1. Introduction
Kilauea is one of 3 active volcanoes on the island of Hawai’i and one of the most active volcanoes on Earth (1). Since the onset of eruption in 1983, it has released at least 300 metric tons of sulfur dioxide (SO2) per day and as much as 30,000 tons per day during vigorous eruptive activity, from Pu’u O’o vent on the east rift zone on the volcano’s flank and Halema’uma’u Crater at the volcano’s summit (2). Even during non-eruptive periods prior to 1983, Kilauea’s SO2 emissions of 50,000–100,000 tons per year was 1000 times greater than the United States Environmental Protection Agency’s (EPA’s) definition of a major pollution source. During active eruption, Kilauea’s output can exceed 2 million metric tons (2 Tg) per year.
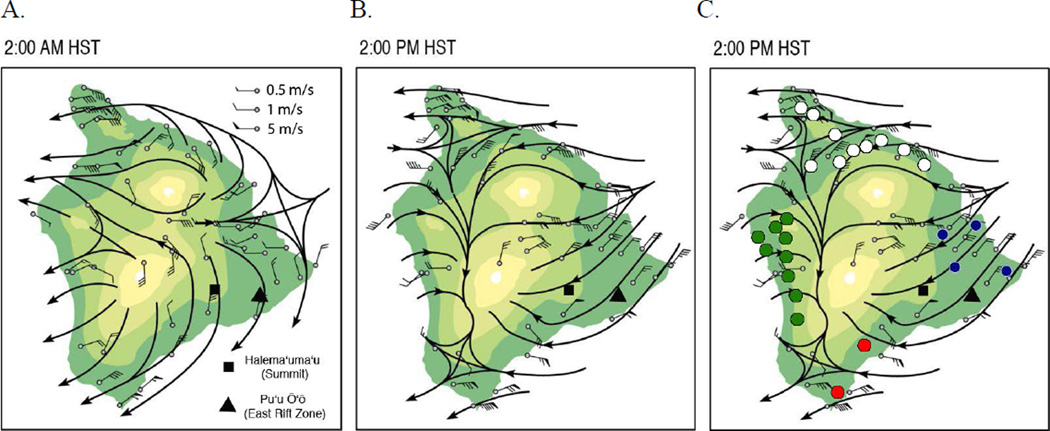
In the presence of sunlight, SO2 and other volcanic gases react chemically with oxygen, water vapor, and dust to yield fine, respirable particles comprised mainly of sulfuric acid and other sulfate species (SOx). The conversion proceeds with a half –life of approximately 6 hr, resulting in a haze of SO2, acid, and other particles known locally as vog (3). Vog is trapped under an inversion layer that is usually 1,500–2,000 m high and is carried from its source by prevailing trade winds from the northeast, around the 4,169-m summit of Mauna Loa and into the seaside towns in the lee of the mountain (Figure 1) (4). Here, volcanic air pollution may linger. At night, vog moves toward the ocean as cool air descends from the mountains (Figure 1a), but is drawn back onshore during the day as air over the coast heats and rises more quickly than the air over the adjacent ocean (Figure 1b).
Figure 1.
Halema’uma’u (Summit) and Pu’u ‘O’o (East Rift Zone) vents, tradewind pattern, July–August, 1990. Colors change with every 1000 m elevation.
A. Wind speed and direction during northeast winds, 2 a.m.
B. Wind speed and direction during northeast winds, 2 p.m.
C. Participating schools in vog exposure zones, 2002–2005: Low (white), Intermittent (blue), Frequent (red), Acid (green).
During periods of low wind speed, vog can accumulate in communities near the volcanic vents. During periods of sustained winds from the south and southwest, vog may be carried to communities on the north and east sides of the island. Thus, because of local topography, Hawai’i Island communities are exposed to different concentrations and composition of volcanic air pollution, depending on the amount of volcanic emissions, the speed and direction of the wind, humidity and precipitation, and the height of the inversion layer.
Studies of the health effects of Hawai’i’s volcanic air pollution are of interest not only to other communities near active volcanoes worldwide, but can also provide insights into the specific effects of individual components, such as SO2 and SO4, in anthropogenic air pollution produced by fossil fuel and biomass combustion. Such air pollution is a complex mixture that includes other gases, organic compounds, oxidants, and heavy metals. To date, Hawai’i Island has relatively few traffic or industrial sources of air pollution that might confound studies of health effects of vog, SO2, and SO4. In contrast to large-scale studies of the association between anthropogenic air pollution and respiratory symptoms and lung function (5–8), studies of health effects of volcanic air pollution have sometimes been limited by sample size and selection, or lack of concurrent measures of air quality and quantitative measures of respiratory health (9–14).
The unique stationary source of outdoor air pollution, and the varying distribution of vog across the island’s communities allowed for performance of a large cross-sectional study that applies the design and methodology of the Children’s Health and Twenty-four Cities Studies (5–7). We present the findings of a multidisciplinary, community-based participatory research effort to investigate association between vog and respiratory symptoms, diagnosed asthma or bronchitis, and reduced lung function.
2. Materials and Methods
To delineate different zones of air pollution, we compiled Kilauea Volcano SO2 emission rates data collected by the U.S. Geological Survey (USGS, 1992–2010), and wind speed and direction at an offshore point northeast of Hawai’i island that determines wind patterns over the island. Community-based researchers then directly measured concentrations of SO2, fine particulate matter (PM2.5), and fine particle acidity between 2002–2005 in Hawai’i Island communities within the putative vog exposure zones. Concurrently, community researchers assessed other environmental factors and the respiratory symptoms and lung function of children who attended schools in each of the zones.
2.1 SO2 gas emissions
SO2 gas emissions were measured regularly by vehicle-based correlation spectroscopy (COSPEC) at the summit of Kilauea between 1979 and 2003 and from Pu'u 'O'o and Kilauea’s east rift zone between 1992 and 2003. This technique uses the strong absorbance of ultraviolet sunlight energy by SO2 gas, and a cross-correlation signal processing technique, to measure the abundance of SO2 in a pollution plume (15). In late 2003, USGS began to augment the traditional COSPEC measurements with data from one of the new generation of miniature spectrometer systems. These measurements are also based on the absorption of ultraviolet energy by SO2 gas, but use the Differential Optical Absorption Spectroscopy (DOAS) method for calculating the amount of SO2 in the volcanic plume. Because of increased emissions from the summit of Kilauea beginning in 2008, the USGS modified the analysis technique to include evaluation of ultraviolet spectra at longer wavelengths, which allows more accurate quantification of high concentrations of SO2 in the plume (16).
2.2 Wind speed and wind direction
2.2.1 1992–2001
We reanalyzed archived data from the National Centers for Environmental Prediction–National Center for Atmospheric Research (NCEP-NCAR). Time series charts of the daily wind speed and wind direction at 20° N, 152.5° W (offshore, northeast of Hilo town) at 0 and 300 m above sea level for both 2 am and 2 pm Hawai’i Standard Time were generated for the 10-year period that coincided with the earliest birth year of the study cohort to the start of the real-time environmental monitoring for this study (1992–2001). The wind data from this grid point represents the prevailing wind conditions over the open ocean upstream of the Island of Hawai’i. These wind conditions drive the distribution of volcanic air pollution over the island terrain and were used to delineate likely vog exposure zones.
2.2.2 2000–2010
To inform our environmental monitoring and health assessments, wind speed and direction data for 2000–2009 were extracted from measurements taken at the summit near the Hawaiian Volcano Observatory and Halema’uma’u Crater (19 ° 25’ 16” N, 155 ° 17’ 13” W), and made publicly available on-line (17). Data was compiled and graphed as wind roses, to depict per month, the proportion of winds of different speed and direction. Note that this land-based monitor reflects local winds affected by mountain barriers and daily warming and cooling of the land mass, and may not reflect the winds driving vog from a particular vent, depending on time of day and the wind direction at 20° N, 152.5° W.
