Abstract
Although ATP is the physiological substrate for cardiac contraction, cardiac contractility is significantly enhanced in vitro when only 10% of ATP substrate is replaced with 2’-deoxy-ATP (dATP). To determine the functional effects of increased intracellular [dATP] ([dATP]i) within living cardiac cells, we used hypertonic loading with varying exogenous dATP/ATP ratios, but constant total nucleotide concentration, to elevate [dATP]i in contractile monolayers of embryonic chick cardiomyocytes. The increase in [dATP]i was estimated from dilution of dye added in parallel with dATP. Cell viability, average contractile amplitude, rates of contraction/relaxation, spontaneous beat frequency, and Ca2+ transient amplitude and kinetics were examined. At total [dATP]i above ~70 μM, spontaneous contractions ceased, and above ~100 μM [dATP]i, membrane blebbing was also observed, consistent with apoptosis. Interestingly, [dATP]i of ~60 μM (~40% increase over basal [dATP]i levels) enhanced both amplitude of contraction and the rates of contraction and relaxation without affecting beat frequency. With total [dATP]i of ~60 μM or less, we found no significant change in Ca2+ transients. These data indicate that there is an “optimal” concentration of exogenously loaded [dATP]i that under controlled conditions can enhance contractility in living cardiomyocytes without affecting beat frequency or Ca2+ transients.
Keywords: cardiomyocyte, contractility, dATP, Ca2+ transient
There are a variety of positive inotropic mechanisms—both physiological and pharmacological—by which cardiac contractility can be enhanced. A recently recognized mechanism of some positive inotropes is direct modulation of actomyosin interactions, both in solution and in the myofilament lattice. One such effector that acts primarily via actomyosin is replacement of ATP by 2-deoxy-ATP (dATP) as substrate for cardiac muscle contraction [Regnier et al., 2000; Schoffstall et al., 2006]. With both α-myosin heavy chain (α-MHC, fast isoform) from untreated rats and β-myosin heavy chain (β-MHC, slow isoform) from propylthiouracil-treated rats, substitution of ATP with dATP as the substrate for cardiac contraction resulted in significant increases of in vitro contractile parameters [Regnier et al., 2000]. Similar enhancements were seen with β-MHC from porcine ventricular tissue, and effects were found to be significant when only 10% of total nucleotide (dATP + ATP) was dATP [Schoffstall et al., 2006]. The in vitro results imply that increased cardiac [dATP]i beyond normal physiological levels could potentially be useful as a positive inotrope in heart failure conditions.
The broader consequences of increasing [dATP]i in living cardiac myocytes—or even whether it enhances actomyosin function in vivo as in vitro—have not been explored. The major cellular role of dATP is as one of the substrates for DNA synthesis; dATP plays an additional role related to DNA replication and repair as an allosteric regulator of ribonucleotide reductase (RNR), the key enzyme for deoxy-nucleotide synthesis. Decreasing normal intracellular [dATP] via inhibition of RNR activity has been utilized as a mechanism for inhibiting DNA replication in the treatment of certain cancers [Cerqueira et al., 2005]. Further, dATP is a necessary component of the apoptosis complex (apoptosome) formed in the mitochondrial-mediated apoptosis pathway [Baines and Molkentin, 2005]. As one of several molecules in the heterogeneous apoptosome complex, dATP alone should not be sufficient to “trigger” apoptosis when present at supra-physiological levels. Mitochondrial-initiated apoptosis is a complex pathway that is triggered first by extra-mitochondrial factors that have no direct effect on, nor are affected directly by the level of dATP. These signaling processes induce mitochondria to release cytochrome C and Bcl-2. Bcl-2 normally binds Apaf-1 to the mitochondrial membrane. Extracellular ligand signaling molecules for apoptosis also must first bind to induce the formation of procaspases [Chereau et al., 2005]. Only if and when all of these factors (cytochrome C, Apaf-1, and procaspases) are released into the cytoplasm does dATP become a necessary component for activation of the apoptosome by binding to Apaf-1 [Budihardjo et al., 1999]. Additionally, Apaf-1 should already be saturated with dATP [Cain et al., 2002] because its affinity (Kd ~1.7 μM) [Jiang and Wang, 2000] is substantially below the normal intracellular level of dATP (~24–46 μM) [Traut, 1994]. It has been suggested that high levels of dATP may alter biochemical pathways in lymphocytes in vitro, resulting in caspase activation and induction of apoptotic death [Genini et al., 2000]. More recently, however, an inhibitory nucleotide binding site was identified in the apoptosome–caspase 9 complex that becomes relevant at high (>1 mM) dATP (or ATP) concentrations [Chereau et al., 2005]. Unlike lymphocytes, cardiomyocytes do not normally divide or cycle. The most direct way to determine if increased [dATP]i will affect the viability (through apoptosis or necrosis) of cardiomyocytes is to load living cardiomyocytes with exogenous dATP.
In addition to affecting cardiac myocyte viability, it is possible that increased [dATP]i would affect ATP utilizing enzymes other than actomyosin in the cardiomyocyte. Of most obvious concern within a cardiac contractility system would be the cardiac sarcoplasmic reticulum ATPase (SERCA2), which is primarily responsible for uptake of cytoplasmic Ca2+ during the “calcium transient” that, in turn, controls the kinetics and strength of cardiac contraction. SERCA2 Ca2+ transport has been shown to be enhanced with dATP in vitro, yet the rate of dATP hydrolysis appears to be approximately the same as ATP hydrolysis [Trumble et al., 1981]. Interestingly, Trumble et al. [1981] found that the NTPase activity and Ca2+ removal by the sarcolemmal (SL) ATPase transporter contrasted with that of SERCA2 by decreasing ~40% when dATP replaced ATP. Liu and Barth [2004] examined structural changes of SERCA1a (fast skeletal) with dATP versus ATP utilizing IR spectroscopy, and found the phosphorylation spectra of both to be very similar. This indicates that the 2’-OH has very little effect on the conformational change of SERCA, at least in skeletal muscle, upon phosphorylation. Neither of these studies, however, gives any indication of how these minor changes might affect function and contractility within a living, contractile cardiomyocyte.
