1. Background
Sepsis remains a significant source of morbidity and mortality in the United States. The National Center for Health Statistics estimates that the number of hospitalizations for sepsis increased from 621,000 in the year 2000 to 1,141,000 in 2008 [1]. In 2010, the National Vital Statistic Reports described septicemia as the 11th leading cause of death [2]. Although the mortality rate from severe sepsis was noted to decrease from 39% in 2000 to 27% in 2007 in the United States, more patients required discharge to a long-term care facility [3]. Due to its high morbidity, mortality, cost, and resource utilization, a number of efforts have been directed towards improving outcomes. In 2001, Rivers et al [4] published a landmark study describing early, aggressive treatment of sepsis in the Emergency Department (ED), with markedly improved patient outcomes. Earlier treatment, in particular appropriate broad-spectrum antibiotics, decreases mortality [5–8]. Moreover, “early goal-directed” therapy in the form of sepsis bundles and standardized order sets has been consistently shown to improve measures such as time to antibiotics, time to fluid resuscitation, lactate clearance, and mortality [9–12]. While more recent multicenter studies have failed to prove a reduction in all-cause mortality or patient outcomes with protocol-based care, the role of early identification, fluid resuscitation, and early appropriate antibiotics remains the mainstay of clinical care for patient with severe sepsis and septic shock [13–15].
We sought to evaluate the efficacy of early, rapid identification of sepsis during Emergency Department (ED) triage, followed by a sepsis work-up and treatment (SWAT) protocol emphasizing rapid mobilization of resources, standardized order sets, and early broad-spectrum antibiotics and fluid resuscitation. We hypothesized that a “best practice alert” (triage sepsis alert) embedded in our electronic health record (Epic Systems Corporation, Verona, Wisconsin) combined with a SWAT protocol, would lead to a significant reduction in door-to-antibiotics time, door-to-intravenous (IVF) bolus time, and overall mortality.
2. Methods
2.1 Design, Setting, and Population
This was a retrospective, quasi-experimental study of adult ED patients (≥18 years of age) before and after our SWAT protocol implementation. The setting was a single, urban, academic ED with an annual census of 48,000. The study was approved by the Institutional Review Board.
2.2 Study Protocol
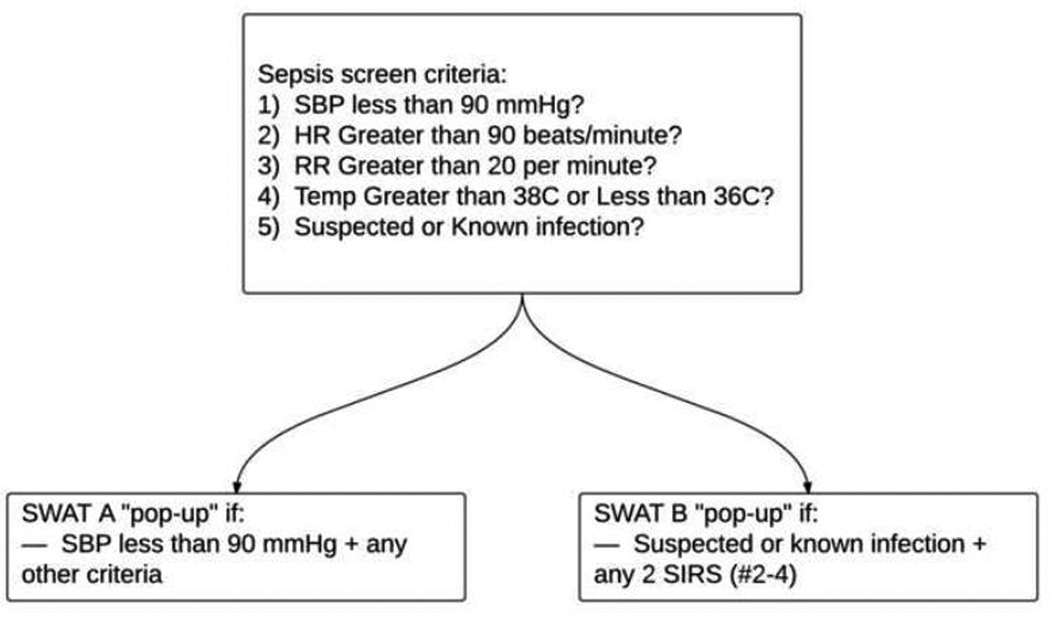
A pre-intervention group (before implementation of the SWAT protocol, or pre-SWAT) and post-intervention group (after implementation of the SWAT protocol, or post-SWAT) were defined. The pre-SWAT and post-SWAT groups were further broken down into SWAT A and SWAT B categories (Figure 1). We determined the implementation of the protocol would have a greater likelihood of success if patterned after an already established trauma alert system at our institution. Similar to a Trauma A, hypotensive patients with findings consistent with sepsis were assigned a SWAT A. Likewise, normotensive patients with 2 or more systemic inflammatory response syndrome (SIRS) criteria and a concern for an infectious source were assigned SWAT B.
Figure 1.
Triage sepsis alert (best practice alert), written into the background of the electronic health record. SWAT A and SWAT B are defined above.
The criteria used to identify pre-and post-intervention patients were the same, but the sequence in which they were used was different. The pre-intervention group was identified by first reviewing the Epic (Epic Systems Corporation, Verona, Wisconsin) electronic health records of all patients aged 18 years and older, who were hospitalized from the ED between November 2012 and March 2013 with an infectious process. Second, only those ED encounters meeting criteria for either a SWAT A or a SWAT B were included (Figure 1). This specific time frame was chosen such that the pre-intervention group patients presented to the ED after implementation of the Epic electronic health record. A report was created using the ICD-9 codes for pneumonia related diagnoses (480–499), skin infections (680–686), pyelonephritis (590), urinary tract infection (595), other bacterial infections (030-041), septic shock (785) and sepsis (995). Chart reviews of the patients on the report generated were conducted to confirm an ED diagnosis consistent with suspected sepsis, severe sepsis, or septic shock, and presence of criteria for either a SWAT A or a SWAT B.
