Abstract
Signaling by ubiquitination regulates virtually every cellular process in eukaryotes. Covalent attachment of ubiquitin to a substrate is catalyzed by the E1, E2 and E3 three-enzyme cascade 1, which links the C terminus of ubiquitin via an isopeptide bond mostly to the ε-amino group of a lysine of the substrate. Given the essential roles of ubiquitination in the regulation of the immune system, it is not surprising that the ubiquitination network is a common target for diverse infectious agents 2. For example, many bacterial pathogens exploit ubiquitin signaling using virulence factors that function as E3 ligases, deubiquitinases 3 or as enzymes that directly attack ubiquitin 4. The bacterial pathogen Legionella pneumophila utilizes approximately 300 effectors that modulate diverse host processes to create a niche permissive for its replication in phagocytes 5. Here we demonstrate that members of the SidE effector family (SidEs) of L. pneumophila ubiquitinate multiple Rab small GTPases associated with the endoplasmic reticulum (ER). Moreover, we show that these proteins are capable of catalyzing ubiquitination without the need for the E1 and E2 enzymes. A putative mono ADP-ribosyltransferase (mART) motif critical for the ubiquitination activity is also essential for the role of SidEs in intracellular bacterial replication in a protozoan host. The E1/E2-independent ubiquitination catalyzed by these enzymes is energized by NAD which activates ubiquitin by the formation of ADP-ribosylated ubiquitin (ADPR-Ub). These results establish that ubiquitination can be catalyzed by a single enzyme whose activity does not require ATP.
Keywords: Legionella, Type IV secretion, mono ADP-ribosyltransferase (mART) motif, Rab small GTPases
The ability of the bacterial pathogen L. pneumophila to replicate within a phagocyte depends completely upon the Dot/Icm type IV secretion system that translocates hundreds of substrates (effectors) into host cells 6-8. The activity of these effectors supports the biogenesis of the Legionella-containing vacuole (LCV) permissive for bacterial replication by manipulating such diverse host processes as vesicle trafficking 5, protein translation 9, autophagy 10, cell migration 11, gene expression 12 and the biosynthesis of signaling lipids 13, often with sophisticated mechanisms 14. With a few exceptions the roles of Dot/Icm effectors in L. pneumophila infection of its host are not fully understood because deletion of these genes individually often does not affect intracellular bacterial replication 5. A biochemical function has been assigned to less than 10% of these effectors 5.
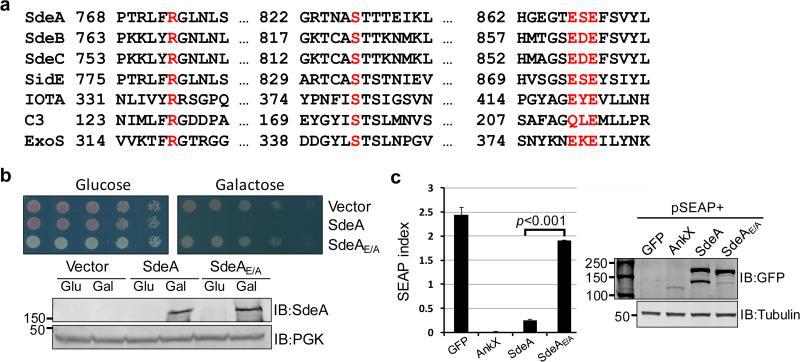
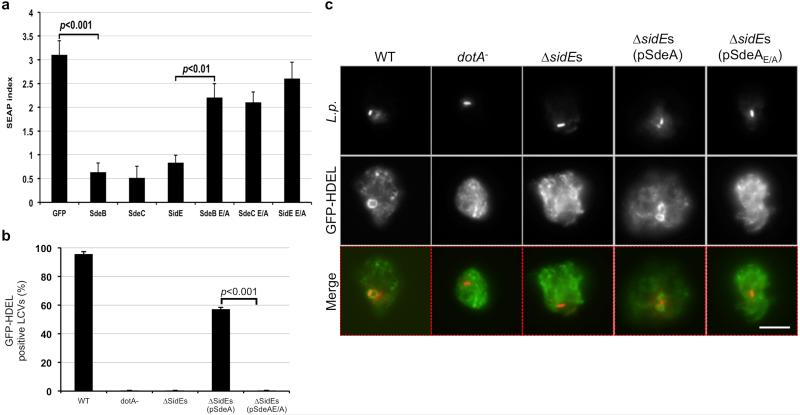
The SidE effector family harbors 4 large proteins that are required for proficient intracellular bacterial replication 6,15. PSI-BLAST analysis identified in the central region of each of these proteins, a putative mono ADP-ribosyltransferase (mART) motif (R-S-ExE) also present in such bacterial toxins as IotA 16, C3 exoenzyme 17 and ExoS 18 (Fig. 1a). Among these, the putative mART element in SdeA is R673-S827-E867S868E869, a catalytic motif found in enzymes that transfer the ADP-ribosyl group from NAD to arginine residues 19. To examine its role in SdeA-mediated yeast toxicity 20,21, we created the SdeAE/A mutant, in which E867 and E869 were mutated to alanine. This mutant has completely lost its toxicity to yeast and was also defective in inhibiting the secretion of the secreted form of the embryonic alkaline phosphatase (SEAP) 22 by mammalian cells (Fig. 1b-c). SidE, SdeB and SdeC also significantly inhibited SEAP secretion in a way that depends upon the predicted mART motif (Extended Data Fig. 1a). These results suggest that the putative mART motif is essential for the activity of the SidE family effectors.
Figure 1. A putative mono ADP-ribosyltransferase (mART) motif is important for yeast toxicity of SdeA.
a. Alignment of the central region of the SidE family members and several toxins with mART activity. Proteins identified by PSI-BLAST were manually aligned. Shown mART toxins are IotA from Clostridium perfringens 16, the C3 exoenzyme from Clostridium botulinum 17 and ExoS from Pseudomonas aeruginosa 18. Residues important for the mART motif were highlighted in red. b-c. The mART is essential for yeast toxicity and for secretion inhibition by SdeA. Yeast cells were spotted on the indicated medium for 3 d before image acquisition. The secretion of SEAP was examined in 293T cells transfected to express SEAP and GFP-tagged testing proteins; the strong SEAP inhibitor AnkX 22 was used as a control. Error bars represent standard error of the mean (s.e.m.) (n=3). The expression of the proteins (the lower panel in b for yeast and the right panel in c for mammalian cells) was probed with indicated antibodies. The PGK (3-phosphoglyceric phosphokinase) and tubulin were probed as a loading control, respectively. SdeAE/A, SdeA with Glu867 and Glu869 mutated to Ala. IB, immunoblotting. The yeast toxicity results in b and protein levels in b and c are from one representative of three independent experiments. The SEAP results in c are one representative done in triplicate from three independent experiments. b, c, Uncropped blots are shown in Supplementary Fig. 1.