By loading contracting embryonic chick cardiomyocytes with exogenous dATP, we performed initial investigations to address several important related questions. We sought to determine: if some level of increased intracellular dATP affects the viability of chick cardiac myocytes; if there exists an optimal [dATP]i that can enhance chick myocyte contractility ex vivo; and if increased [dATP]i affects other ATPases involved in cardiomyocyte calcium cycling. We found that [dATP]i levels above ~70 μM were associated with a reduction in contractile function, with the highest [dATP]i level (~100 μM) causing cell membrane blebbing, consistent with apoptosis. [dATP]i of ~60 μM enhanced the amplitude of contraction and the kinetics of both contraction and relaxation, but not the beat frequency, with no significant change in the Ca2+ transients. These initial cellular data in chick cardiac myocytes indicate that there may be an “optimal” concentration of dATP that results in enhanced contractility in vivo, without affecting beat frequency or Ca2+ transients. These data suggest that enhancement of actomyosin NTPase activity by dATP in cardiac cells is specific to myosin; dATP does not appear to have functionally significant effects on SERCA2 or sarcolemmal ATPase as detected in vivo.
Our findings are especially attractive because earlier strategies for treating heart failure with Ca2+-sensitizing inotropic agents were found to enhance contractility at the expense of changes in Ca2+ signaling, resulting in increased mortality [Perrone and Kaplinsky, 2005]. dATP, and other small molecules that directly enhance cardiac actomyosin activity under controlled conditions, may have therapeutic potential as positive inotropes in human heart failure [Houser and Margulies, 2003; Rodriguez et al., 2007].
MATERIALS AND METHODS
Isolation of Embryonic Chick Ventricular Cardiac Myocytes
Hearts were dissected from 11-day-old chick embryos under sterile conditions essentially as described previously with minor modifications [Ungureanu-Longrois et al., 1997; Wang, 2006]. For each cell preparation, ventricles from five to six hearts were isolated in ice-cold Ca2+/Mg2+-free Hank's balanced salt solution (in mM: 137 NaCl, 5.4 KCl, 0.25 Na2HPO4, 0.44 KH2PO4, and 4.2 NaHCO3) and minced. Ventricular fragments were gently agitated in 4 ml 0.05% Trypsin (w/v) in sterile phosphate buffered saline (PBS) solution (pH 7.0) at 37°C for four cycles of 8 min. each. Cellular suspension containing ventricular myocytes was added to ice-cold 20% Fetal Bovine Serum (FBS) Ca2+/Mg2+-free Hank's solution to inhibit tissue digestion. The cell suspension was filtered through sterile cheesecloth into a sterile conical centrifuge tube and centrifuged for 10 min. at 1,000g. The supernatant was discarded and cell pellet was resuspended in 10 ml plating media. Plating media consisted of M199 (Hyclone, Logan, UT) with 5% FBS and 0.1% penicillin–streptomycin antibiotic solution. Myocytes were purified from fibroblasts by differential adhesion pre-plating on a polystyrene culture dish for 1 h at 37°C. The myocyte-rich supernatant (non-adherent cells) was removed from the culture dish and diluted to ~350,000 cells/ml. Chemicals used in all experiments, unless otherwise indicated, were supplied by Sigma–Aldrich (St. Louis, MO).
Plating of Embryonic Chick Cardiomyocyte Monolayers
Sterile 35 mm × 60 mm No. 1 glass coverslips were separated into sterile polystyrene culture dishes, and pre-treated with laminin (BD Biosciences, Bedford, MA) for 1 h at 37°C. Two milliliters of prepared myocyte suspension was applied directly to the laminin treated glass surface and incubated for 1 h at 37°C. Fifteen milliliters plating media was added to each culture dish, and cells were incubated in a humidified 5% CO2 atmosphere at 37°C. Plating media was exchanged every 24 h. Cell monolayers began spontaneously contracting by approximately day 3 in culture. Contracting monolayers were used to measure contractile amplitude and calcium transients on days 4 and 5 in culture.
Hypertonic Loading of Cell Monolayers
Varying concentrations of dATP and/or ATP were loaded into the chick ventricular myocyte monolayers via a hypertonic solution loading method [Okada and Rechsteiner, 1982] (Fig. 1). Briefly, 2 ml of hypertonic loading media (0.5 M sucrose, 10% polyethylene glycol 1000, and variable concentrations of dATP and/or ATP in plating media) were applied to the surface of each contractile monolayer and incubated at 37°C for 10 min. Total nucleotide concentration ([dATP] + [ATP]) in the hypertonic loading media was kept constant at 6 mM. Nucleotide ratios ([dATP]/[ATP]) were: 0:6, 1:5, 2:4, 3:3, 4:2, 5:1, or 6:0. This approach was necessary for several reasons. It is possible that introduction of additional nucleotide to the cardiomyocytes (whether ATP or dATP) would have some effect on contractile parameters. In addition, because cardiac myosin utilizes the dATP as an alternative substrate to ATP [Schoffstall et al., 2006], it was important to maintain a constant total nucleotide concentration ([dATP] + [ATP]), while replacing [ATP] with [dATP] at different levels. The only way to “replace” nucleotide—in either an in vitro or ex vivo condition—is to control the overall concentration of total nucleotide. Experimentally, maintaining this constant total nucleotide concentration simulates what would be expected if conversion of ATP to dATP were used as a therapeutic treatment in vivo. In our experiments, 6 mM ATP (no dATP) served as a post-hypertonic loading control.