The post-intervention (post-SWAT) group was composed of patients admitted from the ED between April 2013 through October 2014, for whom either a SWAT A or a SWAT B protocol was activated by the treating physician in response to the triage sepsis alert. Specifically, these were ED patients who met the SWAT A or B criteria, and in whom the ED provider suspected an underlying infectious process. A sample size of 130 subjects in the post-intervention group was estimated to be required in order to achieve a 95% confidence interval for a time-to-antibiotics reduction of 30 minutes. Medical records of patients on the SWAT A and SWAT B paging list were reviewed, and patients were excluded who did not meet criteria for SWAT A or SWAT B on ED presentation or were not admitted to the hospital.
Since both groups (pre- and post-intervention) had to meet SWAT A or B criteria, it was assumed that patients with simple (non-sepsis) infections were not included. To support this assumption, the infectious processes for pre- and post-intervention patients were reviewed. In comparing the rates of infectious processes for the 2 groups, no significance difference was found (see Appendix A).
The triage sepsis alert, or “best practice alert,” was derived from rules written in the background of the Epic electronic health record (Epic Systems Corporation, Verona, Wisconsin). If the required fields in the triage process met SWAT A or B criteria (Figure 1), the triage nurse received a pop-up notification prompting a direct communication with the physician. The decision to proceed with a SWAT activation was left to the discretion of the physician, based on a bedside evaluation of the patient. If a SWAT was activated, a standardized sepsis order set was used (computerized physician order entry, or CPOE), and the specified resources were mobilized (Figure 2). The SWAT A or B activation page (as gathered through the paging system, including time and patient medical record number) was recorded by the study investigators, and the medical records were reviewed for inclusion.
Figure 2.
Workflow after the triage sepsis alert for both SWAT A and SWAT B. (CBC/diff = complete blood count with differential, CMP = comprehensive metabolic panel, PT/PTT = prothrombin time/partial thromboplastin time.)
All data were abstracted retrospectively by four reviewers using standardized data collection sheets. Abstracted data was then entered into a secure, online database. Ambiguities were settled by consensus between the three secondary reviewers. The ambiguities included three instances of ED discharge diagnoses which were initially unclear, but agreed upon by the reviewers.
The senior authors on the paper (GH, GH, and RT) re-abstracted 10% of included charts to assess reviewer concordance. Charts were selected for re-review at random. In total, 768 total data points were re-abstracted from 24 charts and agreement was found on 751 data points. The percent of agreement was 97.8% with a 95% confidence interval for a single proportion of 96.4% to 98.6%. There was 100% agreement for ED arrival time, 100% for the time when antibiotics were given, and 100% for the time when intravenous fluids were given. For the 17 data points with discrepancies, the data abstracted by the senior authors were used.
2.3 Measurements
Patient demographic data (age, sex, and race) were obtained, and ED vital signs and laboratory data (white blood cell count and lactate level) were collected to determine severity of illness, SIRS scores, and shock indices. We defined “triage” SIRS as the SIRS criteria available at the time of patient triage (which excluded the white blood cell count), and “total” SIRS included the white blood cell count. Effectiveness of the protocol was determined by specific treatment intervals and patient outcomes: Door-to-bolus (door-to-intravenous fluids bolus), door-to-antibiotics, door-to-admit order, ED length of stay, hospital length of stay, ICU length of stay, and overall mortality (including in-hospital mortality and discharge to hospice care).
2.4 Statistical analysis
Descriptive statistics included numbers and rates for categorical data as well as means, standard deviation, medians and ranges for continuous data. Comparisons between characteristics of subjects pre- and post-intervention were made using chi-square tests (for proportions) and 2-smaple t-tests (for continuous variables). Group differences were calculated, along with their 95% confidence intervals. To assess whether or not the intervention resulted in significant shifts in the time outcomes (i.e. door-to-bolus, door-to-antibiotics, door-to-admit order), a segmented regression modeling approach was used [16] in order to account for any potential secular trends that may have been occurring despite the intervention. In this modeling process, 4 models were considered for each time outcome: 1) a “full” linear regression model, which allowed for a change in means and a change in slope after the intervention; 2) a model that allowed for a change in means but included identical non-zero slopes pre- and post-intervention; 3) a model that allowed for a change in slopes but forced the intercept to be identical pre- and post-intervention; and 4) a model that allowed for a change in means but assumed a zero (flat) slope pre-and post-intervention. Akaike information criterion (AIC) [17] was used to determine which of the 4 models provided the best fit. Once the most appropriate model was selected, patient characteristics identified as being significantly different (pre- vs. post-intervention) were included in the model as covariates, to control for any potential confounding. P-values < 0.05 were considered statistically significant.
3. Results
A total of 238 patient charts were abstracted for the study, including 108 charts in the pre-SWAT group, and 130 charts in the post-SWAT group. All patients were suspected of having an infection by the ED provider team and met SWAT A or SWAT B criteria suggesting sepsis, severe sepsis, or septic shock (independent of the hospital discharge diagnosis).
The pre-SWAT group was composed of 13 patients meeting SWAT A criteria, and 95 patients meeting SWAT B criteria. The post-SWAT group was composed of 32 patients meeting SWAT A criteria, and 98 patients meeting SWAT B criteria.
There were no significant differences between the pre-SWAT and post-SWAT groups in regards to age, sex or race (Table 1). While there was no difference in “triage” SIRS criteria between the two groups (p=0.20), patients in the post-SWAT group had a higher number of “total” SIRS criteria compared to the pre-SWAT group (p=0.04). The shock index was higher in the post-SWAT group with a mean of 1.1 compared to the pre-SWAT shock index of 0.9 (p<0.01). Significant differences were seen between the post-SWAT and pre-SWAT groups in terms of initial temperature and respiratory rate, and systolic blood pressure. The mean systolic blood pressure was, on average, 15.7 mmHg lower in post-SWAT group as compared to the pre-SWAT group (p<0.01), which accounted for a greater percentage of patients classified as SWAT A in the post-SWAT group (25%) as compared to the pre-SWAT group (12%) (p=0.02).
Table 1.