2.3 Community-based participatory research air monitoring, 2002–2005
The island’s terrain, the location of Kilauea’s gas-emitting vents, and wind patterns from 1992–2001, defined 4 zones of different exposures. Using previously described protocols (18, 19) and in partnership with academic investigators, Hawai’i Island community researchers established and maintained filter-based air monitoring of SO2, respirable particulate matter and acidity at representative sites in each zone, avoiding obstructions to airflow, sources of ammonia (livestock, poultry, or fertilizer), and traffic or industrial sources of air pollution.
2.3.1 Sulfur dioxide
SO2 was measured by passive diffusion (20). Electrochemical badges housed in polyvinylchloride caps were exposed in 1- to 4-wk intervals, co-located with Harvard impactors that collected fine particulate matter. After correction for exposure interval, the data is expressed as parts per billion volume (ppb).
2.3.2 Respirable particulate matter (PM2.5)
Ambient air was drawn through Harvard impactors at 10 LPM. Particles ≤ 2.5 µm in diameter were captured on pre-weighed polytetrafluoroethylene (PTFE) filters over 2-wk integrated exposures. Flow rates at the start, mid-point, and end of each 2-wk exposure were measured using calibrated rotameters. Pre- and post-exposure filter weights were measured at the Harvard School of Public Health (HSPH) Environmental Epidemiology Laboratory, with net filter mass per total filtered volume expressed as µg per m3 of filtered air.
2.3.3 Respirable particulate strong acidity
Ambient air was drawn at 4LPM through Harvard impactors specially fitted with denuders coated with a citric acid solution to neutralize ambient ammonia and preserve the acidity of particles captured on the PTFE filter (21). These impactors were co-located with the standard Harvard impactors. All filters were exchanged in a glove box lined with citric acid solution-soaked paper. Exposed filters were stored in vials kept in a similar glove box, and sent to HSPH in citric acid-coated containers for analysis as previously described. Beginning and ending sampling flow rates for each 2- to 4-wk exposure were measured using a calibrated rotameter. Particle strong acidity is expressed as nmol per m3 of filtered air.
2.3.4 Air sampling quality control
Air sampling quality control included the use of field blanks, test blanks, and co-localized monitors, per published protocols (18, 19). All filters were stored refrigerated and shipped on ice for analysis at the HSPH Environmental Epidemiology Laboratory. Community researchers entered filter identification, location, field conditions, and calibrated rotameter readings into a password-protected web-enabled relational database. Laboratory personnel at HSPH entered filter results on a separate table, blinded to the field conditions. Final SO2 (ppb) and mass and acidity per m3 were calculated by the database and reviewed in teleconference by community researchers and academic investigators to ascertain data quality and inclusion for analysis.
2.3.5 X-ray fluorescence
Selected filters with high collected mass were analyzed by x-ray fluorescence (XRF, Desert Research Institute, NV, USA) for component speciation. Elemental masses that were more than twice the mass on unexposed control filter masses were used to calculate concentrations (ng per m3) for statistical analysis. Filters exposed during obvious local confounding activities (such as New Year’s Eve, when fireworks distorted ambient exposures) were excluded from statistical analysis.
2.4. Cross-sectional health study
2.4.1 Design and Recruitment
Our analysis of emissions and wind patterns suggested 4 vog exposure zones. Power analysis and review of census and school enrollment indicated that we would be able to recruit appropriate sample sizes (n≥400) in 3 of the zones. Concern for the rural community that most frequently receives volcanic emissions but had not had systematic monitoring of its air quality led to its inclusion in the study’s air quality and health data collection. Participating schools are shown in Figure 1C. We maintained the age restriction of earlier studies of respiratory health in children (19, 22), but to reflect the population of Hawai’i, and to achieve the necessary sample size, there were no other exclusions for the health study.
Participants were recruited according to community-based participatory research principles and a research protocol approved by the University of Hawai’i Institutional Review Board. Schools were selected to participate based on location in a vog exposure zone and approval of school district superintendents and principals. All students enrolled in the 4th and 5th grades of participating schools were eligible. Study participation was voluntary and required the written legal consent of a parent or legal guardian.
2.4.2 Health Effects Assessment
Participants completed maximal-effort spirometry and standardized questionnaires based on the Harvard Six Cities and Twenty-four Cities Studies (6, 23) and the University of Southern California (USC) Children’s Health Study (19, 24). Specific questions were reviewed and revised by community researchers and a community advisory committee. Parents and guardians responded to initial questionnaires that assessed the child’s personal and family medical history, household, home environment, and current health. Per protocols adapted from the USC Children’s Health Study, Hawai’i Island community researchers trained by the USC field team conducted health assessments in each school in the fall and spring semesters of the school year. Approximately one-half of each school’s participants were tested during each of the 2 visits, to minimize the impact of local widespread illnesses such as the flu. To assess the consistency of testing methods, 10% of participants were tested in both semesters. A brief questionnaire was asked of the child at the time of each testing to assess recent activity, respiratory symptoms, illnesses, and environmental exposure (Initial and Test Questionnaires, Supplemental Information Figures A and B). Participants’ sitting height, standing height, and weight were measured annually, per published protocol (19).
2.4.3 Spirometry
Participants completed 6–8 maneuvers at each testing, using EasyOne Diagnostic Spirometers(ndd Medical Technologies, Zurich, Switzerland, www.nddmed.com) (25, 26), coached by community researchers trained and certified by the USC Children’s Health Study team. Spirometers provided immediate assessment of adequacy and reproducibility of expiratory efforts and comparison to predicted values for children (Dockery). Spirometric information was compiled in a central relational database interfaced with the EasyWare application for additional review for reproducibility and back extrapolated volume according to American Thoracic Society guidelines (27). Data was reviewed weekly by the author and the team for quality assurance. The single best reproducible FEV1 or FVC from each testing cycle was used for analysis.
2.5 Statistical analysis
SO2, particulate mass, acidity, and elemental concentrations were compared between zones by 1-way ANOVA and post hoc testing (SAS JMP v. 12, Cary, NC). Results are expressed as mean ± s.d. Associations of vog exposure and respiratory symptoms or measures of lung function were analyzed by chi square and logistic regression, unadjusted and adjusted for confounding variables (SAS v. 9.2, Cary, NC). Results are expressed as OR (95% CI), as compared to the Low vog exposure group.
3. Results
3.1 Emissions from Kilauea Volcano, 1992–2010
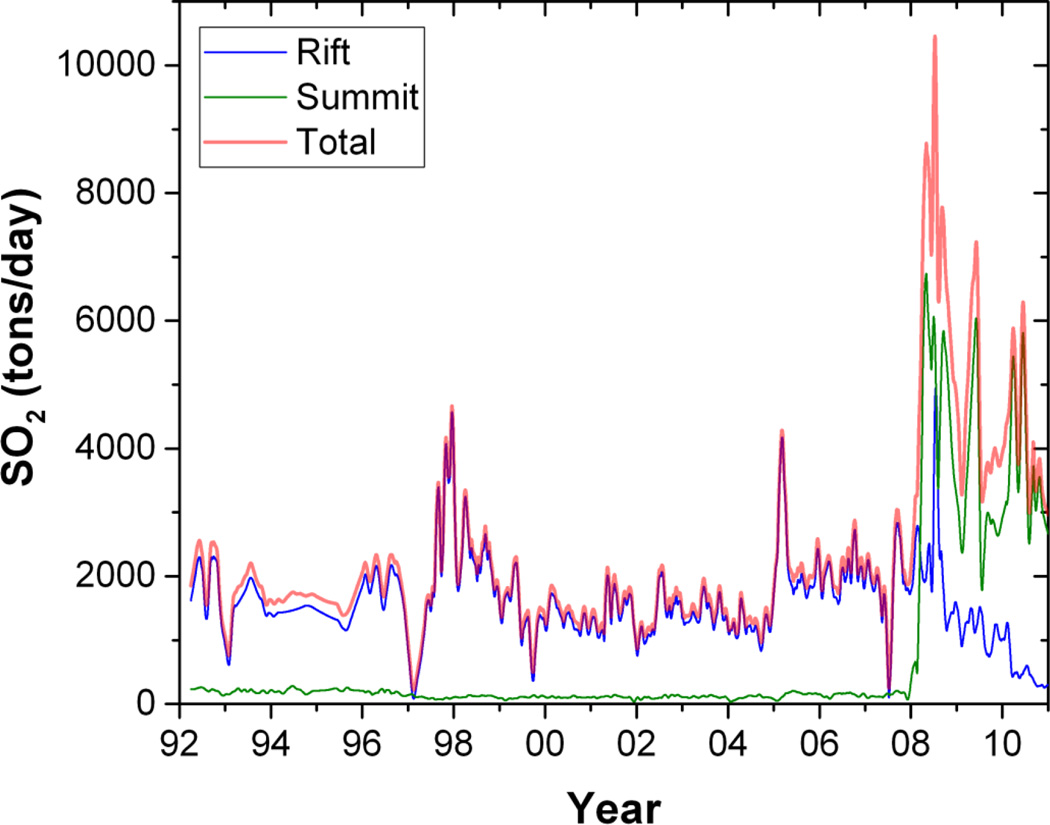
Kilauea emissions have consistently exceeded 1,000 tons per day since 1992 (Figure 2), the earliest birth year of the study cohort. During community-based air monitoring from June 2002 through June 2005 Kilauea emissions averaged 1800±800 tons per day. Total emissions during this period approached 2 million metric tons. Total volcanic emissions increased in March 2008, due to increased emissions from Halema’uma’u crater at the summit as well as from the East Rift Zone (16).