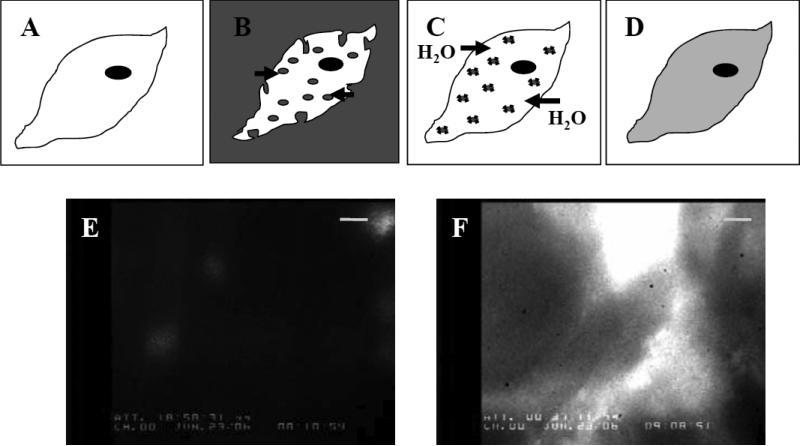
Fig. 1.
Hypertonic loading of chick cardiomyocytes in culture. Panels (A)–(D) are a cartoon representation of the hypertonic loading protocol used to introduce nucleotide into chick cardiomyocytes. A: Chick myocyte in isotonic plating media. B: Hypertonic loading media (see Materials and Methods) containing total 6 mM nucleotide (either ATP, dATP, or a combination of the two) applied to the cell; note that in some instances, loading media also contained a fluorophore (Materials and Methods). Pinocytotic vesicles carry hypertonic loading media into the cell as water is released from the cell to the hypertonic media. C: Hypotonic lysis media applied to the cell. Pinocytotic vesicles lyse within the cell cytoplasm as water enters the cell, thus releasing nucleotide. D: Cell recovery in isotonic plating medium. Panels (E) and (F) demonstrate fluorescein as an indicator of successful hypertonic loading, using fluorescence microscopy with a FITC filter at 100x magnification. Calibration bar represents 10 μm. Panel (F) shows a different region of the same cultured monolayer as panel (E). Panel (E) corresponds to (A), and panel (F) corresponds to (D). E: Chick cardiomyocyte monolayer prior to hypertonic loading. F: Monolayer after hypertonic loading with 20 μM fluorescein in the hypertonic loading media (B), and following pinocytotic lysis (C) and recovery (D). Loading of applied extracellular solution is validated by fluorescein incorporation into the cells during treatment.
As noted below, fluorescent indicators were also added to the loading media in some experiments. Immediately following hypertonic loading, the loading media was removed and 2 ml of hypotonic lysis media (M199 and sterile deionized water in a 6:4 ratio) were applied to the monolayer surface for 1.5 min. Hypotonic lysis media was removed and replaced with 5 ml plating media, then cells were allowed to recover for 30 min. at 37°C. Monolayers were then imaged by bright field and fluorescence microscopy. A pretreatment control was analyzed for each monolayer prior to loading; in most protocols experimental data could be normalized to these control data. Six millimolar ATP (no dATP) served as a post-loading control; experimental data with dATP were compared to the 6 mM ATP control for significance.
Increases in intracellular NTP concentrations in contractile monolayers of chick cardiomyocytes were estimated from a “hypertonic loading ratio” (HLR) that was calculated based on the uptake of 200 μM fluorescein in hypertonic loading media. After loading monolayers (N = 4) with 200 μM fluorescein hypertonic media, remaining extracellular fluorescein was removed by washing, and monolayers were centrifuged to form a cell pellet which was then lysed by rapid freeze-thaw. Samples were centrifuged again to pellet cell debris, and the final absorbance (A480) of each cell lysate was recorded. Two hundred micromolar of fluorescein was also incorporated into isotonic media and treated in parallel as a background control to correct for residual extracellular fluorescein that may not be removed in the washing process. Absorbance of isotonic background control was subtracted from absorbance of hypertonic loaded samples in order to determine the approximate concentration of fluorescein that had been taken up intracellularly. Concentration of each experimental lysate was determined, based on a standard A480 curve for fluorescein ranging from 20 to 200 μM, and the calculated volume of each monolayer. The HLR was the final average intracellular concentration of fluorescein per monolayer to the concentration of fluorescein in hypertonic loading media. Using the assumption that the intracellular concentration of nucleotide internalized via hypertonic loading was proportional to the internalized concentration of intracellular fluorescein, this HLR was used to estimate the increase in intracellular concentrations of dATP and/or ATP following hypertonic loading.
For contractile amplitude studies, 20 μM fluorescein was included in the hypertonic loading media to allow rapid, qualitative assessment of success of loading by fluorescence microscopy (FITC filter set) before observation of contractile amplitude with bright field microscopy (Fig. 1). While ~80% of the monolayers tested indicated successful loading, any monolayer exhibiting little or no fluorescence at this stage was excluded from further analysis.
Calcium transients were measured using Rhod-2 AM (Molecular Probes, Invitrogen, Carlsbad, CA). Although the AM ester is cell permeant, we chose to treat the cells with 1 mM Rhod-2 included as a component of the hypertonic loading medium. No fluorescein was used in these experiments. This all-in-one treatment allowed just one treatment–recovery cycle for each monolayer. In this experiment, the controls used for normalization were nucleotide-free treated samples (i.e. no exogenous nucleotide added) because Ca2+ transients were not detectable in untreated samples (prior to introduction of fluorescent indicator). As with contractile parameters, the 6 mM ATP (no dATP) level served as the post-loading control, and Ca2+ transients with dATP were compared to the ATP control for significance.
Brightfield and Fluorescence Microscopy, Data Acquisition, and Data Analysis
To quantify contractility and Ca2+ transients, cardiomyocyte monolayers were observed at 25°C by bright field or fluorescence microscopy (Hg lamp illumination) on a Nikon Eclipse TE2000-U inverted microscope (Nikon USA, Melville, NY) at 150x magnification, imaging ~5 fields from each monolayer. Field images were recorded as 0.5-1 min. video clips using a VE1000SIT camera (Dage-MTI, Michigan City, IN) and a Sony RDR-GX7 DVD recorder. The DVD clips were processed using iMovie software as AVI files on a Macintosh 2x PowerPC G4 computer with a Miglia video interface (Miglia Technology Ltd., Enfield, UK). AVI movies were processed into movie stacks consisting of ~200–600 frames using Virtual Dub 1.6.11 (Avery Lee, 2005). Stacks were imported into ImageJ 1.34s (National Institutes of Health).