Comparison of Demographics and Severity
| Pre-SWAT (108) |
s | Post-SWAT (130) |
s | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| % SWAT A | 12 | na | 25 | na | 13 | 3 to 23 | 0.02 |
| % SWAT B | 88 | na | 75 | na | 13 | ||
| % Male | 53 | na | 62 | na | 9 | −21.6 to 3.6 | 0.21 |
| % Female | 47 | na | 38 | na | |||
| Age (yrs) | 55 | ±19 | 56 | ±18 | 1 | −5.7 to 3.7 | 0.68 |
| % White | 45 | na | 42 | na | 3 | −9.6 to 15.7 | 0.74 |
| % Black | 50 | na | 55 | na | 5 | −17.1 to 7.1 | 0.50 |
| % Other | 5 | na | 3 | na | 2 | −2.9 to 6.9 | 0.65 |
| Heart Rate (beats/min) | 115.2 | ±21.5 | 118.7 | ±18.2 | 3.5 | −8.6 to 1.6 | 0.18 |
| Temperature (°C) | 37.7 | ±1.6 | 38.3 | ±1.5 | 0.6 | 0.2 to 1 | <0.01 |
| Respiratory Rate (Breath/min) | 22.1 | ±6.1 | 23.9 | ±7 | 1.8 | 0.1 to 3.5 | 0.04 |
| Systolic BP (mmHg) | 130.2 | ±33.6 | 114.5 | ±31.6 | 15.7 | 7.4 to 24 | <0.01 |
| # SIRS Total | 2.8 | ±0.7 | 3 | ±0.8 | 0.2 | 0.01 to 0.4 | 0.04 |
| WBC (thousands/µL) | 14.1 | ±9.8 | 14.4 | ±9.3 | 0.3 | −2.7 to 2.1 | 0.81 |
| Lactate (mmol/L) | 2.4 (n=75) | ±2.4 | 2.8 (n=126) | ±2.4 | 0.4 | −1.1 to 0.3 | 0.25 |
| Lactate performed % | 69.4 | na | 96.9 | na | 27.5 | 18.3 to 36.8 | <0.01 |
| Shock Index (Systolic BP/heart rate) | 0.9 | ±0.3 | 1.1 | ±0.4 | 0.2 | 0.11 to 0.29 | <0.01 |
SIRS = Systemic Inflammatory Response Syndrome; WBC = White blood cell; BP = blood pressure
Of the 108 pre-SWAT patients, a serum lactate test was performed in 75 (69.4%), and of the 130 post-SWAT patients, lactate was performed in 126 (96.9%). This represented a 27.5% increase in lactate tests being performed in the post-SWAT patients (95% CI 18.3 to 36.8%, p<0.01). A comparison of pre-SWAT A (11/13 = 84.6%) to post-SWAT A (30/32 = 93.8%) patients showed an increase of 9.2% (95% CI −27.5 to 9.1%, p=0.69). A comparison of pre-SWAT B (64/95 = 67.4%) to post-SWAT B (96/98 = 98%) patients showed an increase of 30.6% (95% CI 20 to 41.2, p<0.01). No difference, however, was observed in the measured mean lactate levels between the pre-SWAT and post-SWAT groups (p=0.25).
Results of the segmented regression modeling indicated that there were no significant secular trends in any of the time outcomes (i.e. door-to-bolus, door-to-antibiotics, door-to-admit order) and that the best fitting model for each outcome was one that allowed only for a change in means (assuming a zero slope pre- and post-intervention). The post-SWAT group demonstrated marked improvements in both the door-to-intravenous fluids time and the door-to-antibiotics time (Table 2). The mean door-to-bolus time improved by 30.5 minutes (p<0.01), a finding that was slightly attenuated when covariates were added to the model (adjusted difference=22.4 minutes, 95%CI=5.6 to 39.2) (Figure 3). Similarly, the mean door-to-antibiotics time improved by 58.8 minutes (p<0.01), a finding that was also slightly attenuated when covariates were added to the model (adjusted difference=50.6 minutes, 95%CI=34.7 to 66.4) (Figure 4). No significant changes were noted for door-to-pressor given or door-to-admit order times.
Table 2.
Treatment Times (all in minutes)
| Pre-SWAT | s | Post-SWAT | s | Difference | 95% CI | p-value* | |
|---|---|---|---|---|---|---|---|
| Door to Bolus | 81.9 (n=80) | ±66.8 | 51.4 (n=120) | ±47.5 | 30.5 | 14.6 to 46.3 | <0.01 |
| Door to Antibiotics Given | 139.4 (n=108) | ±74.3 | 80.6 (n=130) | ±38.8 | 58.8 | 44 to 73.6 | <0.01 |
| Door to Pressor Given | 139.3 (n=6) | ±218.5 | 125.9 (n=15) | ±132 | 13.4 | −155 to 182 | 0.91 |
| Door to Admit Order | 181.5 (n=108) | ±99.2 | 168.0 (n=129) | ±101.9 | 13.5 | −12.3 to 39.3 | 0.61 |
P-values were obtained from the best-fitting segmented regression model.
Figure 3.
Segmented regression modeling of door-to-bolus administration in pre- and post-SWAT implementation periods. This analysis allowed for a change in means but assumed a zero (flat) slope pre- and post-intervention.
Figure 4.
Segmented regression modeling of door-to-antibiotic (ABX) administration in pre- and post-SWAT implementation periods. This analysis allowed for a change in means but assumed a zero (flat) slope pre- and post-intervention.
Table 3 highlights differences in patient outcomes pre- and post-intervention. There were no statistically significant increases in the proportion of subjects who were admitted to the ICU (35.2% vs. 43.1%, p=0.27) or the proportion who died during their hospital stay (9.3% vs. 13.8%, p=0.38), but there was a notable decline in the proportion discharged to hospice care (7.4% vs. 1.5%, p=0.05). No significant differences between groups were noted in the average length of ICU stay or in the average length of hospital stay. Further analysis is available in Appendix B.
Table 3.
Outcomes
| Pre-SWAT | Post-SWAT | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| % ICU | 35.2 | 43.1 | 7.9 | −20.4 to 4.6 | 0.27 |
| % Mortality | 9.3 | 13.8 | 4.5 | −12.7 to 3.7 | 0.38 |
| % Discharge to Hospice | 7.4 | 1.5 | 5.9 | 0.8 to 11 | 0.05 |
| Average LOS in ICU (days) | 4.1 | 6.2 | 2.1 | −4.9 to 0.7 | 0.14 |
| Average LOS in Hospital (days) | 8.2 | 9.0 | 0.8 | −3.3 to 1.7 | 0.53 |
LOS = length of stay
In comparing pre-SWAT A and post-SWAT A patients, there was no significant difference between demographic, severity of illness or outcome variables such as mortality rate or ED, hospital, or ICU length of stay (analysis available in Appendix C). However, the post-SWAT A mean door-to-antibiotics time was 67.8 minutes less (p=0.02). (Table 4)
Table 4.