Figure 2.
SO2 emissions (metric tons/day) from Kilauea’s East Rift, Summit, and Total, 1992–2010.
The community-based air monitoring was conducted June 2002–June 2005. The cross-sectional health study was conducted September 2002-June 2003.
3.2. Wind speed and wind direction
Our reanalysis confirmed that northeasterly winds prevailed for the 10 years before the start of this health study, and during the health study. Winds of low speed, or with a southerly component, occurred less than 20% of the time, usually during October – May. See Supplemental Information, parts C and D for additional details and plots.
3.3 Community-based air monitoring, 2002–2005
Mean concentrations of SO2, fine particulate mass, and acidity are summarized in Table 1. The temporal relationship to SO2 emissions is shown in Supplemental Information, Figure E, parts A–D.
Table 1.
SO2, PM2.5, and particulate acidity in 4 exposure zones, 2002–2005, 1- to 2-wk integrated exposures, mean ± s.d. (n, range).
| Low | Intermittent | Frequent | Acid | |
|---|---|---|---|---|
| SO2, ppb | 0.3 ± 0.2 (n=15, 0.1–0.8) |
1.6 ± 1.8 (n=42, 0–9.7) |
10.1 ± 5.2* (n=36, 2–18.7) |
1.2 ± 0.4 (n=52, 0.5–2.7) |
| PM2.5, ug/m3 | 2.5 ± 1.2 (n=45, 1.3–5.6) |
2.8 ± 1.5 (n=100, 2.0–6.2) |
4.8 ± 1.9* (n=77, 1.9–11.1) |
7.2 ± 2.3* (n=163, 0.1–16.4) |
| Particulate Acidity, nmol H+/m3 |
0.6 ± 1.1 (n=12, 0.3–15.6) |
4.0 ± 6.8 (n=35, 0.5–23.3) |
4.3 ± 6.7 (n=79, 0.1–43.8) |
25.3 ± 17.9* (n=181, 0.3–100.1) |
significantly different from other zones, p<0.0001
3.3.1 SO2
Mean SO2 concentration was highest in the Frequent exposure zone (Table 1), significantly different from all other zones. Two-wk integrated concentrations of SO2, even in this zone, did not exceed 20 ppb between June, 2002 and June, 2004, when we stopped filter collection (Supplemental Information, Figure E, part B). We thus did not capture by passive filter the increase in emissions in early 2005. SO2 concentrations in the Acid and Intermittent exposure zones of the community-based monitoring can be compared to SO2 concentrations in Kona and Hilo, respectively, determined by the Hawai’i Department of Health (Supplemental Information, Figure F).
3.3.2 Respirable particulate matter
Respirable particulate monitoring demonstrated significant differences between the exposure zones (Table 1). Mean PM2.5 was significantly greater in the “Acid” zone (7.2 ± 2.3 ug/m3) than in all other zones. Mean concentration in the “Frequent” zone (4.8 ± 1.9 ug/m3) was significantly greater than in “Intermittent” and “Low” zones, even between November and May, when calm or southerly winds are more likely to occur. Particulate peaks were associated with fireworks (December 31, 2002), shifts in wind speed or direction, or increased volcanic emissions (early 2005, Supplemental Information Figure E, part C. In early 2005, when Kilauea’s output of emissions was estimated at >4000 tons/day, the highest particulate 2-wk integrated concentration was measured in the Acid zone (16.4 ug/m3). Particulate matter concentrations in the Acid and Intermittent exposure zones of the community-based monitoring can be compared to particulate matter concentrations in Kona and Hilo, respectively, determined by the Hawai’i Department of Health (Supplemental Information, Figure F).
3.3.3 Mean particulate acidity
Mean particulate acidity in the Acid zone (25.3 ± 17.9 nmol H+/m3) was significantly greater than in all other zones; there was no significant difference between the other 3 zones (Table 1). Peak values topped 15, 20, 40, and 100 nmol H+/m3, respectively, in the Low, Intermittent, Frequent, and Acid exposure zones, but the Acid zone was the only zone with sustained levels > 15 nmol H+/m3(Supplemental Information, Figure E, part D)
3.3.4 X-ray fluorescence
Filters with highest collected mass (2–4 filters per zone) were analyzed by XRF for 38 elements. Table 3 presents concentrations of elements with mean concentrations > 10 ng/m3 and selected elements whose concentrations have been reported in recent studies of urban air pollution (28, 29). Sulfur comprised 12–15% and sodium comprised 1–3% of the net mass on each filter. Concentrations of potassium, silica, iron, zinc, copper, lead, nickel, and vanadium in this rural setting were much lower than the concentrations reported in recent studies of traffic-related and industrial air pollution (29).
Table 3.
Participant characteristics. n positive response/N respondents (%).
| All | Low | Intermittent | Frequent | Acid | P-value | |
|---|---|---|---|---|---|---|
| n | 1957 | 531 | 584 | 112 | 730 | |
| Age (mean ± s.d.) |
10.1±0.66 | 10.1±0.69 | 10.0±0.63 | 10.1±0.59 | 10.1±0.67 | 0.11 |
| Girls | 993 (51) | 259 (49) | 282 (48) | 57 (51) | 395 (54) | 0.14 |
| Race | <0.0001 | |||||
| White | 310 (16) | 78 (15) | 40 (7) | 12 (11) | 180 (25) | |
| Asian | 282 (15) | 84 (16) | 117 (20) | 21 (19) | 60 (8) | |
| Mixed | 1218 (63) | 334 (64) | 395 (68) | 66 (60) | 423 (59) | |
| Pacific Isle |
93 (5) | 22 (4) | 23 (4) | 9 (8) | 39 (5) | |
| Other | 35 (2) | 8 (2) | 5 (1) | 2 (2) | 20 (3) | |
| Unknown | 19 (1) | 5 (1) | 4 (1) | 2 (2) | 8 (10) | |
| Premature | 187/1891 (10) | 34/511 (7) | 75/572 (13) | 12/105 (11) | 66/703 (9) | <0.005 |
| Family income >$50,000 |
546/1614 (34) | 156/452 (35) | 119/482 (25) | 15/81 (19) | 256/599 (43) | <0.0001 |
| Mother smoked during pregnancy |
296/1789 (17) | 74/496 (15) | 108/523 (21) | 15/98 (15) | 99/672(15) | <0.05 |
| 0–2 yr, other smokers in home |
733/1789 (41) | 197/490 (40) | 239/525 (46) | 45/98 (46) | 252/676 (37) | <0.05 |
| Current smokers in home |
395/1944 (20) | 111/528 (21) | 146/579 (25) | 16/109 (15) | 122/728 (17) | <0.001 |
| Mold in home in last 12 m |
760/1759 (43) | 220/488 (46) | 265/531 (50) | 28/94 (30) | 247/654 (38) | <0.0001 |
3.4.1 Participant characteristics
Of 2,725 eligible children enrolled in the 4th or 5th grade in 25 schools by August 2002, families of 1,986 children (73%) gave informed consent; 1,957 (72%) returned initial questionnaires (Tables 3 and 4); 1,895 completed measurements of height, weight, and lung function in the first year (Table 5), and 1,836 were able to complete spirometry that met American Thoracic Society guidelines (Tables 5 and 6). Of note, the number of participants in the Frequent exposure zone reflects nearly 100% participation of the 4th and 5th graders in this sparsely populated area most frequently downwind of Kilauea.