Amplitude of contraction was manually tracked over time, frame-by-frame using the ImageJ Manual Tracking plug-in. Eight points per field were tracked, with ~5 fields per monolayer for a total of ~40 points per monolayer that were tracked and then averaged. Kinetic parameters for contractile shortening were measured as maximum dx/dt. Two monolayers for each condition were tested, resulting in ~80 points per condition.
A TRITC filter set was used to detect Ca2+ transients labeled with Rhod-2 AM Ca2+ dye. Because Rhod-2 AM, by design, can also cross organelle membranes, patches of concentrated Rhod-2 dye were observed as spots of very bright fluorescence within the cells. Therefore, cytoplasmic fluorescence intensity measurements were made in specific regions of interest (ROI), where background fluorescence between Ca2+ transients was minimal. Ca2+ transient time courses were analyzed, frame-by-frame using the ImageJ MultiMeasure plug-in, with eight ROI's per field analyzed for average fluorescence intensity per frame. Kinetic parameters for Ca2+ transients were measured as maximum dFL/dt. Because the fluorescence intensity of Rhod-2 increases ~100-fold upon binding to calcium, the level of fluorescence intensity in the intracellular space can be directly correlated with the relative level of intracellular calcium. We tracked time courses for both the Ca2+ transient and the physical contraction in five control fields (Fig. 2). In each case, the peak of the Ca2+ transient preceded peak contraction, as expected. Five fields per monolayer with 8 ROI's per field were assessed, for a total of 40 ROI's per monolayer. Two monolayers for each condition were tested, resulting in 80 ROI's analyzed per condition.
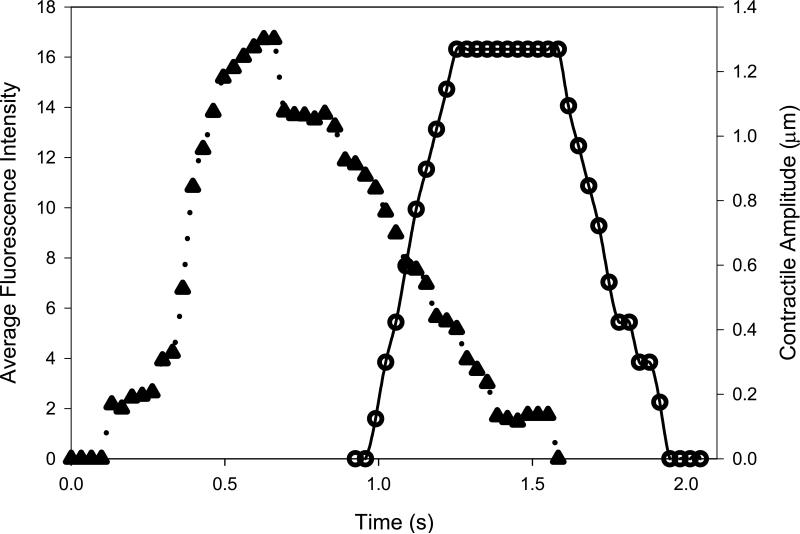
Fig. 2.
Representative cytoplasmic Ca2+ transient and cellular contraction. Time course of one representative control Ca2+ transient (filled triangles and dotted line) followed, after a 0.96 s delay, by contraction (open circles and solid line). Data were obtained from recorded video time course images of the same field under fluorescent (Ca2+ transient) or brightfield (contraction) illumination.
Analyses were performed using Excel (versions 2000 and 2007; Microsoft, Redmond, WA) and data were plotted using either Excel or Sigma Plot 2000 for Windows (version 6.10; SPSS). Average values were calculated as the mean amplitude of contraction or maximum fluorescence. Statistics were calculated using Student's t-test to compare means, and P values ≤0.05 were considered significant.
RESULTS
Estimation of Hypertonic Loading-Induced Increases in Intracellular Nucleotide Concentration in Chick Cardiomyocyte Monolayers
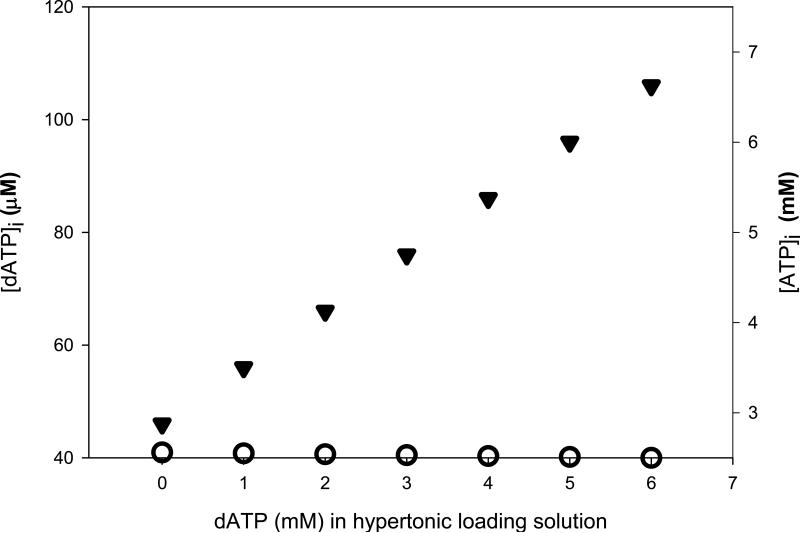
Using the fluorescein hypertonic loading ratio (Materials and Methods), we estimated that [dATP]i increased at least 2.3-fold over normal [dATP]i from ~46 μM to 106 μM when the maximum 6 mM dATP was incorporated into hypertonic loading solution (Fig. 3). The upper end estimate for basal [dATP]i [Traut, 1994] was cited in these experiments in order to report a maximum estimated tolerable [dATP]i for cardiomyocytes. In stark contrast to [dATP]i increases, relative changes in [ATP]i were much smaller. With 6 mM ATP in hypertonic loading media, [ATP]i was increased from ~2.5 to 2.56 mM (Fig. 3). Thus our experimental design enabled us to monitor cellular changes in cardiomyocytes with up to over a 100% increase in [dATP]i, while maintaining the increase in [ATP]i at ~2% or lower.