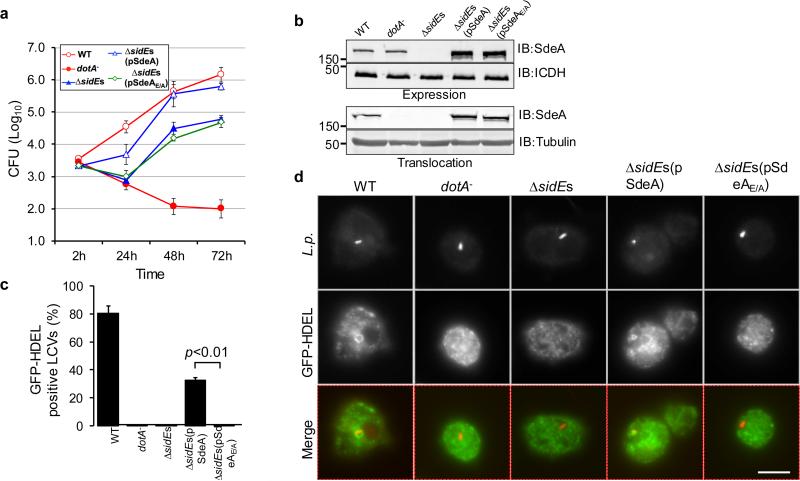
A mutant missing the SidE family (ΔsidEs) shows attenuated virulence against the protozoan host Dictyostelium discoideum 15 (Fig. 2a). Expression of wild type SdeA but not the SdeAE/A mutant in strain ΔsidEs almost completely restored its ability to grow within this host (Fig. 2a-b). In D. discoideum, LCVs containing wild type bacteria efficiently recruit ER markers such as the GFP-HDEL fusion, to their surface, which is a hallmark of L. pneumophila infection 23,24. Similar to its defects in intracellular growth, the ΔsidEs mutant no longer recruited GFP-HDEL to its vacuoles, even at 10 h post infection (Fig. 2c-d and Extended Data Fig. 1b-c). Again, SdeA but not SdeAE/A complemented such defects (Fig. 2c-d). Thus, the putative mART motif is important for the function of the SidEs during bacterial infection.
Figure 2. The predicted mART motif is essential for the role of SdeA in intracellular bacterial growth.
a, The indicated bacterial strains were used to infect D. discoideum and the bacterial yields were monitored at 24-h intervals. Note that SdeA but not the SdeAE/A mutant restored the defect exhibited by the ΔsidEs strain. b, Expression and Dot/Icm-mediated translocation of SdeA and SdeAE/A. The bacteria used for infections were probed for protein expression; the metabolic enzyme isocitrate dehydrogenase (ICDH) was probed as a loading control (upper panels). Saponin-soluble fractions of infected cells were probed for translocated SdeA with tubulin as a loading control (lower panel). c-d, L. pneumophila was used to infect a strain of D. discoideum stably expressing the ER retention fusion GFP-HDEL and the recruitment of the ER marker to the phagosome was evaluated 2 h after infection. IB, immunoblotting. Results in a and c are from one representative experiment done in triplicate from three independent experiments; error bars represent standard error of the mean (s.e.m.) (n=3). Results in b and d are one representative from three independent experiments. Bar, 5 μm. b, Uncropped blots are shown in Supplementary Fig. 1.
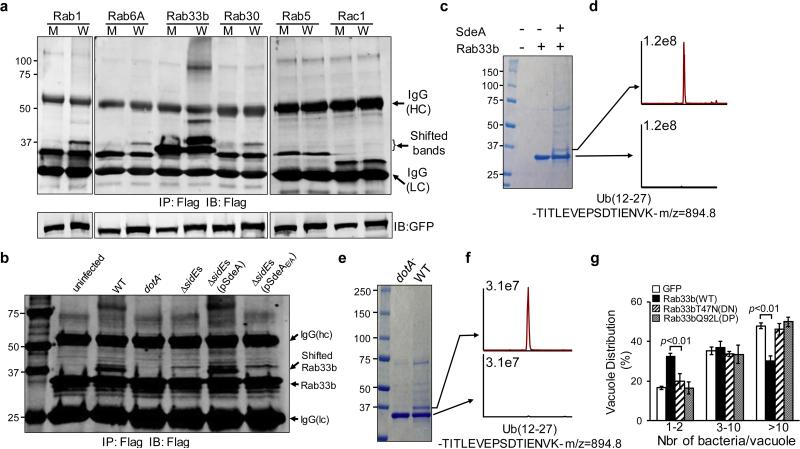
Next we attempted to determine the potential ADP-ribosyltransferase activity of SdeA. Despite extensive efforts, we were unable to detect SdeA-mediated ADP-ribosylation of eukaryotic proteins (Extended Data Fig. 2a), suggesting that this protein possesses a different biochemical activity. During L. pneumophila infection, members of the SidE family are transiently associated with the LCV 15, an organelle resembling the ER 23. Because Rab small GTPases are a common target of L. pneumophila effectors 25, we examined whether SdeA attacks any of the ER-associated Rab proteins 26 by co-expressing 4xFlag-tagged Rab1, Rab6A, Rab30 or Rab33b with this effector in mammalian cells. A clear shift in molecular weight (MW) was observed for all 4 Rab proteins purified from cells co-transfected with SdeA but not SdeAE/A (Fig. 3a, left and middle panels). Such a MW shift did not occur for the endosomal Rab5 or the cytoskeletal small GTPase Rac1 (Fig. 3a, right panel), indicating potential substrate specificity. Among the proteins potentially modified by SdeA, the modification of Rab33b was the most extensive, suggesting that this protein is a preferred substrate. The MW shift in Rab33b also was observed when it was co-expressed with other members of the SidE family (Extended Data Fig. 2b). To determine whether the potential posttranslational modification occurs during bacterial infection, we infected mammalian cells expressing 4xFlag-Rab33b with L. pneumophila. Rab33b of higher MW was detected in samples infected with the wild type strain but not with strains lacking the Dot/Icm transporter or the SidE family (Fig. 3b). The defect in Rab33b modification exhibited by the ΔsidEs strain can be complemented by expressing SdeA but not SdeAE/A (Fig. 3b). Similar SidEs-dependent MW shift also occurred to Rab1 during bacterial infection (Extended Data Fig. 2c). Thus, SdeA induces a biochemical modification of multiple ER-associated Rabs, and at least Rab33b and Rab1 are substrates during bacterial infection.