Comparisons of SWAT A's and B's
| Pre-SWAT | n | SD | Post-SWAT | n | SD | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| SWAT A Door to Bolus (min) | 55.9 | 13 | ±63.8 | 34.6 | 27 | ±31 | 21.3 | −8.8 to 51.4 | 0.27 |
| SWAT A Door to Antibiotics Given (min) | 124.4 | 13 | ±77 | 67.8 | 32 | ±37.6 | 56.6 | 10 to 103 | 0.02 |
| SWAT B Door to Bolus (min) | 87 | 67 | ±67 | 56.3 | 93 | ±50.4 | 30.7 | 12.4 to 49 | 0.00 |
| SWAT B Door to Antibiotics Given (min) | 141.5 | 95 | ±80 | 85.2 | 98 | ±38.8 | 56.3 | 38.6 to 74 | <0.01 |
| SWAT A Mortality or Hospice (%) | 30.8 | 13 | na | 21.9 | 32 | na | 8.9 | −19 to 37 | 0.70 |
| SWAT B Mortality or Hospice (%) | 14.8 | 95 | na | 13.2 | 98 | na | 1.6 | −8.2 to 11.4 | 0.83 |
In comparing pre-SWAT B and post-SWAT B patients, there was no significant difference between demographic and outcome variables (analysis available in Appendix D). However, there was a significant difference in a number of severity of illness variables. Mean pre-SWAT B “triage” SIRS was 2.2 compared to post-SWAT B 2.4 (difference 0.2, 95% CI 0.07 to 0.33, p<0.01). Pre-SWAT mean shock index was 0.9 compared to post-SWAT 1.0 (difference 0.1, 95% CI 0.03 to 0.17, p<0.01). Moreover, pre-SWAT B mean systolic blood pressure (mm Hg) was 137.5 compared to post-SWAT B 126.7 (difference 10.8, 95% CI 2.9 to18.7, p<0.01). There was also a significant difference for the treatment variables. Post-SWAT B mean door-to-bolus time was 30.7 minutes less (p<0.01) and the mean door-to-antibiotic time was 56.3 minutes less (p<0.01). (Table 4)
A sub-analysis was performed regarding an institutional goal of administering antibiotics in presumed sepsis within 60 minutes of ED arrival. Comparing pre-SWAT to post-SWAT patients, antibiotics were administered within 60 minutes in 11.1% (12/108) patients and 36.2% (47/130) patients, respectively, which represented a 25.1% increase (95% CI 14.1 to 36.1%, p<0.01). Comparing pre-SWAT A to post-SWAT A patients, antibiotics were administered within 60 minutes in 15.4% (2/13) patients and 53.1% (17/32) patients, respectively, which represented an absolute 37.7% increase (95% CI 5.9 to 69.5%, p=0.05). Finally, comparing pre-SWAT B to post-SWAT B patients, antibiotics were administered in less than 60 minutes in 10.5% (10/95) patients and 30.6% (30/98) patients, respectively, which represented a 20.1% increase (95% CI 8.7 to 31.5%, p<0.01).
More comprehensive data regarding the pre-SWAT, post-SWAT, and SWAT A and SWAT B sub-groups may be found in Appendices B–D.
4. Discussion
In this retrospective, quasi-experimental study of adult ED patients admitted to the hospital with suspected sepsis, severe sepsis, or septic shock, a sepsis work-up and treatment (SWAT) protocol originating from ED triage (automated ED triage sepsis alert) served to lower the time to intravenous fluids and time to antibiotics. The value of early identification of sepsis is significant, particularly when coupled with evidence-based therapies such as fluid resuscitation and early, appropriate empiric antibiotics [7, 18]. Ferrer et al [5] performed a retrospective analysis of over 28,000 patients with severe sepsis and septic shock and noted a steady increase in hospital mortality for each hour delay in antibiotic administration. Gaieski et al [6] described a similar experience in a study of 261 ED patients, noting that elapsed time to antibiotics served as a primary determinant of mortality in patients with severe sepsis and septic shock. However, beyond early identification, rapid empiric antibiotics, and adequate fluid resuscitation, strict adherence to particular early goal-directed therapy (EGDT) protocols has not led to improvement in outcomes [13–15]. This has consequently led to revisions of the Surviving Sepsis Campaign’s Guidelines and performance improvement indicators [19].
There is an increasing focus on ways to improve sepsis identification. Sepsis screening and alert systems have been studied in both the ED and the intensive care unit, with improvements in early identification of sepsis and the initiation of therapy [20–24]. Computer alerts have also been studied in the context of sepsis, with improved compliance in terms of lactate testing [25]. Various medical informatics solutions have also been investigated, including a clinical decision support tool that predicted lactic acid levels and mortality based on vital signs and white blood cell levels [26]. A recent study evaluated an electronic medical record (EMR) screening tool that triggered a sepsis protocol based on a defined number of systemic inflammatory response (SIRS) criteria present during triage [27]. However, this study was not outcomes-focused, and the impact of their screening tool was not assessed.
Umschied et al [28] described an early warning and response system (EWRS) for sepsis that was used for adult non-ICU patients who were already admitted to the hospital. This “alert” used a combination of SIRS criteria, systolic blood pressure, and lactate. This study evaluated the test characteristics of the EWRS. They did note earlier sepsis care and a trend towards decreased sepsis mortality (not statistically significant). Nguyen et al [29] also evaluated an electronic health record-based sepsis identification tool that incorporated similar features to the Umschied et al [28] study. They evaluated diagnostic accuracy of their sepsis identification tool in an ED setting, but did not report on outcome measures such as time-to-antibiotics or time-to-fluids. Next, Alsolamy et al [30] studied another electronic alert system in ED patients admitted to the ICU, evaluating the diagnostic accuracy of the sepsis alert in detecting severe sepsis or septic shock. Their screen incorporated SIRS criteria and evidence of organ dysfunction (systolic blood pressure, oxygen saturation, and lactate). Similar to the aforementioned studies, our triage sepsis alert used SIRS criteria and blood pressures. However, our alert did not incorporate laboratory testing, as this was unavailable at the time of ED triage. The diagnostic accuracy of the triage sepsis alert was also not evaluated. We did report key outcome measures such as time-to-antibiotics, time-to-fluids, and mortality rates.