Fig. 3.
Final estimated intracellular concentrations of nucleotides following hypertonic loading. Hypertonic cell loading of 6 mM total NTP (6 mM ATP, 6 mM dATP, or a ratio of the two) at varying combinations resulted in indicated final estimated intracellular concentrations. Note differences in dATP (left, in μM) and ATP (right, in mM) y-axes. [dATP]i (solid triangles) was increased more than twofold over normal intracellular levels when 6 mM dATP was incorporated into the hypertonic loading media. Conversely, [ATP]i (open circles) increased by only ~2% when 6 mM ATP was loaded. Each y-axis represents a threefold increase over normal intracellular concentrations. Basal [dATP]i and [ATP]i are assumed to be 46 μM and 2.5 mM, respectively.
Cardiomyocyte Viability
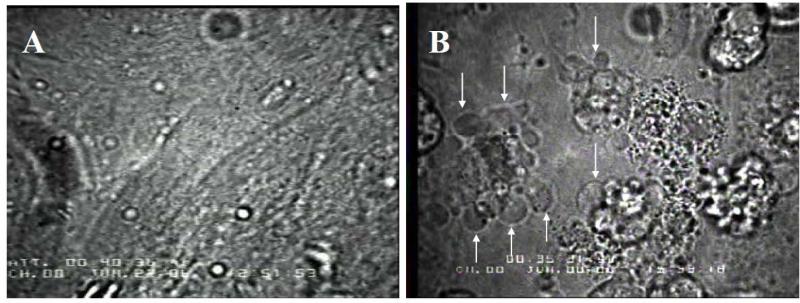
Our ex vivo contractility experiments were designed, first, to determine whether increased [dATP]i is detrimental or toxic to beating monolayers of cultured chick cardiomyocytes. We found that when [dATP]i was increased to ~100 μM via hypertonic loading with 6 mM dATP (Fig. 3), there were evident changes in cell structure—most notably cell rounding and membrane blebbing—suggestive of apoptosis (Fig. 4) [Wyllie et al., 1980; Mills et al., 1998]. Cell blebbing occurred in all of the observed ~100 μM [dATP]i monolayers, with more than 50% of the cells exhibiting structural changes consistent with apoptosis. In our experiments, lower concentrations of [dATP]i did not result in structural changes to the monolayers, thus effects of [dATP]i <100 μM on contractility and Ca2+ handling could be examined. We found that the chick cardiomyocytes could tolerate some increase in [dATP]i without affecting cell viability. The presumptive apoptotic effects of the highest [dATP]i were not investigated further because cardiomyocyte contractility—the primary object of our investigation—was observed at lower [dATP]i.
Fig. 4.
Changes in cell structure induced when intracellular [dATP] was increased to ≥100 μM. A: Representative confluent beating monolayer of embryonic chick cardiomyocytes prior to hypertonic loading. B: Changes in cell structure consistent with apoptosis following treatment to increase the intracellular [dATP] to ≥100 μM (Fig. 2). Cells are no longer confluent, and are rounded with membrane blebbing (arrows). Blebbing was observed in 100% of the monolayers treated with 6 mM hypertonic loading solution, but never at lower levels of dATP loading.
Cardiomyocyte Contractility
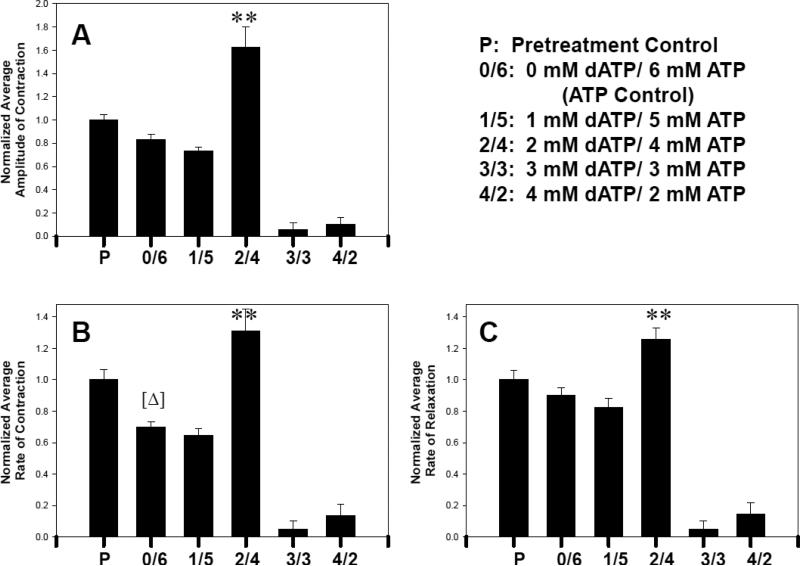
In monolayers with [dATP]i <100 μM, we tested whether increased [dATP]i would enhance cardiomyocyte contractility. Parameters of contractility that were assessed at 25°C included amplitude of contraction, beat frequency, rate of contraction, and rate of relaxation. Hypertonic loading of cardiomyocyte monolayers with 3 mM or 4 mM exogenous dATP resulted in a [dATP]i estimated to be greater than ~60 μM but less than ~100 μM. These increased levels of [dATP]i did not appear to induce apoptosis or necrosis, but did significantly decrease all contractile parameters compared to controls (Fig. 5). At ~60 μM [dATP]i (2mM exogenous dATP), the average amplitude of contraction increased twofold over the ATP control (Fig. 5A). At ~60 μM [dATP]i the rate of contraction, likewise increased two-fold (Fig. 5B), while the rate of relaxation increased by ~1/3 over that of the control (Fig. 5C). While these increases were all statistically significant (P < 0.05), there was no significant difference in beat frequency (data not shown) between dATP treatment levels of 0 mM, 1 mM, and 2 mM dATP.
Fig. 5.