Figure 3. SdeA induces a posttranslational modification on multiple ER-associated Rab proteins.
a, Lysates of 293T cells co-transfected to express SdeA and Flag-tagged small GTPases were subjected to immunoprecipitation with Flag beads and the products were probed with the Flag-specific antibody. Note the appearance of shifted bands for Flag-tagged ER-associated Rabs but not for Rab5 and Rac1. M, SdeAE/A; W, SdeA. b, SdeA-dependent posttranslational modification of Rab33b during bacterial infection. Cells expressing Flag-Rab33b were infected with relevant L. pneumophila strains for 2 h and Flag-Rab33b purified from cell lysates was probed by immunoblotting. c-f, SdeA induces Rab33b ubiquitination. Flag-Rab33b purified from cells co-expressing SdeA (c) or infected with wild type L. pneumophila (e) was subjected to mass spectrometric analysis and tryptic ubiquitin fragments were identified in proteins of the shifted bands (d and f). g, Overexpression of Rab33b restricts intracellular bacterial growth. COS1 cells transfected with Rab33b and the indicated mutants were infected with L. pneumophila and the formation of replicative vacuoles was determined. IB, immunoblotting. Data shown are one representative experiment of three independent experiments (a-f); results in g are one representative done in triplicate from three independent experiments. Error bars represent standard error of the mean (s.e.m.) (n=3). a, b, c, e, Uncropped blots and gel images are shown in Supplementary Fig. 1.
We next determined the nature of the SdeA-induced posttranslational modification by mass spectrometric analysis of 4xFlag-Rab33b purified from 293T cells expressing SdeA. Ubiquitin fragments were only detected in Rab33b of higher MW (Fig. 3c-d and Extended Data Fig. 3a). Similar results were obtained in Rab33b from cells infected with wild type L. pneumophila (Fig. 3e-f). These results suggest that Rab33b is involved in the formation of the LCV and that SdeA induces ubiquitination of Rab33b in a process that requires the putative mART motif. Indeed, overexpression of wild type Rab33b but not its dominant negative or dominant positive mutants 27, inhibits the formation of vacuoles containing large number (>10) of bacteria (Fig. 3g and Extended Data Fig. 3b).
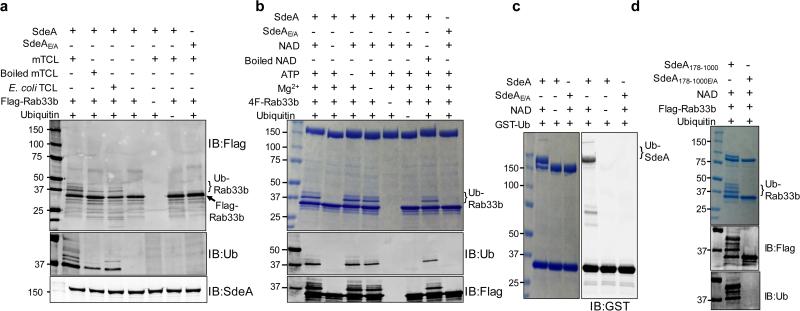
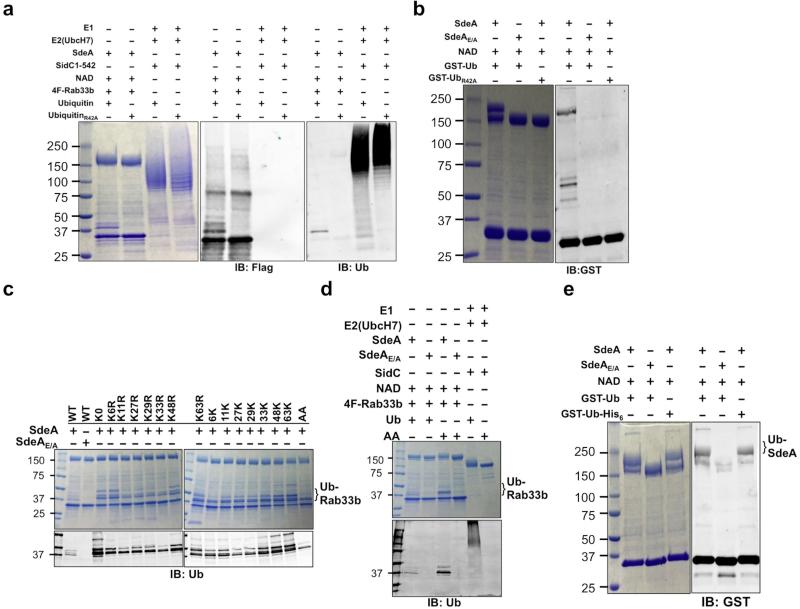
Ubiquitination requires enzymes E1, E2 and E3 which activates, conjugates and transfers the ubiquitin molecule to the substrate, respectively 1. We thus used in vitro reactions to determine whether SdeA directly participates in the ubiquitination of Rab33b. In a series of reactions each containing E1 and one of several E2 enzymes, no ubiquitination of Rab33b was detected (Extended Data Fig. 3c). We thus tested the hypothesis that an unknown E2 is required for the activity of SdeA by adding cell lysates to the reactions, which indeed led to ubiquitination of Rab33b in an mART-dependent manner (Fig. 4a). Unexpectedly, ubiquitination still occurred in reactions receiving heat-treated cell lysates (Fig. 4a, lane 3), suggesting that both E1 and the putative SdeA-specific E2 are heat stable or that SdeA is able to catalyze ubiquitination by itself but only in the presence of heat stable molecule(s) from cells. To distinguish between these two possibilities, we added E. coli lysates to the reaction. Intriguingly, ubiquitination of Rab33b occurred (Fig. 4a, lane 4). These results demonstrate that SdeA catalyzes E1/E2-independent ubiquitination in a process that requires one or more heat stable molecules present in cells.
Figure 4. SdeA catalyzes ubiquitination independent of E1 and E2.
