 800 mg/day of I3C [53] or 500 mg/day of artemisinin without any adverse side effects [54]. Artemisinin and I3C have anti-cancer activity both in vitro and in vivo [22–30, 51], and the development of highly potent derivatives of these natural phytochemicals [22, 24, 26, 37, 51, 55] in combination with our current studies suggest that the stimulated expression and/or activity of tumor suppressive microRNAs, such as miR-34a, provide a potential means to elucidate the efficacy and therapeutic potential of newly developed artemisinin-based and I3C-based cancer therapeutic strategies.
Acknowledgments
Grant Support: This study was initially supported by NIH Public Service grant CA102360 awarded from the National Cancer Institute, and in the later stages of the work this study was supported by CA164095 awarded from the National Cancer Institute. KH was supported by a dissertation fellowship from the University of California.
We thank KM Poindexter and R Flores of the Firestone laboratory, and YJ Choi and CP Lin, of the He laboratory, for their technical assistance and thoughtful insight on our study.
Abbreviations
- CDK
cyclin dependent kinase
- DNp53
Dominant Negative p53
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- I3C
indole-3-carbinol
References
- 1.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 3.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 5.Friedman RC, Farth KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network-another piece in the tumor-suppression puzzle. Nat Rev Cancer. 2007;7:819–822. doi: 10.1038/nrc2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croce CM, Calin GA. MicroRNA-Cancer Connection: The beginning of a new tale. Cancer Research. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 10.Olivier M, Hollsetin M, Hainaut P. Tp53 mutations in human cancers: origins, consequences and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juntilla M, Evan G. p53-a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 12.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death and Differentiation. 2009;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 13.Wong MYW, Yu Y, Walsh WR, Yang JL. microRNA-34 family and treatment of cancers with mutant or wild-type p53. International Journal of Oncology. 2011;38:1189–1195. doi: 10.3892/ijo.2011.970. [DOI] [PubMed] [Google Scholar]
- 14.He L, He X, Lim LP, et al. A microRNA component of the p53 tumor suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogt M, Munding J, Grüner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 16.Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Li Y, Gao J, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013:324294–4303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 18.Peurala H, Greco D, Heikkinen T, et al. miR-34a expression has an effect for lower risk of metastasis and associates with expression patterns predicting clinical outcome in breast cancer. PLoS One. 2011;6:e26122. doi: 10.1371/journal.pone.0026122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackiewicz M, Huppi K, Pitt JJ, et al. Identification of the receptor tyrosine kinase Axl in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat. 2011;130:663–679. doi: 10.1007/s10549-011-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Ji M, Zhong S, et al. MicroRNA-34a modulates chemosensitivity of breast cancer cells to adriamycin by targeting Notch1. Arch Med Res. 2012;43:514–521. doi: 10.1016/j.arcmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Yuan L, Luo J, Gao J, Guo J, Xie X. miR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2013;13:109–117. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 22.Firestone GL, Sundar SN. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol Endocrinol. 2009;23:1940–1947. doi: 10.1210/me.2009-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng JR, Tsai CH, Kulp S, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Letters. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:1–15. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- 25.Liu WM, Gravatt AM, Dalgleish AG. The antimalarial agent artesunate possess anticancer properties that can be enhanced by combination strategies. Int J Cancer. 2011;128:1471–1480. doi: 10.1002/ijc.25707. [DOI] [PubMed] [Google Scholar]
- 26.Efferth T. Willmar Schwabe Award 2006: Antiplasmodial and antitumor activity of artemisinin-from bench to bedside. Planta Med. 2007;73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 27.Brew CT, Aronchik I, Hsu JC, et al. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int J Cancer. 2006;118:857–868. doi: 10.1002/ijc.21445. [DOI] [PubMed] [Google Scholar]
- 28.Tin AS, Sundar SM, Tran KQ, Park AH, Poindexter KM, Firestone GL. Anti-proliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anticancer Drugs. 2012;4:370–379. doi: 10.1097/CAD.0b013e32834f6ea8. [DOI] [PubMed] [Google Scholar]
- 29.Willoughby JA, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang HT, Wang YL, Zhang J, Zhang QX. Artemisinin inhibits gastric cancer cell proliferation through upregulation of p53. Tumour Biol. 2014;35:1403–1409. doi: 10.1007/s13277-013-1193-1. [DOI] [PubMed] [Google Scholar]