Contractile amplitude (A), rate of contraction (B) and rate of relaxation (C) obtained from chick cardiomyocytes in culture. Forty points were tracked per monolayer, as illustrated in Figure 3, and analyzed as described in Materials and Methods. Data from two monolayers for each nucleotide level of treatment were averaged and normalized to the average pre-treatment amplitude or rate of contraction. Error bars represent the SEM. ** Represents P≤0.01 where 2 mM dATP/4 mM ATP (2/4) is compared to 6 mM ATP control (0/6). In (A) and (C), ATP control (0/6) was not significantly different from pretreatment control (P), while in (B) ATP control (0/6) was significantly lower than P. [Δ] Represents P≤0.05 where ATP control was compared to P. Temperature was 25°C.
Calcium Transients
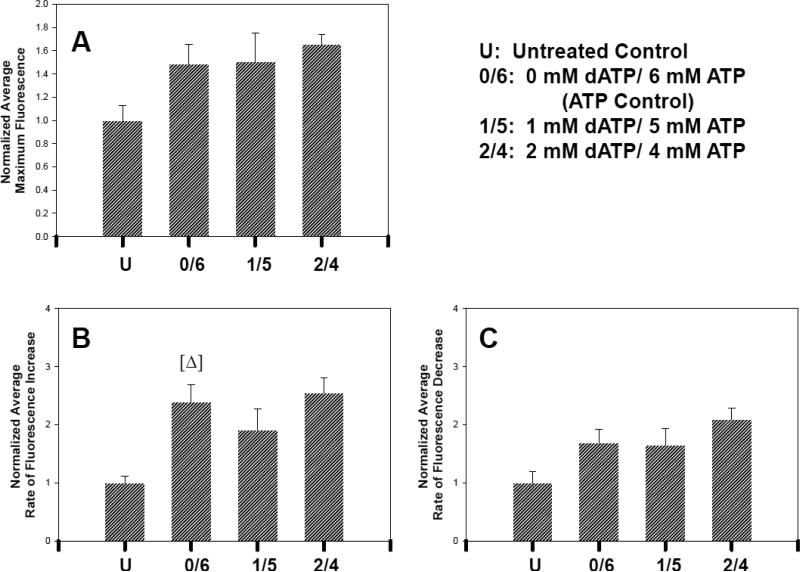
We used the Ca2+ sensitive dye Rhod-2 to examine Ca2+ transients of contracting embryonic chick cardiomyocyte monolayers (Fig. 6 and Materials and Methods) to determine if increased [dATP]i affected the function of SERCA-2 and/or sarcolemmal ATPases such that the Ca2+ transient was altered. We examined Ca2+ transients only at [dATP]i levels of ~60 μM or lower because contractile activity was not observed at higher levels. We found no significant changes in maximum Rhod-2 fluorescence intensity (Fig. 7A), rate of fluorescence increase, or rate of fluorescence decrease (Fig. 7B,C) between treatments with 0 mM dATP, 1 mM dATP, or 2 mM dATP. Even at the treatment level (2 mM dATP) which yielded ~60 μM [dATP]i and increased amplitude of contraction, there were no significant changes in Ca2+ transient parameters over that of the ATP control.
Fig. 6.
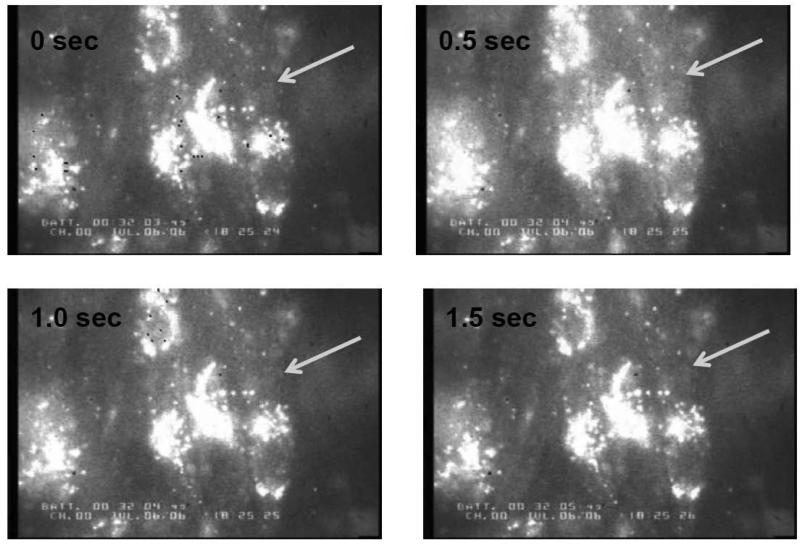
Recording of cytoplasmic Ca2+ in cultured chick cardiomyocytes using Rhod-2 AM calcium indicator. Changes in cytoplasmic Ca2+ were tracked over time by observing the increase in cytoplasmic fluorescence from time 0 to the peak of the Ca2+ transient (0.5 s), and decrease back to baseline. Arrow points to cytoplasmic region of interest (ROI). ROI's were chosen to avoid Rhod-2 accumulation within organelles, which appear as bright spots within the cell monolayer (Materials and Methods). Representative images are from ATP control following hypertonic loading at 25°C.
Fig. 7.
Ca2+ transient amplitude (A), rate of increase (B), and rate of decrease (C) obtained from Rhod-2 fluorescence in chick cardiomyocytes in culture. Forty regions of interest (ROI's) were tracked per monolayer and analyzed as described in Materials and Methods. Data from two monolayers for each nucleotide level—that exhibited contractility—were averaged and normalized to the average untreated (U; no nucleotide added) control. Error bars represent the SEM. In (A) and (C), ATP (0/6) control was not significantly different from untreated control, while in (B) ATP control (0/6) was significantly higher than U. [Δ] Represents P≤0.05 where ATP control was compared to U.