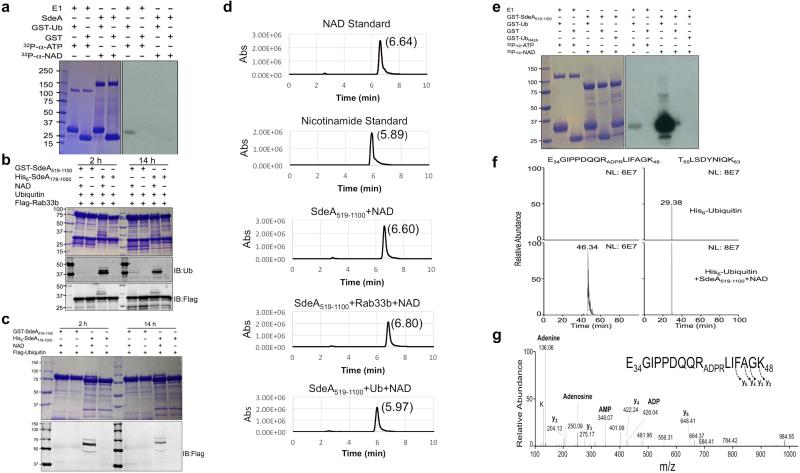
a, A heat stable molecule from cells is required for ubiquitination induced by SdeA. Reactions resolved by SDS-PAGE were probed with the indicated antibodies. Note the production of ubiquitinated Rab33b in reactions containing boiled mammalian cell lysates and E. coli lysates. TCL, total cell lysates. b, NAD is required for SdeA-catalyzed ubiquitination. Ubiquitinated Rab33b and SdeA were probed by Coomassie staining or by immunoblotting with antibodies specific for ubiquitin or Flag. c, Self-ubiquitination by SdeA. SdeA or SdeAE/A was incubated with GST-ubiquitin and NAD; ubiquitination was detected by immunoblotting or by Coomassie staining. Note the formation of the high molecular weight self-ubiquitinated SdeA when GST-ubiquitin was included in the reactions. d, Ubiquitination catalyzed by the central domain of SdeA. SdeA178-1000 or SdeA178-1000E/A was used for ubiquitination of Rab33b and the products were probed by Coomassie staining or by immunoblotting. IB, immunoblotting. a-d, similar results were obtained from four experiments. a, b, c, d, Uncropped blots are shown in Supplementary Fig. 1.
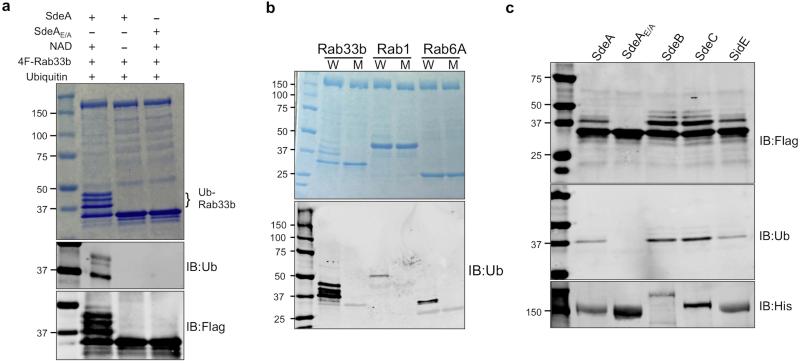
Classic ubiquitination requires the conserved E1 that activates ubiquitin in a process powered by hydrolysis of ATP, which binds the enzyme in a Mg2+-dependent manner 1. We thus determined the requirement of these molecules in SdeA-mediated ubiquitination. Because of the importance of the mART motif in the cleavage of NAD by canonical ADP-ribosyltransferases 19, we included this compound in our reactions. In reactions containing NAD, Mg2+ and ATP, ubiquitination of Rab33b occurred (Fig. 4b lane 2). Yet, when NAD was withdrawn, no ubiquitination was detected (Fig. 4b lane 3). In line with this observation, ubiquitination occurred in reactions containing NAD but not ATP or Mg2+ (Fig. 4b lanes 4 and 5). Heat-treated NAD is active, which is consistent with the fact that boiled cell lysates allowed SdeA to function (Fig. 4b lane 8). Exogenous NAD is sufficient for the activity of SdeA that had been dialyzed against a buffer containing EDTA (Extended Data Fig. 4a), suggesting that this compound is the only co-factor required for the activity. SdeAE/A is unable to catalyze the modification even in the presence of NAD (Fig. 4b lane 9). Under this condition, both Rab1 and Rab6A were ubiquitinated by SdeA (Extended Data Fig. 4b). Similarly, SidE, SdeB and SdeC ubiquitinated Rab33b (Extended Data Fig. 4c). Consistently, SdeA does not detectably ADP-ribosylate Rab33b or Rab1 (Extended Data Fig. 5a).
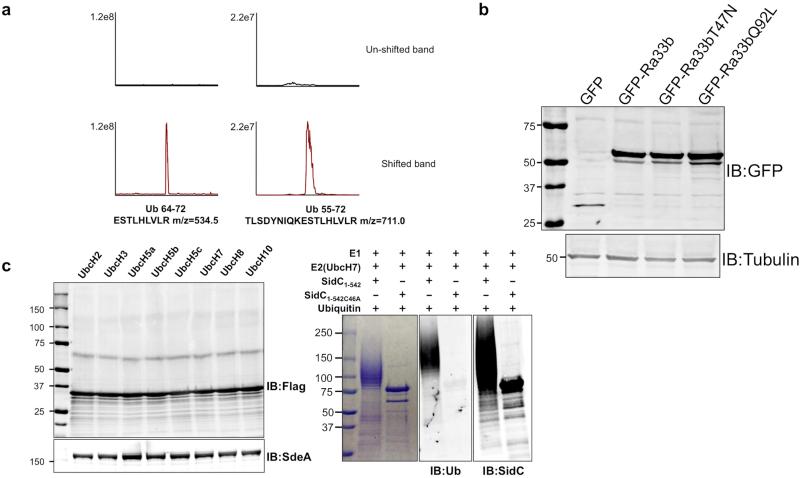
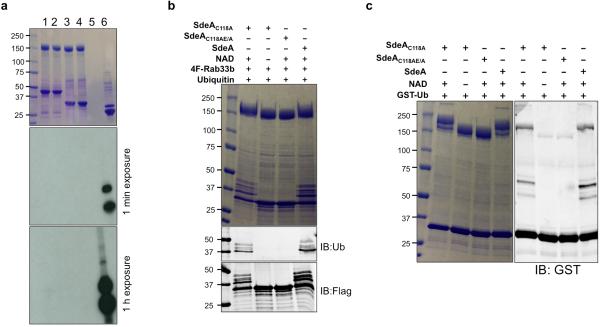
Since ubiquitin ligases often self-modify 1, we incubated SdeA with GST-ubiquitin to probe such self-ubiquitination. Proteins of higher MW were detected in reactions containing SdeA but not SdeAE/A, again in a NAD-dependent manner (Fig. 4c). The central domain of SdeA remains toxic to yeast 20, suggesting that it is still biochemically active. Indeed, SdeA178-1000 robustly ubiquitinates itself and Rab33b in a manner that requires both NAD and the mART motif (Fig. 4d). These results demonstrate that the N-terminal deubiquitinase (DUB) domain 28 of SdeA does not interfere with its ubiquitin conjugation activity. Indeed, the SdeAC118A mutant defective in the DUB activity 28 catalyzes ubiquitination indistinguishably to that of wild type protein (Extended Data Fig. 5b-c).