Specifically in this study, we evaluated a SWAT protocol which incorporates an electronic health record-based triage sepsis alert (best practice alert), direct communication between ED triage nurse and the ED physician, and standardized orders and a mobilization of resources. Implementation of the SWAT protocol led to an approximately 31 minute decrease in door-to-bolus and a 59 minute decrease in door-to-antibiotics, in the setting of a more ill post-SWAT group who had more SIRS criteria, a higher mean shock index, and lower mean systolic blood pressures. Segmented regression modeling did not identify secular trends in any of these time outcomes, which confirms that these significant differences are more likely a result of the intervention. The lack of significance difference in mortality rates could be attributed to the post-SWAT group being sicker.
While there was a significant difference in the performance of lactate assessment between the pre-SWAT and post-SWAT groups, there was no difference in the lactate levels between the two groups. Of note, no standardized diagnostic work-up was in place for patients with suspected sepsis in the pre-SWAT group, while lactate assessment was included on the SWAT protocol in the post-SWAT group. This is the likely explanation for a higher percentage of lactate assessments performed in the post-SWAT group, though a difference in illness severity between the two groups is also possible.
When pre-SWAT A and post-SWAT A groups were compared, the improvement in door-to-antibiotics was maintained, although the door-to-bolus times were no different. Since hypotension upon presentation is a core criterion for SWAT A, it is not surprising that a rapid fluid bolus was also observed in both groups. For the pre-SWAT B and post-SWAT B groups, significant improvement in both the door-to-bolus time and door-to-antibiotics time was still observed.
In all post-intervention groups, the percentage of patients receiving antibiotics within 60 minutes of ED arrival was significantly higher. This institutional goal is base on the Surviving Sepsis Campaign Guidelines from 2012 [7] which recommend “administration of effective intravenous antimicrobials within the first hour of recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C) as the goal of therapy.” As of 2010, only 68% of patients in the SSC registry received antibiotics within 3 hours. While no definitive causal link between early antibiotics and patient outcomes has been established, there is an association in observational data between early antibiotics and survival outcomes in severe sepsis and septic shock. The absolute 25.1% increase in post-intervention patients in this study who received antibiotics within 60 minutes is significant.
5. Limitations
A number of limitations exist to this study, in addition to its retrospective and single site design. Different methods were used to identify pre and post intervention groups, and the groups were not matched for illness severity. The post-SWAT group had significantly greater severity of illness, and this along with implementing the SWAT protocol likely contributed to improved mean times to bolus and antibiotics. Thus severity of illness was a confounding factor that may have limited the internal validity of the results.
In regards to the post-SWAT group, the study did not measure adherence to individual elements of the SWAT protocol, which is a limitation of the retrospective design. Also the study did not track performance of the triage sepsis alert, so false positives and false negative rates for SWAT activations are not known. If the number of false positives was significant, then the effectiveness of the SWAT protocol treatment would be difficult to measure since these patients were not septic, and good outcome rates would be falsely elevated. If the number of false negatives was significant, then all potential patients were not included in the study, resulting in selection bias.
6. Conclusion
In conclusion, this study demonstrates a significant reduction in the time-to-intravenous fluids and time-to-antibiotics in ED patients admitted with suspected sepsis, severe sepsis, or septic shock, following implementation of an EHR-based triage sepsis alert and SWAT protocol.
Acknowledgments
Dr. Nietert’s time is funding in part by a grant from the National Center for Advancing Translational Sciences (award number UL1 TR000062).
APPENDIX A: COMPARISON OF PRE-SWAT AND POST-SWAT INFECTION SOURCES AND POSITIVE CULTURES
| Pre-SWAT (108 patients) |
Post-SWAT (130 patients) |
Difference (%) | 95% CI | p-value | |
|---|---|---|---|---|---|
| Blood culture + (%) | 20.4 (20) | 28.5 (37) | 8.1 | −19.1 to 2.9 | 0.20 |