DISCUSSION
Replacement of ATP by dATP as contractile substrate in vitro, using rat or porcine cardiac muscle, increases the number of strongly attached cross-bridges, which in turn activate the cardiac thin filament to a level over that achievable with ATP and saturating Ca2+ [Regnier et al., 2000; Regnier et al., 2004; Schoffstall et al., 2006]. Here, embryonic chick cardiomyocytes were used for preliminary determination of effects of increased intracellular dATP on living, contractile cardiac cells. We found that high levels of increasing intracellular dATP by hypertonic loading can be toxic or detrimental to chick cardiac myocyte viability and/or function, but there appears to be an “optimal” increase in intracellular [dATP] that can be tolerated by cardiomyocytes ex vivo that enhances contractility without affecting other ATPases involved in the cardiac Ca2+ transient.
To determine if the positive inotropic effects of dATP that we previously observed in vitro [Schoffstall et al., 2006] could be translated into living cardiac cells, we loaded contractile monolayers of embryonic chick cardiomyocytes with varying concentrations of nucleotide (ATP, dATP, or a combination of the two). We introduced nucleotides via hypertonic loading followed by osmotic lysis of pinocytotic vesicles [Okada and Rechsteiner, 1982] such that the resultant dATP concentration within the cardiac myocytes was greater than physiological (Fig. 3). Successful cell loading was verified by addition of 20 μM fluorescein as indicator (Fig. 1). We found that when total [dATP]i was ~100 μM, apoptosis appeared to be induced as evidenced by structural changes in cardiomyocytes (Fig. 4). Increased [dATP]i levels greater than 100 μM have previously been reported to induce apoptosis in HL-60 granulocytic leukemia cells and were cytotoxic to lymphocytes and erythrocytes of adenosine deaminase deficient patients [Cohen et al., 1978; Katona et al., 2006]. Our original hypothesis was that dATP alone should not be sufficient to induce apoptosis (Introduction). It is possible that we and others [Katona et al., 2006] have seen this apparent induction of apoptosis as a side effect of the methods used to increase [dATP]i. Perhaps there is mitochondrial compromise in either of these preparations due to either our hypertonic/hypotonic treatment (which temporarily weakens membranes) or due to demand for phosphorylation of increased levels of dAMP [Katona et al., 2006] (which would increase the demands on the mitochondria for ATP production). If the mitochondria are compromised, there may be increased cytochrome C leakage, leading to apoptosis. While plausible, we find this mechanism not entirely consistent with our results because cell rounding and membrane blebbing was not observed at all [dATP]i. Interestingly, Cohen et al. [1978] and Katona et al. [2006] suggested that the inhibition of ribonucleotide reductase (RNR) activity caused by increased [dATP]i may contribute to dATP's apoptotic effect. We [Schoffstall et al., 2006] and others [Regnier et al., 2000] have suggested that overexpression and enhanced activity of RNR may be used as a therapeutic treatment for heart failure by increasing [dATP]i in cardiomyoctes.
Our data indicate that there is an “optimal” total [dATP]i, ~60 μM, that causes an increase in the amplitude of contraction and rate of contraction and relaxation with contractile monolayers of embryonic chick cardiomyocytes (Fig. 5). This positive inotropic response occurs with little or no effect on beat frequency (data not shown) or Ca2+ transients during the contractile cycle (Fig. 7). The intracellular dATP/ATP ratio at this level is lower than that used in our in vitro assays with porcine cardiac muscle [Schoffstall et al., 2006]; the intracellular ratio is estimated to be ~3%. With our in vitro assays, we reported a significant enhancement of contractile parameters at 10% dATP/ATP. In our porcine studies, a 5% ratio yielded a significant increase in contractility as well, but only if analyzed using a one-tailed P value (unpublished data). Because [dATP]i and [ATP]i are estimates (Fig. 3), it is possible that the intracellular dATP/ATP ratio achieved in these experiments was somewhat higher than 3%. Even at 3%, contractile enhancement seen at the 60 μM [dATP]i correlates well with our in vitro results with porcine cardiac muscle [Schoffstall et al., 2006]. Above this optimal [dATP]i, contractile function was impaired (Fig. 5), suggesting that >70 μM total [dATP]i may affect aspects of cellular function other than actomyosin in chick cardiomyocytes.
We predicted that increased [dATP]i would produce a positive inotropic response without affecting the activity of other ATPases involved in cardiomyocyte contraction, the two most evident being SERCA-2 and the sarcolemmal ATPase. This prediction was based, in part, on our previous in vitro findings that porcine cardiac myosin has an apparent higher affinity for dATP than ATP [Schoffstall et al., 2006]; the higher affinity may be key to dATP's enhancement of myosin activity. Other ATPases would not necessarily be expected to have relative affinities for ATP and dATP similar to myosin because the affinity difference may be unique to myosin's nucleotide hydrolysis site. We examined Ca2+ transients at [dATP]i levels of ~60 μM or lower, the levels at which contractile activity was observed after treatment. These data (Fig. 7) suggest that increased [dATP]i does not enhance or otherwise alter the activity of SERCA-2 and/or sarcolemmal ATPase sufficiently to affect Ca2+ transients in embryonic chick cardiomyocytes.
In developing avian cardiomyocytes, the relative contributions of sarcoplasmic reticulum Ca2+ release and extracellular Ca2+ influx to the overall Ca2+ transient are somewhat different from that of an adult human. In early embryonic stages of all mammals, the SR content is sparse, thus extracellular Ca2+ is the main contributor to the Ca2+ transient. As development proceeds, SR content increases in a species-dependent manner. By embryonic development day 11 (ED11), the SR content of embryonic chick cardiomyocytes is nearly at the adult chick level [Creazzo et al., 2004]. From the time of hatching and beyond, SR contribution to the Ca2+ transient is greater than sarcolemmal (extracellular Ca2+) [Vetter et al., 1986]. Thus in the cultured cardiomyocytes used for our studies (ED11), we can estimate that there exists at least some contribution of activity from both SERCA-2 and the sarcolemmal ATPase to the Ca2+ transient. If the activity of either of these ATPases was significantly affected, we would expect to see significant changes in the Ca2+ transient parameters. Because Ca2+ transient amplitude and kinetics were not different from ATP controls at the tested dATP concentrations (Fig. 7), our results suggest no significant effect of increased [dATP]i on the activity of these ATPases in chick cardiac myocytes.