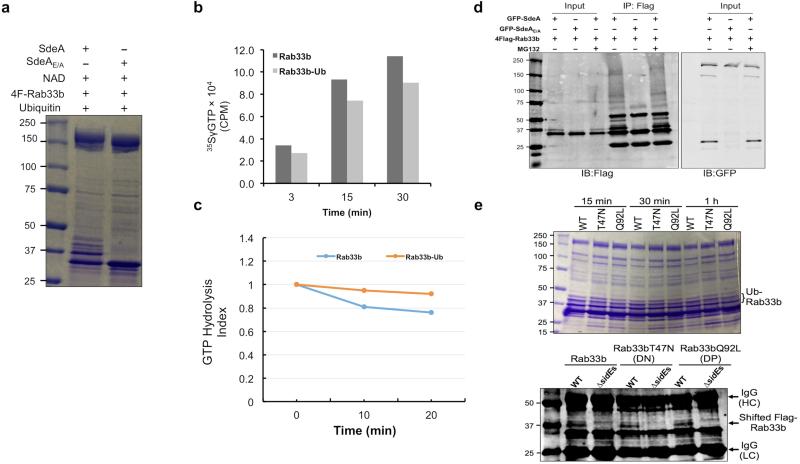
Mass spectrometric and mutational analyses revealed that Arg42 of ubiquitin is important for SdeA-mediated, but not for canonical ubiquitination catalyzed by the E1-E2-E3 cascade (Extended Data Fig. 6a-b). Consistent with these results, SdeA ubiquitinates Rab33b with all lysine variants of ubiquitin, as well as the ubiquitin derivative harboring an alanine substitution in the last two glycine residues or with 6 histidine residues attached to its carboxyl terminus (Extended Data Fig. 6c-e). Further, ubiquitination catalyzed by SdeA is insensitive to the cysteine alkylation agent maleimide, suggesting that a cysteine conjugation of ubiquitin does not form during the reaction (Extended Data Fig. 7). Finally, ubiquitination by SdeA affected the GTP loading and hydrolysis activity of Rab33b but did not detectably affect its stability (Fig. 3a and Extended Data Fig. 8). The nucleotide binding status of Rab33b did not affect its suitability as the substrate of SdeA (Extended Data Fig. 8e).
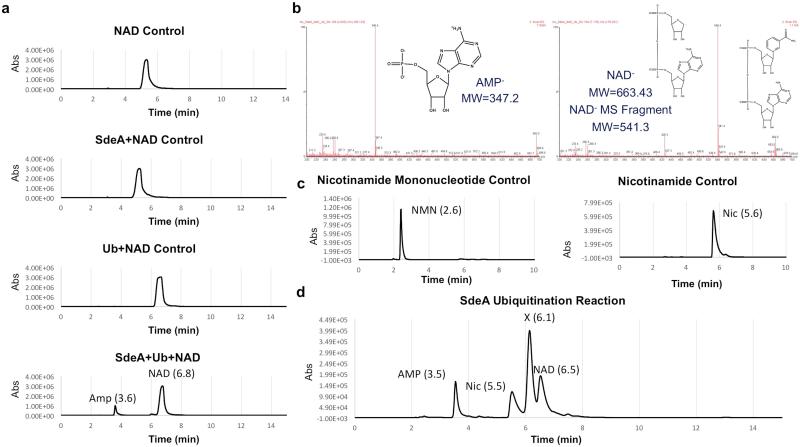
We detected AMP, nicotinamide, ubiquitin and NAD in SdeA-catalyzed reactions (Extended Data Fig. 9). The release of AMP suggests the formation of an ubiquitin-AMP adduct during the reaction. Yet, the ubiquitin-AMP adduct could not be detected by 32P-α-NAD or by TCA precipitation followed by HPLC-MS (Extended Data Fig. 10a). The release of nicotinamide and the requirement of Arg42 of ubiquitin implied ADP-ribosylation of this side chain as a possible step prior to ubiquitin conjugation, which is consistent with the requirement of the R-S-ExE motif found in members of the SidE protein family. Thus, we probed the reaction intermediate by obtaining SdeA519-1100, a fragment that retained the ability to modify Rab33b but had lost the self-ubiquitination activity (Extended Data Fig. 10b-c). Incubation of SdeA519-1100 with NAD and ubiquitin led to the release of nicotinamide (Extended Data Fig. 10d), suggesting the formation of ADP-ribosylated ubiquitin. Furthermore, inclusion of 32P-α-NAD in the reaction produced 32P-labeled ubiquitin in an Arg42-dependent manner and the ADP-ribosyl moiety linked to Arg42 of ubiquitin can be detected by mass spectrometric analysis (Extended Data Fig. 10e-g). Thus, ADP-ribosylated ubiquitin is the reaction intermediate. The production of AMP in reactions with full-length SdeA could be a subsequent step in the attack of an acceptor nucleophile (from the Rab proteins or SdeA itself in the self-conjugation reaction) on the ADP-ribosylated ubiquitin leading to the modification of the target protein.
In a canonical ubiquitination reaction, ubiquitin activated by E1 is delivered to E2 to form the E2~Ub thioester. For the E3 ligases of the RING family, ubiquitin is directly transferred from the E2 to a substrate facilitated by the ligases, whereas members of the HECT and RBR E3 families transfer ubiquitin to a catalytic cysteine in the E3 before delivering it to the substrate 1. Clearly, SdeA defines an all-in-one ubiquitin conjugation enzyme that directly activates ubiquitin; the fact that SdeA519-1100 defective in auto-ubiquitination can still modify Rab33b suggests that the activated ubiquitin is directly transferred to the substrate.
The discovery that ubiquitin can be modified by ADP-ribosylation expands the post-translational modification on this prevalent signaling molecule, which has been shown to be modified by acetylation and phosphorylation 29. Whether ADPR-Ub itself is directly used to modify proteins is unknown, but it is clear that such modifications can potentially lead to significant expansion of the ubiquitin code and its functions in cellular processes and disease development 29. The mART motif is present in a family of mammalian proteins, some of which are unable to catalyze ADP-ribosylation 30. In light of the mART-dependent ubiquitination activity of SdeA, it will be interesting to determine whether any of these mART-containing proteins is capable of catalyzing ubiquitination, and if so, whether the reaction requires E1 and E2. The identification of eukaryotic mART proteins with such a capability will surely expand the spectrum of cellular processes regulated by ubiquitination.