Table 4.
Respiratory symptoms reported on initial questionnaire, n=1957 respondents
| Vog exposure |
n | % Yes | Unadjusted OR |
95% CI | Adjusted OR* |
95% CI | Adjusted OR** |
95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Chronic cough |
Low | 485 | 8 | 1.0 | 1.0 | ||||
| Intermittent | 533 | 10 | 1.2 | 0.76–1.80 | 1.0 | 0.63–1.67 | 1.0 | 0.61–1.67 | |
| Frequent | 95 | 11 | 1.3 | 0.61–2.64 | 1.7 | 0.75–3.97 | 1.9 | 0.80–4.50 | |
| Acid | 683 | 11 | 1.4 | 0.94–2.08 | 1.5 | 0.98–2.38 | 1.7 | 1.06–2.67 | |
| Chronic cough in asthmatics |
Low | 76 | 25 | 1.0 | |||||
| Intermittent | 106 | 28 | 1.2 | 0.61–2.31 | 1.1 | 0.50–2.38 | |||
| Frequent | 14 | 29 | 1.2 | 0.34–4.28 | 2.8 | 0.63–12.41 | |||
| Acid | 91 | 30 | 1.3 | 0.64–2.52 | 1.3 | 0.58–2.77 | |||
| Chronic cough in non- asthmatics |
Low | 407 | 5 | 1 | |||||
| Intermittent | 421 | 5 | 0.97 | 0.53–1.77 | .93 | 0.47–1.84 | |||
| Frequent | 81 | 7 | 1.4 | 0.55–3.57 | 1.5 | 0.49–4.77 | |||
| Acid | 591 | 9 | 1.7 | 0.99–2.77 | 2.0 | 1.11–3.57 | |||
| Physician- diagnosed asthma |
Low | 529 | 16 | 1.0 | |||||
| Intermittent | 575 | 21 | 1.4 | 1.04–1.91 | 1.1 | 0.77–1.54 | |||
| Frequent | 111 | 14 | 0.9 | 0.49–1.57 | 0.9 | 0.43–1.76 | |||
| Acid | 727 | 14 | 0.8 | 0.60–1.13 | 0.8 | 0.58–1.17 | |||
| Persistent wheeze in last 12 months |
Low | 478 | 8 | 1.0 | |||||
| Intermittent | 524 | 9 | 1.1 | 0.71–1.76 | 1.2 | 0.72–1.94 | |||
| Frequent | 89 | 4 | 0.6 | 0.20–1.62 | 0.8 | 0.22–2.58 | |||
| Acid | 650 | 6 | 0.8 | 0.51–1.27 | 0.8 | 0.50–1.38 |
adjusted for age, race, sex, premature birth, maternal smoking during pregnancy, current ETS exposure, mold and sitting height
adjusted also for physician-diagnosed asthma
Table 5.
Respiratory symptoms, height, weight, BMI, and lung function at first testing, n=1,895
| All | Low | Intermittent | Frequent | Acid | P-value | |
|---|---|---|---|---|---|---|
| 1895 | 523 | 562 | 103 | 707 | ||
| Diagnosed asthma (%) | 23 | 23 | 29 | 18 | 19 | <0.001 |
| Asthma, last 12 m (%)* | 12 | 13 | 15 | 7 | 8 | <0.001 |
| Head cold in last month (%) |
53 | 47 | 59 | 52 | 52 | <0.005 |
| Chest cold in last month (%) |
34 | 33 | 36 | 31 | 34 | 0.65 |
| Standing height (cm) | 139.6±8.3 | 139.1±8.4 | 138.7±8.3 | 137.4±7.8 | 140.9±8.2 | <0.0001 |
| Sitting height (cm) | 74.5±4.4 | 74.3±4.5 | 74.4±4.5 | 73.0±4.5 | 74.9±4.2 | <0.0001 |
| Weight (kg) | 38.2±12.0 | 37.5±11.2 | 38.0±11.5 | 36.3±11.1 | 39.1±13.0 | <0.05 |
| BMI (mean) | 19.3±4.4 | 19.1±4.3 | 19.5±4.2 | 19.0±4.5 | 19.4±4.6 | NS |
| BMI percentile | <0.01 | |||||
| <50th | 63 (3) | 23 (4) | 14 (2) | 9 (9) | 17 (2) | |
| Normal | 1150 (61) | 322 (62) | 327(58) | 67 (63) | 436 (62) | |
| >85th | 314 (17) | 73 (14) | 109 (19) | 15 (15) | 117 (17) | |
| >95th | 368 (19) | 105 (20) | 112 (20) | 14 (14) | 137 (19) | |
| Lung Function | ||||||
| n | 1836 | 504 | 551 | 103 | 678 | |
| FEV1 (L) | 1.92±0.36 | 1.93±0.36 | 1.89±0.34 | 1.83±0.34 | 1.95±0.36 | <0.005 |
| FVC (L) | 2.26±0.43 | 2.26±0.44 | 2.23±0.41 | 2.13±0.41 | 2.29±0.44 | <0.005 |
| FEV1/(sitting height**)2 | 3.44±0.47 | 3.48±0.45 | 3.41±0.47 | 3.41±0.46 | 3.44±0.47 | NS |
| FEV1/FVC | 0.85±0.07 | 0.85±0.06 | 0.85±0.07 | 0.86±0.07 | 0.85±0.07 | NS |
226/429 respondents with asthma responded to this question. Others did not respond or responded, “don’t know.”
sitting height in m
Table 6.
Prevalence of FEV1/FVC<0.8 or FEV1/FVC ≤0.75 in different vog exposure zones
| Vog Exposure |
n | % Yes | Unadjusted OR |
95% CI |
*Adjusted OR |
95% CI | |
|---|---|---|---|---|---|---|---|
| FEV1/FVC <0.80 |
Low | 501 | 17.4 | 1 | 1 | ||
| Intermittent | 547 | 18.8 | 1.10 | 0.81–1.51 | 1.22 | 0.86–1.75 | |
| Frequent | 103 | 13.6 | 0.75 | 0.41–1.38 | 0.91 | 0.44–1.89 | |
| Acid | 670 | 19.7 | 1.17 | 0.87–1.58 | 1.32 | 0.94–1.86 | |
| FEV1/FVC ≤0.75 |
Low | 501 | 5.4 | 1 | 1 | ||
| Intermittent | 547 | 6.4 | 1.2 | 0.72–2.01 | 1.1 | 0.63–2.02 | |
| Frequent | 103 | 3.9 | 0.7 | 0.24–2.07 | 0.9 | 0.26–3.17 | |
| Acid | 670 | 7.2 | 1.4 | 0.83–2.20 | 1.5 | 0.88–2.57 |
adjusted for age, race, sex, premature birth, maternal smoking during pregnancy, current ETS exposure, mold, sitting height, and diagnosed asthma
Participants in the 4 vog exposure zones differed significantly in several respects, including race, prematurity, family income, rates of maternal smoking during pregnancy, early childhood and current environmental tobacco smoke (ETS) exposure, and visible mold in homes in the prior 12 months (Table 3). Overall, the population was highly admixed; at least 60% were of more than 1 race. The percentage of white children was highest in the Acid zone (25%), and the percentage of Asian children highest in the Intermittent exposure zone (20%, mostly of Japanese, Filipino, or Chinese descent). Participants in the Intermittent zone were most likely to report premature birth, maternal smoking during pregnancy, smoking in the home when participants were <2 years old, current smokers in the home, and visible mold in the home. Participants in this zone were also more likely to have a BMI > 85Th percentile (Table 6). In the Frequent exposure zone, participants were more likely to be mixed, Asian, or Pacific Islander, least likely to report more than $50,000 in family income in the prior year, or to report maternal smoking during pregnancy, early or current smokers or visible mold in the home. Mean height and weight, and the percentage of students with BMI >85th percentile, were also lowest in this group (Table 6).