| Urine culture + | 25.0 (27) | 26.9 (35) | 1.9 | −13.1 to 9.3 | 0.85 |
| Any culture + | 50.0 (54) | 59.2 (77) | 9.2 | −21.9 to 35.0 | 0.20 |
| Source: Lungs | 46.3 (50) | 36.2 (47) | 10.1 | −2.4 to 22.6 | 0.15 |
| Source: Urine | 28.7 (31) | 34.6 (45) | 5.9 | −17.8 to 6.0 | 0.41 |
| Source: Soft Tissue | 15.7 (17) | 14.6 (19) | 1.1 | −8.0 to 10.2 | 0.46 |
| Source: Gastrointestinal | 8.3 (9) | 7.7 (10) | 0.6 | −6.3 to 7.5 | 0.94 |
| Source: Venous Line | 3.7 (4) | 3.1 (4) | 0.6 | −4.0 to 5.2 | 0.92 |
| Source: Other/Unknown | 2.8 (3) | 6.9 (9) | 4.1 | −9.7 to 1.5 | 0.25 |
APPENDIX B: COMPARISON OF PRE-SWAT AND POST-SWAT DATA
| PRE-SWAT | PRE-SWAT (±SD) | POST-SWAT | POST-SWAT (±SD) | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Total patients | 108 | na | 130 | na | na | na | na |
| % Male | 53% | na | 62% | na | 9% | −21.6 to 3.6 | 0.21 |
| % Female | 47% | na | 38% | na | na | na | na |
| Age (yrs) | 55 | ± 19 | 56 | ± 18 | 1 | −5.7 to 3.7 | 0.68 |
| % White | 45% | na | 42% | na | 3% | −9.6 to 15.7 | 0.74 |
| % Black | 50% | na | 55% | na | 5% | −17.1 to 7.1 | 0.50 |
| % Other | 5% | na | 3% | na | 2% | −2.9 to 6.9 | 0.65 |
| % SWAT A | 12% | na | 25% | na | 13% | 3 to 23 | 0.02 |
| % SWAT B | 88% | na | 75% | na | na | na | na |
| # SIRS on ED arrival | 2.2 | ± 0.6 | 2.3 | ± 0.6 | 0.10 | −0.3 to 0.1 | 0.20 |
| # SIRS Total | 2.8 | ± 0.7 | 3.0 | ± 0.8 | 0.20 | 0.01 to 0.4 | 0.04 |
| Temperature (°C) | 37.7 | ± 1.6 | 38.3 | ± 1.5 | 0.6 | 0.2 to 1 | 0.00 |
| Respiratory Rate (Breath/min) | 22.1 | ± 6.1 | 23.9 | ± 7.0 | 1.8 | 0.1 to 3.5 | 0.04 |
| Systolic BP (mmHg) | 130.2 | ± 33.6 | 114.5 | ± 31.6 | 15.7 | 7.4 to 24 | <0.01 |
| Heart Rate (beats/min) | 115.2 | ± 21.5 | 118.7 | ± 18.2 | 3.5 | −8.6 to 1.6 | 0.18 |
| WBC (thousands/µL) | 14.1 | ± 9.8 | 14.4 | ± 9.3 | 0.3 | −2.7 to 2.1 | 0.81 |
| % Central Line | 2.8% | na | 10.8% | na | 8% | 1.4 to 14.6 | 0.03 |
| % Pressors given | 4.6% | na | 11.5% | na | 6.9% | −14 to 0.2 | 0.09 |
| % ICU | 35.2% | na | 43.1% | na | 7.9% | −20.4 to 4.6 | 0.27 |
| % Mortality | 9.3% | na | 13.8% | na | 4.5% | −12.7 to 3.7 | 0.38 |
| % Discharge to Hospice | 7.4% | na | 1.5% | na | 5.9% | 0.8 to 11 | 0.05 |
| % Mortality or Hospice | 16.7% | na | 15.3% | na | 1.4% | −7.9 to 11 | 0.91 |
| Door to bolus (min) | 81.9 (80) | ± 66.8 | 51.4 (120) | ± 47.5 | 30.5% | 14.6 to 46.4 | <0.01* |
| Door to Antibiotic order (min) | 103.1 | ± 70.9 | 46.0 | ± 32.3 | 57.1 | 43.4 to 70.8 | <0.01 |
| Door to Antibiotic given (min) | 139.4 | ± 74.3 | 80.6 | ± 38.8 | 58.8 | 44 to 73.6 | <0.01* |
| Order to Antibiotic given (min) | 36.4 | ± 21.3 | 34.3 | ± 26.1 | 2.1 | −4.1 to 8.3 | 0.50 |
| Door to Pressor order (min) | 139.3 (5) | ± 218.5 | 125.9 (15) | ± 132.0 | 13.4 | −155 to 182 | 0.87 |
| Door to Pressor given (min) | 139.3 (5) | ± 218.5 | 125.9 (15) | ± 132.0 | 13.4 | −155 to 182 | 0.87* |
| Door to Admit order (min) | 181.5 | ± 99.2 | 168.0 | ± 101.9 | 13.5 | −12.3 to 39.3 | 0.30* |
| ED Admit Order to OTF (min) | 279.1 | ± 381.0 | 214.3 | ± 281.4 | 64.8 | −19.9 to 149.5 | 0.13 |
| ED LOS (min) | 460.7 | ± 387.0 | 382.1 | ± 305.2 | 78.6 | −9.8 to 167 | 0.08 |
| LOS ICU (days) | 4.1 (38) | ± 2.9 | 6.2 (56) | ± 8.4 | 2.1 | −4.9 to 0.7 | 0.14 |
| LOS Hospital (days) | 8.2 | ± 9.2 | 9.0 | ± 10.1 | 0.8 | −3.3 to 1.7 | 0.53 |
| Shock Index (Systolic BP/heart rate) | 0.9 | ± 0.3 | 1.1 | ± 0.4 | 0.2 | 0.11 to 0.29 | <0.01 |
SIRS = Systemic Inflammatory Response Syndrome; WBC = White blood cell; BP = blood pressure; LOS = length of stay; OTF = off-the-floor
Corresponding p-values were obtained from the best-fitting segmented regression model.
APPENDIX C: COMPARISON OF PRE-SWAT A AND POST-SWAT A DATA
| PRE-SWAT | PRE-SWAT (±SD) | POST-SWAT | POST-SWAT (±SD) | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Total patients | 13 | na | 32 | na | na | na | na |
| % Male | 62% | na | 72% | na | 10% | −40 to 20 | 0.76 |
| % Female | 38% | na | 28% | na | na | na | na |
| Age (yrs) | 59 | ± 16 | 52 | ± 20 | 7 | −5.6 to 19.6 | 0.27 |
| % White | 46% | na | 38% | na | 8% | −24 to 40 | 0.87 |
| % Black | 54% | na | 59% | na | 5% | −37 to 27 | 0.98 |
| % Other | 0% | na | 3% | na | 3% | −12 to 6 | 0.61 |
| # SIRS on ED arrival | 1.8 | ± 1.0 | 1.8 | ± 0.7 | 0 | −0.5 to 0.5 | 1.00 |
| # SIRS Total | 2.3 | ± 1.1 | 2.6 | ± 0.8 | 0.3 | −0.9 to 0.3 | 0.31 |
| Temperature (°C) | 36.6 | ± 3.0 | 37.3 | ± 1.9 | 0.7 | −2.2 to 0.8 | 0.35 |
| Respiratory Rate (Breath/min) | 21.5 | ± 10.7 | 22.1 | ± 6.7 | 0.6 | −5.9 to 4.7 | 0.82 |
| Systolic BP (mmHg) | 77.5 | ± 7.4 | 77.2 | ± 8.9 | 0.3 | −5.3 to 5.9 | 0.92 |
| Heart Rate (beats/min) | 105.5 | ± 29.0 | 116.7 | ± 22.8 | 11.2 | −27.6 to 5.2 | 0.18 |