Methods
Bacterial, yeast strains and plasmid construction
L. pneumophila strains used in this study were derivatives of the Philadelphia 1 strain Lp02 31 and were grown and maintained on CYE medium or in AYE broth as previously described 31. When necessary antibiotics were included as described 31. The ΔsidEs strain was made by step-wise deletion of the 4 members using an established method 6. For complementation experiments, the genes were inserted into pZL507 32. All infections were performed with bacterial cultures grown to the post-exponential phase as judged by optical density of the cultures (OD600=3.3-3.8) as well as increase of bacterial motility. For expression in mammalian cells, genes were cloned into pEGFPC1 (Clontech) or a 4xFlag vector 32. The integrity of all constructs was verified by sequencing analysis.
Cell culture, infection, transfection and co-immunoprecipitation
HEK293 or 293T cells (ATCC) were cultured in Dulbecco's modified minimum Eagle's medium (DMEM) supplemented with 10% FBS. Cells grown to about 80% confluence were transfected with Lipofectamine 3000 (Life Technology) following manufacturer's instructions. U937 cells (ATCC) were differentiated into macrophages as described 33. D. discoideum strains AX4 and AX4-HDEL::GFP were cultured in HL-5 medium as described earlier 34. Strains of L. pneumophila used for infection were grown in AYE to post-exponential phase judged by optical density (OD600=3.2-4.0) and by increase in motility. 2×105 D. discoideum cells seeded in 24-well plates were infected with an MOI of 0.05 for growth experiments and of 5 for immunostaining. In all cases, one hour after adding bacteria to cultured cells, infections were synchronized by washing the infected cells three times with warm PBS buffer. Total bacterial counts at indicated time points were determined by plating serially diluted saponin lysates onto bacterial media. To determine the development of the LCV in COS1 cells (ATCC) expressing Rab33b and its mutants, cells transfected for 14 h were infected with wild type L. pneumophila and samples were fixed 14 h after bacterial uptake. Intracellular and extracellular bacteria were differentially stained with a Legionella-specific antibody and secondary antibodies conjugated to different fluorescence dyes. The category of LCVs was scored visually under a fluorescence microscope. All cell lines used were directly purchased from ATCC and were free of mycoplasma contamination by monthly testing using the PlasmoTest Kit (Invivogen).
For infections to determine the modification of Rab33b, HEK293 cells were transfected to express 4xFlag-Rab33b and FCγRII for 24 h with Lipofectamine 3000 (Life Technology). Bacteria of relevant L. pneumophila strains were opsonized with rabbit anti-Legionella antibodies 32 at 1:500 for 30 min before infecting the cells at an MOI of 10 for 2 h. Lysates prepared from infected cells with RIPA buffer (Thermo Fisher Scientific) were subjected to immunoprecipitation with Flag beads (Sigma-Aldrich).
To determine protein translocation by L. pneumophila, cells infected with the indicated bacterial strains were lysed with 0.2% saponin, which lyses membranes of mammalian cells but not of bacterial cells. The lysates were directly probed for SdeA with a specific antibody.
The secretion of SEAP was measured 24 h after cells were transfected with plasmids carrying the testing genes and pSEAP 22,35. The alkaline phosphatase activity was determined with Tropix phosphalight System kit (Applied Biosystems) per the manufacturer's instructions.
Yeast toxicity assays
All yeast strains used were derived from W303 36; yeast was grown at 30°C in YPD medium or in appropriate amino acid dropout synthetic media with glucose or galactose at a final concentration of 2% as the sole carbon source. Yeast transformation was performed according to a standard procedure 37. Inducible protein toxicity was assessed by the galactose-inducible promoter on pSB157 38. SdeA or its mutant was inserted into pSB157 and the resulting plasmids were linearized before transforming into yeast strain W30336. Yeast strains grown in liquid selective medium containing glucose were serially diluted fivefold, and 10 μL of each dilution was spotted onto selective plates containing glucose or galactose. Plates were incubated at 30 °C for 3 days before the images were acquired.
Protein purification
To purify Flag-Rab33b from mammalian cells, 293T cells transfected with the indicated plasmids for 24 h were lysed with RIPA buffer. Flag-antibody-coated beads were added to cleared lysates and obtained by centrifugation at 12,000×g for 10 min. The mixtures were incubated at 4 °C with agitation for 4 h. Unbound proteins were removed by washing the beads 3 times with RIPA buffer and the Flag-tagged proteins were eluted with 450 μg/mL 3×Flag peptide solution. To purify modified Rab33b from infected cells, HEK293 cells transfected to express 4xFlag-Rab33b and FCγRII were infected with wild type L. pneumophila for 2 h. The samples were lysed with RIPA buffer. Flag-Rab33b from the infection samples were purified followed the same protocol used for transfection samples.
Unless otherwise specified, the E. coli strain BL21(DE3) was used as the host for expression and purification of recombinant proteins. Rab1 was purified as GST-tagged protein, while all other proteins were purified as His6-tagged proteins. pQE30::4×Flag-Rab33b was sub-cloned from the mammalian expression vector p4×Flag::Rab33b to produce His6-4×Flag:: Rab33b. For protein production, 30 mL of overnight culture of the E. coli strain harboring the appropriate plasmid was transferred to 750 mL LB medium (Ampicillin 100 μg/mL) and grown until OD600 of 0.6~0.8 was reached. After adding IPTG (isopropyl thio-D-galactopyranoside) to a final concentration of 0.2 mM, the cultures were further incubated in a shaker at 18°C for 16~18 h. Bacterial cells were harvested by spinning at 12,000×g and lysed by sonication in the presence of protease inhibitors. The soluble fractions were collected by centrifugation at 12,000×g twice at 4 °C. His-tagged proteins were purified with Ni2+-NTA beads (Qiagen), and eluted with PBS containing 300 mM imidazole; GST-Rab1 were purified with Glutathione Sepharose 4 Fast Flow beads (GE healthcare), and proteins bound to beads were eluted with 25 mM reduced glutathione in 20 mM Tris-HCl, pH 8.0, 100 mM NaCl. Purified proteins were dialyzed in a buffer containing 25 mM Tris-HCl, pH7.5, 150 mM NaCl, 5% Glycerol, 1 mM DTT. To determine the potential involvement of the ions and other co-factors in the activity of SdeA, the protein was dialyzed against the same buffer containing 10 mM EDTA for 14 h at 4 °C. Protein concentrations were determined by the Bradford assay. For proteins used in in vitro biochemical assays, extensive dialysis was performed with at least two buffer changes. The purity of proteins was larger than 95% as assessed by Coomassie brilliant blue staining.