Thus, all analyses included adjustments for age, race, sex, sitting height, BMI, premature birth, maternal smoking during pregnancy, current smokers in the home, and visible mold in the home. Selected analyses also included adjustment for physician-diagnosed asthma.
3.4.2 Respiratory Symptoms
Overall, 10% of participants reported chronic cough on the initial questionnaire, with zone-specific rates of 8–11%. Compared to the prevalence of cough in the Low exposure zone, univariate analysis suggested a trend to increased prevalence of cough in the Frequent and Acid vog exposure zones (Table 4). This remained non-significant even after adjustment for age, sex, race, sitting height, premature birth, maternal smoking during pregnancy, current smokers in the home, and mold or mildew in the home in the prior 12 months. Increased cough in the Acid exposure zone reached statistical significance when physician-diagnosed asthma was included in the adjustment. Stratified analysis of asthmatic and non-asthmatic participants indicated that the odds of cough doubled among the non-asthmatic participants (9% in the Acid vs 5% in the Low exposure zones). There was a non-significant trend to increased cough among asthmatics, 25% of whom reported cough even in the Low exposure zone (Table 3).
Nearly 20% of all participants reported physician-diagnosed asthma on the initial questionnaire, with zone-specific rates of 14–21%. On univariate analysis, the asthma prevalence in the Intermittent exposure zone was significantly greater than in the Low exposure zone, but this increase was reduced by adjustment for the specified variables. Interestingly, we noted a non-significant trend of lower odds of physician-diagnosed asthma in the Frequent and Acid exposure zones compared to the Low exposure zone. This did not change with adjustment for the selected variables.
Persistent wheeze in the last 12 months was reported by 8% of participants, with zone-specific rates of 4–9%. No significant association was found, but we again noted a non-significant pattern of lower odds of this symptom in the Frequent and Acid exposure zones.
Only 60 participants (3%) reported bronchitis in the past 12 months, with zone-specific rates of 3% in the Acid exposure zone, and 4% in the other zones. No significant association with vog exposure was detected.
Chi-square analysis on participants’ responses to the Test Questionnaire provides additional insights (Table 5). In response to the Test Questionnaire, 23% of participants reported a diagnosis of asthma. Again, the prevalence was highest in the Intermittent exposure zone (29%), and lowest in the Frequent (18%) and Acid (19%) zones. Only 226 of the participants with asthma were able to recall their most recent asthma attack. Their report of asthma in the last 12 months followed the same pattern of highest prevalence in the Intermittent zone and lower in the Frequent and Acid zones. More than 50% of the children answered “yes” in response to the question, “In the last month, have you had a head cold, headache, stuffy nose, or runny nose?” Chi square analysis indicated a significant difference in zone-specific rates (P<0.005). Prevalence in the Intermittent exposure zone (59%) was greater than in the Frequent or Acid (52%) and Low (47%) exposure zones. In contrast, there was no significant difference in percentage of affirmative responses to the question “In the last month, have you had a chest cold, cough, or other chest illness?” Overall, 34% reported chest cold symptoms. Again, there was a trend of highest in the Intermittent and lowest in the Frequent exposure zone.
3.4.3 Height, weight, and lung function
Participants in the vog exposure zones differed significantly in height and weight (Table 5). Participants in the Frequent exposure zone were lowest in these measures whereas those in the Acid exposure zone were tallest and heaviest. After applying ATS criteria, spirometric data from 3% the participants were excluded from analysis; rates of exclusion were similar between the zones. As expected from the differences in height, the FEV1 and FVC of participants in the different vog exposure groups were significantly different (Table 5). However, mean FEV1/(sitting height)2 and mean FEV1/FVC did not differ significantly between the zones.
We explored whether vog exposure is associated with reduced FEV1/FVC ratio. This ratio reflects changes in airway caliber and, in growing children, maturation of the lung and airway. Moreover, it is less affected by racial differences in body build or sitting/standingheight ratio. We selected FEV1/FVC <0.8 (lower than the mean FEV1/FVC of our participants) and FEV1/FVC ≤0.75 as dependent variables. Overall, the rates of FEV1/FVC <0.8 or FEV1/FVC≤0.75 were 18% and 6%, respectively (Table 6). There was a trend toward FEV1/FVC <0.8 in the Acid exposure zone (OR 1.32, CI0.94–1.86). No difference was seen with FEV1/FVC≤0.75. Although not statistically significant, we noted that prevalence of reduced FEV1/FVC, at either cut point, was lowest among participants in the Frequent exposure zone.
4. Discussion
We describe the output, dispersal, and composition of emissions from Kilauea Volcano, and the findings of a cross-sectional study of respiratory health effects associated with volcanic air pollution. Our findings have health implications for the estimated 10% of the world population near active or historically active gas-emitting volcanoes (26, 30). The findings also provide insight into the specific effects of SO2 and acid particulates in more familiar and complex anthropogenic air pollution.
We emphasize that Kilauea’s major emission is SO2 gas, similar to Miyakejima Island, Japan (14, 31), but different from the volcanic ash released during the eruptions of Mount Saint Helens, Mount Etna, or Eyjafjallajokull (32–36), the mixture of CO2 and radon released in fumarolic fields in the Azores (26, 37, 38)or H2S released from geothermal vents in Rotorua, New Zealand (39–41). Kilauea’s estimated SO2 output was 0.5–1 million metric tons (0.5–1 Tg) per year during this cross-sectional study, and increased further beginning in 2008 (Figure 2). To compare with a recognized example of anthropogenic pollution, China’s estimated SO2 emissions in 2002–2005 were 22–25 Tg per year, largely from power plants and industry, and some from biomass burning and transportation (42). Thus, the annual output from Kilauea, a single stationary source, is 5–10% of China’s annual SO2 anthropogenic emissions.
The dispersal and distribution of Kilauea’s emissions are defined by Hawai’i Island’s topography and meteorology, and the rate of conversion of SO2 to sulfate aerosols is influenced by sunlight, humidity, and other atmospheric conditions. These complex and dynamic factors were considered in defining the 4 vog exposure zones for the cross-sectional study. Wind data collected at an offshore point northeast of Hawai’i Island 1992–2001 and at Kilauea’s summit for the period 2000–2009 (Supplemental Information, Figures C and D) indicates that calm or southerly winds occurred less than 20% of the time, exposing communities near or northeast of the vents only intermittently. Filter-based monitoring confirmed that the zones differ significantly in concentrations of SO2, respirable particulate mass, and acidity. SO2 levels measured in the Frequent exposure zone by our community researchers were comparable to levels measured by other investigators for 3 weeks in Summer 2003 in the same area [Ka’u District, (43)]. Concentrations of SO2 in the Intermittent and Acid zones reported here are also consistent with 24-hr concentrations measured 2002–2005 in Hilo and Kona, respectively, by the Hawai’i Department of Health (Supplemental Information, Figure C). Concentrations are comparable to Miyakejima Island, Japan, where volcanic SO2 emissions are estimated at 300–700 tons per day and mean concentrations in its most polluted area decreased from 75 ppb in 2006 to 13 ppb in 2011 (14). To compare with concentrations reported for North American cities, the mean SO2 concentration in our Frequent exposure zone (10.1 ± 5.2 ppb) is similar to the mean concentrations measured 1988–1991 in Penn Hill, PAor Athens, OH, in the sulfate belt in the 24-Cities Study (18).