| WBC (thousands/µL) | 11.2 | ± 6.1 | 15.0 | ± 10.5 | 3.8 | −10.1 to 2.5 | 0.23 |
| % Central Line | 7.7% | na | 28.1% | na | 20.3% | −47 to 6 | 0.28 |
| % Pressors given | 15.4% | na | 18.8% | na | 3.4% | −28 to 21 | 0.87 |
| % ICU | 61.5% | na | 65.6% | na | 4.1% | −35 to 27 | 0.93 |
| % Mortality | 7.7% | na | 21.9% | na | 14.2% | −39 to 10 | 0.49 |
| % Discharge to Hospice | 23.1% | na | 0.0% | na | 23.1% | 7 to 39 | 0.03 |
| % Mortality or Hospice | 30.8% | na | 21.9% | na | 8.9% | −19 to 37 | 0.81 |
| Door to bolus (min) | 55.9 | ± 63.8 | 34.6 (27) | ± 31.0 | 21.3 | −8.8 to 51 | 0.16 |
| Door to Antibiotic order (min) | 95.9 | ± 72.9 | 40.4 | ± 30.7 | 55.5 | 25 to 86 | 0.00 |
| Door to Antibiotic given (min) | 124.4 | ± 77.0 | 67.8 | ± 37.6 | 56.6 | 10 to 103 | 0.02 |
| Order to Antibiotic given (min) | 28.5 | ± 21.6 | 44.7 | ± 20.0 | 16.2 | 30 to 3 | 0.02 |
| Door to Pressor order (min) | 53.5 (2) | ± 58.7 | 111.5 (24) | ± 147.8 | 58 | −281 to 165 | 0.60 |
| Door to Pressor given (min) | 53.5 (2) | ± 58.7 | 111.5 (24) | ± 147.8 | 58 | −281 to 165 | 0.60 |
| Door to Admit order (min) | 162.8 | ± 124.0 | 148.7 | ± 115.3 | 14.1 | −64 to 92 | 0.72 |
| ED Admit Order to OTF (min) | 118.4 | ± 118.0 | 149.6 | ± 173.7 | 31.2 | −137 to 75 | 0.56 |
| ED LOS (min) | 281.2 | ± 232.6 | 298.3 | ± 219.3 | 17.1 | −165 to 131 | 0.82 |
| LOS ICU (days) | 4.3 (8) | ± 3.2 | 5.9 (21) | ± 10.3 | 1.6 | −9.3 to 6.1 | 0.67 |
| LOS Hospital (days) | 10.6 | ± 8.4 | 8.8 | ± 9.3 | 1.8 | −4.2 to 7.8 | 0.55 |
| Shock Index (Systolic BP/heart rate) | 1.4 | ± 0.4 | 1.5 | ± 0.4 | 0.1 | −0.4 to 0.2 | 0.45 |
SIRS = Systemic Inflammatory Response Syndrome; WBC = White blood cell; BP = blood pressure; LOS = length of stay; OTF = off-the-floor
APPENDIX D: COMPARISON OF PRE-SWAT B AND POST-SWAT B DATA
| PRE-SWAT | PRE-SWAT (±SD) | POST-SWAT | POST-SWAT (±SD) | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Total patients | 95 | na | 98 | na | na | na | na |
| % Male | 52% | na | 59% | na | 7% | −21 to 7 | 0.41 |
| % Female | 48% | na | 41% | na | na | na | na |
| Age (yrs) | 54 | ± 19 | 57 | ± 18 | 3 | −8.3 to 2.3 | 0.26 |
| % White | 45% | na | 44% | na | 1% | −13 to 15 | 1.00 |
| % Black | 49% | na | 53% | na | 4% | −18 to 10 | 0.68 |
| % Other | 6% | na | 2% | na | 4% | −1.5 to 9.5 | 0.29 |
| # SIRS on ED arrival | 2.2 | ± 0.4 | 2.4 | ± 0.5 | 0.2 | 0.07 to 0.33 | 0.00 |
| # SIRS Total | 2.9 | ± 0.7 | 3.2 | ± 0.7 | 0.3 | 0.10 to 0.5 | 0.00 |
| Temperature (°C) | 37.8 | ± 1.3 | 38.6 | ± 1.2 | 0.8 | 0.44 to1.2 | 0.00 |
| Respiratory Rate (Breath/min) | 22.2 | ± 5.3 | 24.5 | ± 7.0 | 2.3 | 0.53 to 4.1 | 0.01 |
| Systolic BP (mmHg) | 137.5 | ± 29.0 | 126.7 | ± 26.3 | 10.8 | 2.9 to 18.7 | 0.01 |
| Heart Rate (beats/min) | 116.6 | ± 20.1 | 119.3 | ± 16.5 | 2.7 | −7.9 to 2.5 | 0.31 |
| WBC (thousands/µL) | 14.5 | ± 10.1 | 14.2 | ± 9.0 | 0.3 | −2.4 to 3.0 | 0.83 |
| % Central Line | 2.1% | na | 5.1% | na | 3% | −8.3 to 2.3 | 0.47 |
| % Pressors given | 4.2% | na | 7.1% | na | 2.9% | −9.4 to 36 | 0.58 |
| % ICU | 31.6% | na | 35.7% | na | 4.1% | −17 to 9 | 0.65 |
| % Mortality | 9.5% | na | 11.2% | na | 1.7% | −10 to 7 | 0.88 |
| % Discharge to Hospice | 5.3% | na | 2.0% | na | 3.3% | −2 to 8.6 | 0.40 |
| % Mortality or Hospice | 14.8% | na | 13.2% | na | 1.6% | −8.2 to 11.4 | 0.91 |
| Door to bolus (min) | 87.0 (67) | ± 66.7 | 56.3 (93) | ± 50.4 | 30.7 | 12.4 to 49 | 0.00 |
| Door to Antibiotic order (min) | 104.1 | ± 74.9 | 48.2 | ± 33.0 | 55.9 | 40 to 72 | 0.00 |
| Door to Antibiotic given (min) | 141.5 | ± 79.8 | 85.2 | ± 38.8 | 56.3 | 39 to 74 | 0.00 |
| Order to Antibiotic given (min) | 37.4 | ± 22.3 | 36.1 | ± 28.1 | 1.3 | −5.1 to 8.5 | 0.72 |
| Door to Pressor order (min) | 182.2 (4) | ± 266.5 | 142.4 (7) | ± 120.7 | 39.8 | −219 to 299 | 0.74 |
| Door to Pressor given (min) | 182.2 (4) | ± 266.5 | 142.4 (7) | ± 120.7 | 39.8 | −219 to 299 | 0.74 |
| Door to Admit order (min) | 195.9 | ± 100.8 | 176.6 | ± 97.9 | 19.3 | −9 to 48 | 0.18 |
| ED Admit Order to OTF (min) | 274.2 | ± 379.8 | 231.6 | ± 302.5 | 42.6 | −55 to 140 | 0.39 |
| ED LOS (min) | 470.0 | ± 390.7 | 407.7 | ± 322.4 | 62.3 | −39 to 164 | 0.23 |
| LOS ICU (days) | 3.9 (30) | ± 2.5 | 6.4 (35) | ± 7.2 | 2.5 | −5.3 to 0.26 | 0.08 |
| LOS Hospital (days) | 7.6 | ± 5.7 | 9.1 | ± 10.7 | 1.5 | −3.9 to 1 | 0.23 |
| Shock Index (Systolic BP/heart rate) | 0.9 | ± 0.3 | 1.0 | ± 0.2 | 0.1 | 0.17 to 0.03 | 0.01 |
SIRS = Systemic Inflammatory Response Syndrome; WBC = White blood cell; BP = blood pressure; LOS = length of stay; OTF = off-the-floor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial associations or sources of support that might pose a conflict of interest. No source of support for this study.