In vitro ubiquitination assays
E1, E2s and ubiquitin were obtained from Boston Biochem and were used at 100 nM for each 50-μl reaction. Ubiquitination assays were performed at 37°C for 2 h in a reaction buffer containing 50 mM Tris-HCl (pH 7.5), 0.4 mM β-Nicotinamide adenine dinucleotide (β-NAD) (Sigma-Aldrich) and 1 mM DTT. Each 50-μl reaction contains 10–μg ubiquitin, 5-μg SdeA, SdeB, SdeC, SidE or their mutant proteins and 5-μg substrates. When necessary, ATP and Mg2+ were added to a final concentration of 2 mM and 5 mM, respectively. When needed, 50 μg of mammalian or E. coli lysates were added. Heat treatment of cell lysates or NAD was performed at 100°C for 5 min. When necessary maleimide (MEM) was added to in vitro reactions at a final concentration of 50 μM.
Antibodies, immunostaining and Immumobloting
Antibodies against Legionella and GFP were described elsewhere32. Antibodies specific for SdeA were prepared by injecting rabbits with purified protein (Pocono Rabbit Farm and Laboratory, Canadensis, PA) following a standard procedure used by the service provider. When necessary, antibodies were affinity-purified against the same proteins covalently coupled to an Affigel matrix (Bio-Rad) using standard protocols 39. Cell fixation, permeabilization and immunostaining were performed as described 40. For immunostaining, anti-Legionella antisera were used at 1:10,000 32. Intracellular bacteria were distinguished from extracellular bacteria by differential immunostaining with secondary antibodies of distinct fluorescence dyes. Processed samples were inspected and scored using an Olympus IX-81 fluorescence microscope.
For immunoblotting, samples resolved by SDS-PAGE were transferred onto nitrocellulose membranes. After blocking with 5% milk, membranes were incubated with the appropriate primary antibody: anti-GFP (Sigma, cat# G7781), 1:10,000; anti-GST (Sigma, cat# G6539), 1:10,000; anti-Flag (Sigma, F1804), 1: 2000; anti-ICDH, 1:10,000; anti-PGK (Life Technology, cat# 459250), 1:3000; anti-SdeA, 1:10,000; anti-SidC 6, 1:10,000; anti-Ub (Santa cruz, cat# sc-8017), 1:1000; anti-His (Sigma, cat# H1029), 1:10,000. Tubulin (DSHB, E7), 1:10,000. Membranes were incubated with an appropriate IRDye infrared secondary antibody (Li-Cor's Biosciences Lincoln, Nebraska, USA) and the signals were obtained by using the Odyssey infrared imaging system.
GTP loading assay
For 35SγGTP incorporation assays, 20 μg of 4xFlag-Rab33b was loaded with unlabeled GDP (5 mM) before ubiquitination as described 22. GDP loaded 4xFlag-Rab33b was used for ubiquitination assays in the presence of either SdeA (10 μg) or SdeAE/A (10 μg) for 2 h at 37°C. 20% of the samples were withdrawn to test for the extent of ubiquitination of 4xFlag-Rab33b by SDS-PAGE and Coomassie staining. Ubiquitinated or non-ubiquitinated 4xFlag-Rab33b was incubated in 50 μL nucleotide exchange buffer containing 25 mM Tris·HCl (pH 7.5), 50 mM NaCl, 5 mM MgCl2, and 0.1 mM EDTA with 5 μCi 35SγGTP (Perkin-Elmer). GTP-loading reactions were performed at 22°C. Aliquots of reactions were withdrawn at indicated time points, passed through nitrocellulose membrane filters (Hawp02500; Millipore) and placed onto a vacuum platform attached to a waste liquid container. Membranes were washed three times using the exchange buffer to remove the free nucleotides, and were then transferred into scintillation vials containing 8 mL scintillation fluid (Beckman). Incorporated 35SγGTP was detected by a scintillation counter at 1 min per count.
GTPase assay
20 μg of 4xFlag-Rab33b was used for ubiquitination assays in the presence of either SdeA (10 μg) or SdeAE/A (10 μg) for 2 h before 5 μCi of 32PγGTP (Perkin-Elmer) was added to the reactions. Nucleotide loading was performed at 22°C for 30 min. Aliquots of the reactions were withdrawn and passed through membranes as described in the GTP loading assay. The reading of these aliquots served as starting points for different reactions. Samples withdrawn at later time points were measured for 32PγGTP and retained by 4xFlag-Rab33b-bound with a scintillation counter. The GTP hydrolysis index was calculated by dividing the readings obtained in later time points by the values of the starting point.
ADP-ribosylation assay
5 μg of SdeA or SdeAE/A was incubated with 5 μg of GST-Rab1, 4xFlag-Rab33b or 100 μg of 293T cell lysate in the presence of 10 mM Tris-HCl (pH 7.5), 20 mM NaCl. 5 μCi of 32P-α-NAD (Perkin-Elmer) was added to each reaction. ADP-ribosylation assays were performed at 22°C for 1 h and were stopped by adding 5xSDS loading buffer. A reaction containing ExoS78-453 (200 ng), FAS (factor activating ExoS) (2 μg), Rab5 (5 μg) or 293T cell lysates (100 μg) was used as positive control. The incorporation of 32P-α-ADPR into proteins was detected by autoradiography.
Detection of reaction intermediates by 32P-labeled ATP and NAD
To detect the ubiquitin intermediate, 5 μg of SdeA or SdeA519-1100 was incubated with 10-μg GST-Ubiquitin, GST-UbiquitinR42A or GST in the presence of 32P-α-NAD (5 μCi) in a reaction buffer containing 50 mM Tris-HCl (pH 7.5). The reaction was performed at 37°C for 6 h and stopped by adding 5xSDS loading buffer. A reaction containing the E1 activating enzyme (1 μg), GST-Ubiquitin or GST (10 μg), 32P-α-ATP (5 μCi) in the presence of 50 mM Tris-HCl (pH 7.5) and 2 mM MgCl2 was used as a positive control. The 32P-labeled intermediates were detected by autoradiography.
Detection of Reaction Intermediates
To detect AMP generated in reactions catalyzed by SdeA, reactions were set up with 50-μg SdeA178-1000, 10 mM NAD and 450-μg ubiquitin in reaction buffer (50 mM Tris pH 7.6, 50 mM NaCl, 1 mM DTT) and allowed to react for 2 h at 22°C. To detect all reaction intermediates, a reaction was set up with 100-μg SdeA178-1000, 1 mM NAD and 100-μg ubiquitin in reaction buffer (50 mM Tris pH 7.6, 50 mM NaCl, 1 mM DTT) and allowed to react for 16 h at 22°C. The reaction was then separated on an Agilent C8 column using a Waters 600 HPLC system with a linear gradient of 0-5% (v/v) acetonitrile in water over 25 minutes at 1 ml/minute. The intermediates were detected with a Waters 2487 dual wavelength detection system with wavelengths set to 260 nm and 280 nm. The mixture was then directly analyzed with a Waters micromass ZQ spectrometer in negative electrospray ionization mode. The detection range was set from 100-700 (m/z) with a scans at 1 sec intervals. Standard samples of AMP, ADP, NMN, and nicotinamide were set up in parallel and analyzed following the same method to determine the elution profile of each possible intermediate.