Mean PM2.5 in the Frequent exposure zone was similar to that measured by other investigators in the summer of 2003 in the same area [Ka’u District,(43)]. Mean PM2.5 concentrations in the Intermittent and Acid zones are consistent with 24-hr averages in Hilo and Kona, respectively, reported by the state’s Department of Health (Supplemental Data, Figure C). Note that the highest mean PM2.5 values, measured in the Frequent and Acid zones, are comparable to concentrations measured in 1988–1991 in Monterey, CA or Aberdeen, SD, selected as clean or “background” cities in the Twenty-four Cities study (18), or to the mean 2-week average PM2.5 of Alpine and Lompoc, CA, 1994, in the Children’s Health Study (19). Notably, despite PM2.5 comparable to “background” North American cities, particle strong acidity measured in Hawai’i Island’s Acid zone is comparable to concentrations measured in 1988–1991 in Uniontown, PA, which had the strongest particle acidity in the 24-Cities study (5, 18).
XRF analysis filtrates collected in this study contained much lower concentrations of trace elements reported for agricultural, industrial, or traffic-related air pollution (Table 2). Low levels were measured of copper and iron (associated with brake and tire wear), zinc (tire wear), silica (soils), vanadium and nickel (oil combustion), and potassium (biomass burning) (29). Of these, the heavy metals copper, vanadium, iron, and nickel, have been implicated in the development of respiratory or cardiovascular disease (44, 45).
Table 2.
Particulate elemental composition in vog exposure zones, determined by energy dispersive X-ray fluorescence, ng/m3 (mean ± s.d.)
| Low | Intermittent | Frequent | Acid | P-value | Traffic-related air pollution (29) |
|
|---|---|---|---|---|---|---|
| Filters (n) | 2 | 4 | 4 | 2 | ||
| Mean net mass (ug) |
826±695 | 1082±553 | 1685±411 | 2378±766 | NS | * |
| Sulfur | 580±501 | 777±361 | 1001±305 | 1572±478 | NS | 912.7 |
| Sodium | 45±1 | 85±23 | 110±80 | 95±35 | NS | * |
| Potassium | 9±1 | 33±28 | 47±27 | 18±6 | NS | 195.2 |
| Silica | 6±4 | 31±20 | 49±26 | 25±18 | NS | 137.7 |
| Iron | 5±2 | 21±8 | 31±2 | 16±9 | NS | 144.8 |
| Zinc | 0.4±0.2 | 1.4±0.3 | 2.5±0.4 | 1.0±0.2 | <0.001 | 26.4 |
| Copper | 0.2±0.03 | 1.2±0.2 | 1±0.7 | 0.4±0.2 | <0.01 | 6.4 |
| Lead | 0.2±0.05 | 0.6±0.05 | 1.47±1 | 0.4±0.2 | <0.05 | * |
| Nickel | 0.06±0.06 | 0.41±0.13 | 0.06±0.03 | 0.14±0.15 | <0.05 | 1.6 |
| Vanadium | 0.04±0.07 | 0.81±0.4 | 0.11±0.16 | 0±0 | <0.01 | 2.9 |
not reported
Combined, our measurements confirm that the 4 vog exposure zones differ from one another, that the respirable particles in the Acid exposure zone are strongly acid, and that many co-pollutants associated with anthropogenic sources of air pollution were relatively low at our sampling sites.
Our school-based health study, conducted according to community-based participatory research principles, effectively recruited and retained participants in all zones. The 73% participation rate suggests that study findings are likely to be applicable to the local community.
The ethnic diversity and racial admixture of our participants is a distinctive feature of this study. Participants reflect the population of Hawai’i and are unlike the study cohorts of the Six Cities, Twenty-four Cities, or Children’s Health Studies, who were all or mostly white (6, 19, 23, 24). U.S. Census 2000 reported children in Hawai’i as 15% White, 2% Black, 12% Hispanic, 28% Asian, 12% Hawaiian, 30% of 2 or more races, and other (46). Our Hawai’i Island participants identified their racial background, which researchers classified as 16% White, 15% Asian, 5% Pacific Islander (including Hawaiian), 63% of 2 or more races (including part-Hawaiian or part-Pacific Islander), 3% Other or Unknown. The issue of race became more compelling with emerging awareness of gene-environment interactions in the development of asthma and other respiratory conditions (47) and, among our Asian and Pacific Islander participants, likely higher prevalence of gene variants implicated in the pathogenesis of asthma (48). Even more fundamental than different susceptibilities to respiratory morbidity, are the racial differences in anthropometry and rates of airway and lung development, which can confound interpretation of lung function measures in growing children. The Global Lungs Initiative (GLI) continues to report the challenges and progress in reporting and interpreting pediatric lung function in ethnically mixed groups and individuals (49–51). Accordingly, we applied the GLI’s recommendation to take into consideration ratios (for example, FEV1/FVC), as well as sex, age, ethnic group, height and, particularly in ethnically mixed groups, sitting height.
The prevalence of premature birth (10% overall), family income > $50,000 (34%), maternal smoking during pregnancy (17%), smokers in the home during early childhood (41%) and currently (20%), and visible mold in the home (43% overall) varied between the vog exposure zones. We thus included age, sex, race, and sitting height, as well as premature birth, maternal smoking during pregnancy, current ETS exposure, and visible mold in home, in all adjustments for respiratory symptoms and lung function changes. We report on the respiratory symptoms of cough (described by 8–11% of respondents), physician-diagnosed asthma (14–21% of respondents), persistent wheeze in the last 12 months (4–9% of respondents), and bronchitis in the 12 months before testing (4% of respondents).
Only after additional adjustment for physician-diagnosed asthma, was an association with chronic cough detected among the participants in the Acid exposure group (adjusted OR 1.7, CI 1.06–2.67, Table 4). Stratification indicated that this increase was significant in the non-asthmatic participants (adjusted OR 2.0, CI 1.11–3.57), in whom the prevalence of cough nearly doubled from 5% in the Low exposure zone to 9% in the Acid zone (among asthmatic participants, prevalence increased from 25% to 30%). In similar studies of children on Miyakejima Island, cough was significantly increased in high exposure areas and decreased as air pollution improved over 6 years (14). In comparison, an association between high acid exposure and cough was not observed in the Six Cities or Twenty-four Cities studies of anthropogenic air pollution (5, 6).
Increased unadjusted odds for physician-diagnosed asthma were noted in the Intermittent exposure group but were reversed after adjusting for confounding factors. There was no increased risk for diagnosed asthma in the Frequent and Acid exposure zones. In fact, adjusted OR’s were lower than for the Low exposure zone. No association was seen with persistent wheeze in the last 12 months. The lack of association between high acid exposure and asthma or persistent wheeze in the prior 12 months is similar to findings of the Six Cities and Twenty-four Cities studies (5, 6).
The overall rate of reported bronchitis in the last 12 months was just 3% overall, including in the Acid exposure zone. This contrasts with a major finding of the Twenty-four Cities Study, which showed an association between particle strong acidity and the prevalence of any bronchitic symptom in the year before testing (8 to15%, adjusted OR 1.66, 1.11–2.48). That we did not observe this association with highly acid, but relatively “pure” volcanic air pollution may implicate co-contaminants in the anthropogenic air pollution, rather than the sulfate itself, in the development of bronchitis.. In studies of volcanic air pollution, “bronchitis” was not directly assessed among children on Miyakejima Island (14), but prevalence of bronchitis or bronchitis-like symptoms was increased in high exposure areas or episodes in several studies of adults exposed to volcanic air pollution with predominant CO2 and radon (26, 38) or SO2 and SO4 (11, 31, 52).
We attempted to address many complexities in interpreting and analyzing lung function in this racially and socioeconomically diverse population, in addition to the technical challenges of conducting spirometry for the first time in young children. We focused on FEV1/FVC <0.8 or ≤ 0.75 as the dependent variables in lung function analyses. The FEV1/FVC ratio was selected because it is solely dependent upon sex, age, and height and can be measured in the field. It is not affected by the sitting to standing height ratio, which differs between races. The zone-specific rates of FEV1/FVC <0.8 or for FEV1/FVC ≤ 0.75 were 14–20% and 4–7%, respectively. The OR of FEV1/FVC <0.8 in the Acid exposure group (OR 1.32, CI 0.94–1.86) suggests a trend toward reduced lung function in this zone. However, in the absence of an increase in the diagnosis or symptoms of asthma, it is possible that the prevalence of “reduced” FEV1/FVC ratio in this zone reflects normal lung maturation rather than airway narrowing. We note that participants in the Acid exposure zone are taller and heavier. They may well be more advanced in lung maturation, associated with a normal decline in FEV1/FVC to pubertal levels (49). Participants in the Acid exposure zone were more likely to have private health insurance and family income >$50,000, so undiagnosed asthma is less likely in this zone, and the socioeconomic status more conducive to healthy growth and maturation of lungs. Further study and follow-up are needed to discern the airway effects of vog or other pollutants vs normal developmental changes.