- 31.Sertel S, Eichhorn T, Simon CH, Plinkert PK, Johnson SW, Efferth T. Pharmacogenomic identification of c-Myc/Max-regulated genes associated with cytotoxicity of artesunate towards human colon, ovarian and lung cancer cell lines. Molecules. 2010;15:2886–2910. doi: 10.3390/molecules15042886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efferth T, Oesch F. Oxidative stress response of tumor cells: microarray-based comparison between artemisinins and anthracyclines. Biochem Pharmacol. 2004;68:3–10. doi: 10.1016/j.bcp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staub RE, Feng C, Onisko B, Bailey GS, Firestone GL, Bjeldanes LF. Fate of indole-3-carbinol in cultured human breast tumor cells. Chem Res Toxicol. 2002 Feb;15(2):101–9. doi: 10.1021/tx010056m. [DOI] [PubMed] [Google Scholar]
- 35.Voljtĕšek B, Lane DP. Regulation of p53 protein expression in human breast cancer cell lines. J Cell Sci. 1993;105:607–612. doi: 10.1242/jcs.105.3.607. [DOI] [PubMed] [Google Scholar]
- 36.Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol. 2007;27:8284–8295. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes R, Chan HW, Lung CM, Ng NC, Wong HN, Shek LY. Artesunate and dihydroartemisinin (DHA): unusual decomposition products formed under mild conditions and comments on the fitness of DHA as an anti-malarial drug. Chem Med Chem. 2007;2:1448–1463. doi: 10.1002/cmdc.200700064. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010;6:1027–1041. doi: 10.1007/s11095-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar FH, Li Y, Wang Z, Kong D, Ali S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resistance Updates. 2010;13:57–66. doi: 10.1016/j.drup.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paik WH, Kim HR, Park JK, Song BJ, Lee SH, Hwang JH. Chemosensitivity induced by down-regulation of microRNA-21 in gemcitabine-resistant pancreatic cancer cells by indole-3-carbinol. Anticancer Res. 2013;3:1473–1481. [PubMed] [Google Scholar]
- 41.Izzotti A, Calin GA, Steele VE, Cartiglia C, Longobardi M, Croce CM. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev Res. 2010;3:62–72. doi: 10.1158/1940-6207.CAPR-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melkamu T, Zhang X, Tan J, Zeng Y, Kassie F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2009;31:252–258. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- 43.Deng X, Cao M, Zhang J, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Hui C, Yujie F, Lijia Y, et al. MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast Cancer Res. 2012;22:R80. doi: 10.1186/bcr3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao G, Guo J, Li D, et al. microRNA-34a suppresses cell proliferation by targeting LMTK3 in human breast cancer MCF-7 cell line. DNA and Cell Biology. 2013;0:1–9. doi: 10.1089/dna.2013.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun H, Meng X, Han J, et al. Anticancer activity of DHA on gastric cancer-an in vitro and in vivo study. Tumour Biol. 2013;34:3791–3800. doi: 10.1007/s13277-013-0963-0. [DOI] [PubMed] [Google Scholar]
- 47.Christoffersen NR, Shalgi R, Frankel LB, et al. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17:236–245. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 48.Tran KQ, Tin AS, Firestone GL. Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin-dependent kinase-4 promoter activity and expression by disrupting nuclear factor-κB transcriptional signaling. Anticancer Drugs. 2014;25:270–281. doi: 10.1097/CAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thanaketpaisarn O, Waiwut P, Sakurai H, Saiki I. Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-κB and PI3K/Akt signaling pathways. Int J Oncol. 2011;39:279–285. doi: 10.3892/ijo.2011.1017. [DOI] [PubMed] [Google Scholar]
- 50.McGovern PE, Christofidou-Solomidou M, Wang W, Dukes F, Davidson T, El-Deiry WS. Anticancer activity of botanical compounds in ancient fermented beverages. International Journal of Oncology. 2010;37:5–14. doi: 10.3892/ijo_00000647. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 52.Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, Hudson EA, Manbson MM. Predicting the physiological relevance of in vitro cancer preventative acivities of phytochemicals. Acta Pharmacol Sin. 2007;28:1274–1304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 53.Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- 54.Gordi T, Huong DX, Hai TN, Nieu NT, Ashton M. Artemisinin pharmacokinetics and efficacy in uncomplicated-malaria patients treated with two different dosage regimens. Antimicrob Agents Chemother. 2002;46:1026–1031. doi: 10.1128/AAC.46.4.1026-1031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aronchik I, Chen T, Durkin KA, Horwitz MS, Preobrazhenskaya MN, Bjeldanes LF, Firestone GL. Target protein interactions of indole-3-carbinol and the highly potent derivative 1-benzyl-I3C with the C-terminal domain of human elastase uncouples cell cycle arrest from apoptotic signaling. Molecular Carcinogenesis. 2012;51:881–894. doi: 10.1002/mc.20857. [DOI] [PubMed] [Google Scholar]