Early therapeutic drugs targeted at increasing cardiac contractility by increasing Ca2+ affinity of cardiac troponin were found to overstimulate signaling pathways, such as cAMP and Ca2+-calcineurin [Houser and Margulies, 2003]. This overstimulation led to an increase in patient mortality, presumably due to increased apoptosis and arrhythmia. Therefore, identifying therapeutic agents—such as dATP—that can increase contractility without interfering with calcium signaling cascades could be the answer for improving hemodynamics in human heart failure. The direct effects that dATP has on actomyosin interaction and cardiac contractility in vitro, along with our preliminary data with living, contractile cardiomyocytes, suggest that increased [dATP]i may have therapeutic potential as a positive inotropic agent in human heart failure conditions. Actomyosin activator drug compounds have been shown to be effective in the treatment of heart failure [Rodriguez et al., 2007]; exploration of various methods to therapeutically increase [dATP]i in cardiomyocytes should also be explored as possible treatments for the symptoms of heart failure. Overexpression (via gene transfection) of RNR and/or a constitutively active D57N RNR mutant [Eriksson et al., 1981; Reichard et al., 2000] may be one avenue for controlled therapeutic increase of [dATP]i in cardiomyocytes for treatment of heart failure symptoms.
ACKNOWLEDGMENTS
We thank Drs. L. Keller and T. Keller for suggesting the use of embryonic chick cardiac myocytes, Dr. K. Harper and Mr. M. Wiltshire for assistance with obtaining fertilized eggs, Ms. N.S. Sweeney for Rhod-2 AM, and Mr. B. Bauerband with assistance in cell preparations and fluorescein experiments. Supported by NIH HL63972 and a Graduate Fellowship from the Florida State University Center for Materials Research and Technology (MARTECH).
REFERENCES
- Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: A large caspase-activating complex. Biochimie. 2002;84:203–214. doi: 10.1016/s0300-9084(02)01376-7. [DOI] [PubMed] [Google Scholar]
- Cerqueira NM, Pereira S, Fernandes PA, Ramos MJ. Overview of ribonucleotide reductase inhibitors: An appealing target in anti-tumour therapy. Curr Med Chem. 2005;12:1283–1294. doi: 10.2174/0929867054020981. [DOI] [PubMed] [Google Scholar]
- Chereau D, Zou H, Spada AP, Wu JC. A nucleotide binding site in caspase-9 regulates apoptosome activation. Biochemistry. 2005;44:4971–4976. doi: 10.1021/bi047360+. [DOI] [PubMed] [Google Scholar]
- Cohen A, Hirschhorn R, Horowitz SD, Rubinstein A, Polmar SH, Hong R, Martin DW., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci USA. 1978;75:472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creazzo TL, Burch J, Godt RE. Calcium buffering and excitation-contraction coupling in developing avian myocardium. Biophys J. 2004;86:966–977. doi: 10.1016/S0006-3495(04)74172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Gudas LJ, Ullman B, Clift SM, Martin DW., Jr DeoxyATP-resistant ribonucleotide reductase of mutant mouse lymphoma cells. Evidence for heterozygosity for the protein M1 subunits. J Biol Chem. 1981;256:10184–10188. [PubMed] [Google Scholar]
- Genini D, Budihardjo I, Plunkett W, Wang X, Carrera CJ, Cottam HB, Carson DA, Leoni LM. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J Biol Chem. 2000;275:29–34. doi: 10.1074/jbc.275.1.29. [DOI] [PubMed] [Google Scholar]
- Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- Katona K, Herczegh P, Kappelmayer J, Fesus L, Aradi J. Deoxy-adenosinemonophosphate (dAMP) di-n-butylester induces apoptosis by increasing the dATP level in HL-60 cells. Cancer Lett. 2006;235:281–290. doi: 10.1016/j.canlet.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Liu M, Barth A. Phosphorylation of the sarcoplasmic reticulum Ca2+-ATPase from ATP and ATP analogs studied by infrared spectroscopy. J Biol Chem. 2004;279:49902–49909. doi: 10.1074/jbc.M408062200. [DOI] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada CY, Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982;29:33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Perrone SV, Kaplinsky EJ. Calcium sensitizer agents: A new class of inotropic agents in the treatment of decompensated heart failure. Int J Cardiol. 2005;103:248–255. doi: 10.1016/j.ijcard.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000;86:1211–1217. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004;87:1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P, Eliasson R, Ingemarson R, Thelander L. Cross-talk between the allosteric effector-binding sites in mouse ribonucleotide reductase. J Biol Chem. 2000;275:33021–33026. doi: 10.1074/jbc.M005337200. [DOI] [PubMed] [Google Scholar]
- Rodriguez MF, Sakowicz R, Hartman J. The cardiac myosin activator, CK-1827452, accelerates the enzymatic step gating entry of myosin into its force generating state.. Biophysical Society 51st Annual Meeting; Baltimore, MD. 2007. [Google Scholar]
- Schoffstall B, Clark A, Chase PB. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophys J. 2006;91:2216–2226. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Trumble WR, Sutko JL, Reeves JP. Cardiac sarcolemmal and sarcoplasmic reticulum membrane vesicles exhibit distinctive (Ca-Mg)-ATPase substrate specificities. J Biol Chem. 1981;256:7101–7104. [PubMed] [Google Scholar]
- Ungureanu-Longrois D, Bezie Y, Perret C, Laurent S. Effects of exogenous and endogenous nitric oxide on the contractile function of cultured chick embryo ventricular myocytes. J Mol Cell Cardiol. 1997;29:677–687. doi: 10.1006/jmcc.1996.0310. [DOI] [PubMed] [Google Scholar]
- Vetter R, Will H, Kuttner I, Kemsies C, Will-Shahab L. Developmental changes of Ca++ transport systems in chick heart. Biomed Biochim Acta. 1986;45:S219–S222. [PubMed] [Google Scholar]
- Wang Y-L. Yu-li Wang's lab protocols, p Web Page of lab protocols. 2006 URL: http://ylwang.umassmed.edu/protocol/cc/cardiac.htm.
- Wyllie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]