We compare lung function changes in this study to those described in earlier studies of children, with caution. As we described earlier, our participants are racially more diverse and admixed, and the vog exposure zones racially and socioeconomically disparate. Having no race-specific predicted values for lung volumes, we did not analyze or report volume changes in this cross-sectional study, but focused on the FEV1/FVC ratio instead. In the Twenty-four Cities Studies, this ratio was preserved, despite decrements in FEV1, FVC, peak expiratory flow rate, mid-expiratory flow rate (all relative to predicted values), or increases in the number of children with FVC<85% of predicted, that were associated with particle strong acidity and respirable particulate matter (23). The investigators hypothesized that this combination of lung function changes might reflect submaximal inspiration (perhaps due to airway blockage or sensory inhibition related to inflammation), submaximal exhalation (due to airway closure), or reduced total lung capacity (due to slowed growth rate). Longitudinal study may help discern the mechanism, if we find that volume effects worsen and reverse with acute episodes. The FEV1/FVC ratio was not reported in the study of children on Miyakejima Island, but there was no significant air pollution-associated decrement in lung function (14).
We observed an intriguing apparent reduction in the Frequent vog exposure group in report of asthma diagnosis and persistent wheeze on the initial questionnaire (by guardians and parents), in report of asthma diagnosis in structured interview before testing (by the participants), and a trend toward lower odds of reduced FEV1/FVC <0.8 or ≤ 0.75. Although no single dependent variable achieved statistical significance, the consistency of the trend raises the possibility of some other protective factor(s) in this community or a direct salutary effect of this blend of SO2 and acid particulates (53). Longitudinal follow up can determine if the trends persist, and discern protective factors, if any. A similar phenomenon was noted in a study of the effects of long-term H2S among 1639 adult residents of Rotorua, New Zealand. Not only was there no decrement in spirometric function, nor increased asthma or COPD risk, there was also a suggestion that H2S mitigates lung damage in smokers (41). We conclude that Kilauea’s SO2 emissions, Hawai’i Island’s topography and meteorology produce 4 vog exposure zones that allowed for a cross-sectional study of effects on respiratory health. Our data indicates that chronic exposure to strongly acid respirable particulates is associated with cough and a trend to reduced FEV1/FVC ratio, but not with diagnosis of asthma, or persistent wheeze or bronchitis in the last 12 months. Longitudinal study can help discern interactions between volcanic air pollution and other environmental factors in the development of respiratory symptoms and function, identify new onset asthma, and determine acute effects of episodes of even greater volcanic emissions. Even before longitudinal studies are complete, insights from the current collaboration indicate susceptible communities and increase our understanding of volcanic and other sources of air pollution.
Supplementary Material
Highlights.
Kilauea volcano on the Island of Hawai’i has erupted continuously since 1983; it released 1800 to 10000 metric tons of sulfur dioxide per day between 2000–2010.
Volcanic emissions, wind patterns and mountains produce Low, Intermittent, Frequent, and Acid exposure zones on the island.
In 2002–2003, 1,957 4th and 5th grade students were recruited into a study to test the hypothesis that high concentrations of sulfur dioxide and sulfate particulates (“vog”) is associated with respiratory symptoms, asthma or bronchitis, and reduced lung function.
High (Acid) vog exposure was associated with increased cough and a suggestion of reduced lung function, but not with physician-diagnosed asthma, persistent cough or wheeze
Acknowledgments
Acknowledgments and grant support
This work was supported by NIH/NIEHS R01-ES11346, CDC R01-EH000326, the American Lung Association of Hawai’i, and Leahi Fund. J. Davis is funded in part by NIH/NCRR U54MD007584. The authors gratefully acknowledge the HICLASS Research Team (E. Fernandez, J. Kometani, A. Petersen, J. Sutherland, M. Thomason, E. Wong, J. Yoshioka); the Hawai’i Department of Education and families and staff of participating schools; the Ka’u Rural Health Community Association, Inc. and the Hawai’i Island Rural Health Association (J. Marques) for community advisory support; the Hawai’i Department of Health (J. Kunimoto), Clean Air Branch (L.Young) and Hazard Evaluation and Emergency Response Program (B. Brooks); M. Davey, M. Wolfson, and J.P. Michaud for technical and scientific support, and E. Wong, J. Shrader, Q. Le, P. Namnama, and R. Grattan for manuscript preparation.
Abbreviations
- EPA
Environmental Protection Agency
- PM2.5
particulate matter ≤ 2.5 micron in diameter
- SO2
sulfur dioxide gas
- SO4
sulfate
- FEV1
Forced Expiratory Volume in 1 s
- FVC
Forced Vital Capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holcomb RTDRW, Wright TL, Stauffer PH. Eruptive history and long-term behavior of Kilauea Volcano. US Geological Survey Professional Paper. 1987;1(1350):261–350. [Google Scholar]
- 2.Elias T, Sutton AJ. Sulfur dioxide emission rates of Kiluaea Volcano, Hawaii 1979–1997. US Geological Survey Open File Report 98–642. 1998 [Google Scholar]
- 3.Clarke AD, Porter JN. Volcanic haze-physicochemistry and transport. Hilo, HI: UH-Hilo; 1991. [Google Scholar]
- 4.Chen Y-L, Nash AJ. Diurnal variation of surface airflow and rainfall frequencies on the island of Hawaii. Mon Wea Rev. 1994;122:34–45. [Google Scholar]
- 5.Ware JH, Ferris BG, Dockery DW, Spengler JD, Stram DO, Speizer FE. Effects of ambient sulfur oxides and suspended particles on respiratory health of preadolescent children. Am Rev Respir Dis. 1986;133(5):834–842. [PubMed] [Google Scholar]
- 6.Dockery DW, Cunningham J, Damokosh AI, Neas LM, Spengler JD, Koutrakis P, et al. Health effects of acid aerosols on North American children: respiratory symptoms. Environ Health Perspect. 1996;104(5):500–505. doi: 10.1289/ehp.96104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 8.Dimakopoulou K, Samoli E, Beelen R, Stafoggia M, Andersen ZJ, Hoffmann B, et al. Air pollution and nonmalignant respiratory mortality in 16 cohorts within the ESCAPE project. Am J Respir Crit Care Med. 2014;189(6):684–696. doi: 10.1164/rccm.201310-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaud JP. Investigation of Possible Acute Health Effects in Children Exposed to Vog [in Hilo] on the Island of Hawai'i. Honolulu, HI: Hazard Evaluation and Emergency Response Office, Hawaii Department of Health; 2000. [Google Scholar]
- 10.Longo BM, Yang W. Acute bronchitis and volcanic air pollution: a community-based cohort study at Kilauea Volcano, Hawai'i USA. Journal of toxicology and environmental health Part A. 2008;71(24):1565–1571. doi: 10.1080/15287390802414117. [DOI] [PubMed] [Google Scholar]
- 11.Longo BM, Rossignol A, Green JB. Cardiorespiratory health effects associated with sulphurous volcanic air pollution. Public health. 2008;122(8):809–820. doi: 10.1016/j.puhe.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Longo BM, Yang W, Green JB, Crosby FL, Crosby VL. Acute health effects associated with exposure to volcanic air pollution (vog) from increased activity at Kilauea Volcano in 2008. Journal of toxicology and environmental health Part A. 2010;73(20):1370–1381. doi: 10.1080/15287394.2010.497440. [DOI] [PubMed] [Google Scholar]
- 13.Chow DC, Grandinetti A, Fernandez E, Sutton AJ, Elias T, Brooks B, et al. Is volcanic air pollution associated with decreased heart-rate variability? Heart Asia. 2010;2(1):36–41. doi: 10.1136/ha.2009.001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasawa S, Nakano M, Tsuboi T, Kochi T, Tanaka S, Katsunuma T, et al. Effects of sulfur dioxide on the respiratory system of Miyakejima child residents 6 years after returning to the island. International archives of occupational and environmental health. 2015 doi: 10.1007/s00420-015-1037-y. [DOI] [PubMed] [Google Scholar]
- 15.Milan MM, editor. Absorption correlation spectrometry: IAVCEI, Methods in Volcanology. 2008. [Google Scholar]
- 16.U.S. Geological Survey. Gas numbers are up but emission rates are not 2013. [Available from: http://hvo.wr.usgs.gov/volcanowatch/view.php?id=207.