REFERENCES
- 1.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. National Center for Health Statistics Data Brief. 2011;(62):1–8. [PubMed] [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. National Vital Statistics Reports. 2013 May;61(4):1–118. [PubMed] [Google Scholar]
- 3.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide Trends of Severe Sepsis in the 21st Century (2000–2007) CHEST. 2011;140(5):1223–1229. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock From the First Hour: Results From a Guideline-Based Performance Improvement Program. Crit Care Med. 2014 Apr 8; doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department*. Crit Care Med. 2010 Apr;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. 2013:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010 Sep;38(9):1773–1785. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 9.Seoane L, Winterbottom F, Nash T, Behrhorst J, Chacko E, Shum L, et al. Using quality improvement principles to improve the care of patients with severe sepsis and septic shock. Ochsner J. 2013;13(3):359–366. [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, Holanda MS, Ortiz F, Llorca J, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010 Apr;38(4):1036–1043. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 11.Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH, et al. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009 Mar;37(3):819–824. doi: 10.1097/CCM.0b013e318196206b. [DOI] [PubMed] [Google Scholar]
- 12.Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, et al. Before-after study of a standardized hospital order set for the management of septic shock*. Crit Care Med. 2006 Nov;34(11):2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 13.ARISE Investigators and the ANZICS Clinical Trials Group et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014 Oct 16;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 14.ProCESS Investigators et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014 May 1;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N Engl J Med. 2015 Apr 2;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 16.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38–S44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 18.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic Review and Meta-Analysis of the Efficacy of Appropriate Empiric Antibiotic Therapy for Sepsis. Antimicrob Agents Chemother. 2010 Oct 14;54(11):4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surviving Sepsis Campaign “Updated Bundles in Response to New Evidence”. (Accessed on April 13, 2015 at http://www.survivingsepsis.org/SiteCollectionDocuments/SSC_Bundle.pdf)
- 20.LaRosa JA, Ahmad N, Feinberg M, Shah M, Dibrienza R, Studer S, et al. The Use of an Early Alert System to Improve Compliance with Sepsis Bundles and to Assess Impact on Mortality. Critical Care Research and Practice. 2012;2012(1):1–8. doi: 10.1155/2012/980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawyer AM, Deal EN, Labelle AJ, Witt C, Thiel SW, Heard K, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011 Mar;39(3):469–473. doi: 10.1097/CCM.0b013e318205df85. [DOI] [PubMed] [Google Scholar]
- 22.Moore LJ, Jones SL, Kreiner LA, McKinley B, Sucher JF, Todd SR, et al. Validation of a Screening Tool for the Early Identification of Sepsis. J Trauma. 2009 Jun;66(6):1539–1547. doi: 10.1097/TA.0b013e3181a3ac4b. [DOI] [PubMed] [Google Scholar]
- 23.Girardis M, Rinaldi L, Donno L, Marietta M, Codeluppi M, Marchegiano P, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care. 2009;13(5):1–8. doi: 10.1186/cc8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell J. The effect of nurse champions on compliance with Keystone Intensive Care Unit Sepsis-screening protocol. Crit Care Nurs Q. 2008 Jul;31(3):251–269. doi: 10.1097/01.CNQ.0000325050.91473.0b. [DOI] [PubMed] [Google Scholar]
- 25.Berger T, Birnbaum A, Bijur P, Kuperman G, Gennis P, et al. A Computerized Alert Screening For Severe Sepsis In Emergency Department Patients Increases Lactate Testing But Does Not Improve Inpatient Mortality. Applied Clinical Informatics. 2010;1(4):394–407. doi: 10.4338/ACI-2010-09-RA-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gultepe E, Green JP, Nguyen H, Adams J, Albertson T, Tagkopoulos I, et al. From vital signs to clinical outcomes for patients with sepsis: a machine learning basis for a clinical decision support system. J Am Med Inform Assoc. 2014 Mar;21(2):315–325. doi: 10.1136/amiajnl-2013-001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumont L, Harding AD. Development and implementation of a sepsis program. J Emerg Nurs. 2013 Nov;39(6):625–630. doi: 10.1016/j.jen.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Umscheid CA, Betesh J, VanZandbergen C, Hanish A, Tait G, Mikkelsen ME, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. Journal of Hospital Medicine. 2015;10(1):26–31. doi: 10.1002/jhm.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen SQ, Mwakalindile E, Booth JS, Hogan V, Morgan J, Prickett CT, et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ. 2014;2:e343-10. doi: 10.7717/peerj.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsolamy S, Salamah MA, Thagafi MA, Al-Dorzi HM, Marini AM, Aljerian N, et al. Diagnostic accuracy of a screening electronic alert tool for severe sepsis and septic shock in the emergency department. BMC Medical Informatics and Decision Making. 2014;14(1):1–6. doi: 10.1186/s12911-014-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]