- 17.U.S. Department of the Interior National Park Service. Access to gaseous pollutant and meteorological data. [Available from: http://ard-request.air-resource.com/data.aspx. [Google Scholar]
- 18.Spengler JD, Koutrakis P, Dockery DW, Raizenne M, Speizer FE. Health effects of acid aerosols on North American children: air pollution exposures. Environ Health Perspect. 1996;104(5):492–499. doi: 10.1289/ehp.96104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JM, Avol E, Navidi W, London SJ, Gauderman WJ, Lurmann F, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159(3):760–767. doi: 10.1164/ajrccm.159.3.9804143. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, Spengler JD, et al. Acute effects of summer air pollution on respiratory symptom reporting in children. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1234–1242. doi: 10.1164/ajrccm.150.5.7952546. [DOI] [PubMed] [Google Scholar]
- 21.Koutrakis P, Wolfson JM, Spengler JD. An improved method for measuring aerosol strong acidity: results from a nine-month study in St. Louis, Missouri and Kingston, Tennessee. Atmospheric Environment. 1988;22(1):157–162. [Google Scholar]
- 22.Speizer FE. Studies of acid aerosols in six cities and in a new multi-city investigation: design issues. Environ Health Perspect. 1989;79:61–67. doi: 10.1289/ehp.897961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raizenne M, Neas LM, Damokosh AI, Dockery DW, Spengler JD, Koutrakis P, et al. Health effects of acid aerosols on North American children: pulmonary function. Environ Health Perspect. 1996;104(5):506–514. doi: 10.1289/ehp.96104506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 25.Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11(3):306–310. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 26.Linhares D, Ventura Garcia P, Viveiros F, Ferreira T, dos Santos Rodrigues A. Air Pollution by Hydrothermal Volcanism and Human Pulmonary Function. Biomed Res Int. 2015;2015:326794. doi: 10.1155/2015/326794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ATS. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 28.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 29.Tsai MY, Hoek G, Eeftens M, de Hoogh K, Beelen R, Beregszaszi T, et al. Spatial variation of PM elemental composition between and within 20 European study areas - Results of the ESCAPE project. Environment international. 2015;84:181–192. doi: 10.1016/j.envint.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 30.National Research Council. Active Tectonics: Impact on Society. Washington, DC: 1986. [Google Scholar]
- 31.Ishigami A, Kikuchi Y, Iwasawa S, Nishiwaki Y, Takebayashi T, Tanaka S, et al. Volcanic sulfur dioxide and acute respiratory symptoms on Miyakejima island. Occupational and environmental medicine. 2008;65(10):701–707. doi: 10.1136/oem.2007.033456. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein RS, Baxter PJ, Falk H, Ing R, Foster L, Frost F. Immediate public health concerns and actions in volcanic eruptions: lessons from the Mount St. Helens eruptions, May 18–October 18, 1980. Am J Public Health. 1986;76(3 Suppl):25–37. doi: 10.2105/ajph.76.suppl.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newhall CG, Fruchter JS. Volcanic activity: a review for health professionals. Am J Public Health. 1986;76(3 Suppl):10–24. doi: 10.2105/ajph.76.suppl.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudmundsson G. Respiratory health effects of volcanic ash with special reference to Iceland. A review. The clinical respiratory journal. 2011;5(1):2–9. doi: 10.1111/j.1752-699X.2010.00231.x. [DOI] [PubMed] [Google Scholar]
- 35.Lombardo D, Ciancio N, Campisi R, Di Maria A, Bivona L, Poletti V, et al. A retrospective study on acute health effects due to volcanic ash exposure during the eruption of Mount Etna (Sicily) in 2002. Multidisciplinary respiratory medicine. 2013;8(1):51. doi: 10.1186/2049-6958-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monick MM, Baltrusaitis J, Powers LS, Borcherding JA, Caraballo JC, Mudunkotuwa I, et al. Effects of Eyjafjallajokull volcanic ash on innate immune system responses and bacterial growth in vitro. Environ Health Perspect. 2013;121(6):691–698. doi: 10.1289/ehp.1206004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral A, Rodrigues V, Oliveira J, Pinto C, Carneiro V, Sanbento R, et al. Chronic exposure to volcanic environments and cancer incidence in the Azores, Portugal. The Science of the total environment. 2006;367(1):123–128. doi: 10.1016/j.scitotenv.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Amaral AF, Rodrigues AS. Chronic exposure to volcanic environments and chronic bronchitis incidence in the Azores, Portugal. Environ Res. 2007;103(3):419–423. doi: 10.1016/j.envres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Durand M, Grattan J. Effects of volcanic air pollution on health. Lancet. 2001;357(9251):164. doi: 10.1016/S0140-6736(00)03586-8. [DOI] [PubMed] [Google Scholar]
- 40.Bates MN, Garrett N, Crane J, Balmes JR. Associations of ambient hydrogen sulfide exposure with self-reported asthma and asthma symptoms. Environ Res. 2013;122:81–87. doi: 10.1016/j.envres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bates MN, Crane J, Balmes JR, Garrett N. Investigation of hydrogen sulfide exposure and lung function, asthma and chronic obstructive pulmonary disease in a geothermal area of New Zealand. PloS one. 2015;10(3):e0122062. doi: 10.1371/journal.pone.0122062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su S, Li B, Cui S, Tao S. Sulfur dioxide emissions from combustion in china: from 1990 to 2007. Environmental science & technology. 2011;45(19):8403–8410. doi: 10.1021/es201656f. [DOI] [PubMed] [Google Scholar]
- 43.Longo BM, Grunder A, Chuan R, Rossignol A. SO2 and fine aerosol dispersion from the Kilauea plume, Kau District, Hawaii, USA. Geology. 2005;33(3):217–220. [Google Scholar]
- 44.Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, et al. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Human & experimental toxicology. 2006;25(10):559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- 45.Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118(3):363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CensusScope-2010 Census. [Available from: http://www.censusscope.org/us/s15/chart_multi.html. [Google Scholar]
- 47.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002;166(4):457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 48.Sangrajrang S, Jedpiyawongse A, Srivatanakul P. Genetic polymorphisms of CYP2E1 and GSTM1 in a Thai population. Asian Pac J Cancer Prev. 2006;7(3):415–419. [PubMed] [Google Scholar]
- 49.Quanjer PH, Stanojevic S, Stocks J, Hall GL, Prasad KV, Cole TJ, et al. Changes in the FEV(1)/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J. 2010;36(6):1391–1399. doi: 10.1183/09031936.00164109. [DOI] [PubMed] [Google Scholar]
- 50.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quanjer PH, Stanojevic S, Stocks J, Cole TJ. Ethnically specific norms for ventilatory function. International journal of epidemiology. 2012;41(5):1490. doi: 10.1093/ije/dys153. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 52.Iwasawa S, Kikuchi Y, Nishiwaki Y, Nakano M, Michikawa T, Tsuboi T, et al. Effects of SO2 on respiratory system of adult Miyakejima resident 2 years after returning to the island. J Occup Health. 2009;51(1):38–47. doi: 10.1539/joh.l8075. [DOI] [PubMed] [Google Scholar]
- 53.Wang XB, Du JB, Cui H. Signal pathways involved in the biological effects of sulfur dioxide. European journal of pharmacology. 2015;764:94–99. doi: 10.1016/j.ejphar.2015.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.