For experiments using SdeA519-1100 defective in autoubiquitination, 50 μg of SdeA519-1100 was incubated with 15-μg ubiquitin and 1 mM NAD in reaction buffer (50 mM Tris pH 7.6, 50 mM NaCl, 1 mM DTT) at 22°C for 18 h. The reaction was then applied directly to an Agilent C8 column on a Waters 600 HPLC system. The products of the reaction were separated with a linear gradient of 0-5% (v/v) acetonitrile in water with a flow rate of 1 ml/min over 25 min. The products were detected with a Waters 2487 dual wavelength detection system set to 260 nm and 280 nm. Controls used were 1 mM solutions containing only NAD, nicotinamide or AMP.
Samples for mass spectrometric analysis were obtained by using His6-ubiquitin in reactions containing SdeA519-1100 and NAD for 2 h, SdeA519-1100 and other components were removed by Ni2+ beads chromatography. Eluted proteins were separated in SDS-PAGE and the band corresponding ubiquitin was excised and digested with trypsin. Resulting peptides were analyzed in a NanoAcquity nanoHPLC system (Waters) by loading peptides into a trap column (5 cm × 150 μm i.d. column packed in-lab with 5-μm Jupiter C18 stationary phase) and separated in a 40 cm × 75 μm i.d. column packed in-lab with 3-μm Jupiter C18 stationary phase. The elution was carried out at 300 nL/min with the following gradient: 0-8% B solvent in 2 min, 8-20% B in 18 min, 12-30% B 55 min, 30-45% B in 22 and 97-100% B in 3 min, before holding for 10 min at 100% B. Eluting peptides were introduced to the mass spectrometer (Q-Exactive HF, Thermo Fisher Scientific) using electrospray ionization and aass spectra were collected from 400-2000 m/z with 100k resolution at m/z 400. HCD tandem-mass spectra were collected by data-dependent acquisition of the 12 most intense ions using normalized collision energy of 30%. A dynamic exclusion time of 45 s was used to discriminate against previously analyzed ions. Spectra were analyzed manually by de novo sequencing.
Data quantitation and statistic analyses
Student's t-test (two-sided) was used to compare the mean levels between two groups each with at least three independent samples.
Extended Data
Extended Data Figure 1. Inhibition of the secretion of SEAP by SidE, SdeB and SdeC and the recruitment of an ER marker by the L. pneumophila mutant lacking the sidE family.
a, GFP-fusions of the indicated proteins were co-expressed with SEAP in 293T cells for 24 h. The SEAP index was determined by measuring alkaline phosphatase activity in culture supernatant or in cells. Similar results were obtained in three independent experiments, and data shown are from one representative experiment done in triplicate. Note that mutations in the putative mART motif abolished the inhibitory effects. Error bars represent standard error of the mean (s.e.m.) (n=3). b, Quantitation of the vacuoles positive for GFP-HDEL. The indicated bacterial strains were used to infect a line of D. discoideum stably expressing GFP fusion to the ER retention signal HDEL and the recruitment of the GFP-HDEL signal to the phagosome was evaluated 10 h after infection. At least 150 phagosomes were scored in each sample done in triplicate. Results shown are from one representative experiment done in triplicate and similar results were obtained from three independent experiments. Error bars represent standard error of the mean (s.e.m.) (n=3). c, Representative images of L. pneumophila phagosomes associated with GFP-HDEL. Images are from one representative of three independent experiments with similar results. Bar, 5 μm.
Extended Data Figure 2. SdeA does not ADP-ribosylate mammalian proteins, the modification of Rab33b by other members of the SidE family and SdeA-mediated posttranslational modification of Rab1 during bacterial infection.
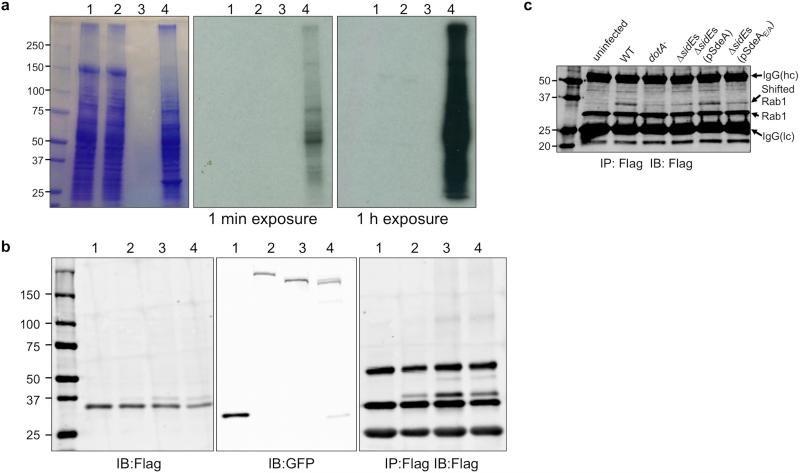
a, SdeA, SdeAE/A or ExoS and 5 μCi 32P-NAD were added to 100 μg total proteins of 293T cells. After incubation at 22°C for 1 h, samples were separated by SDS-PAGE. Gels were stained with Coomassie brilliant blue (left panel) and then by autoradiography for the indicated time duration (middle and right panels). In samples receiving SdeA, no ADP-ribosylation signal was detected in many experiments performed in various reaction conditions. Lane 1: 32P-α-NAD+SdeA+293T lysates; Lane 2: 32P-α-NAD+SdeAE/A+293T lysates; Lane 3: no sample; Lane 4: 32P-α-NAD+ExoS78-453 +FAS+293T lysates. b, Flag-tagged Rab33b was co-expressed with GFP-tagged testing proteins in 293T cells for 24 h. Cell lysates were subjected to immunoprecipitation with Flag beads and the precipitated products were probed with the Flag antibody (right panel). 5% of each lysate was probed for the expression of Rab33b (left panel) or for GFP fusions (middle panel). Proteins used: 1, GFP; 2, GFP-SdeB1-1751; 3, GFP-SdeC; 4, GFP-SidE. c, 293T cells transfected to express Flag-Rab1 were infected with the indicated L. pneumophila strains for 2 h and the Rab1 enriched by immunoprecipitation was probed by immunoblotting. For all panels, similar results were obtained from three experiments. a, b, c, Uncropped blots and autoradiograph images are shown in Supplementary Fig. 1.
Extended Data Figure 3. The extracted ion chromatograms of ubiquitin tryptic fragments detected by mass spectrometry, expression of Rab33b and its mutants in COS1 cells, and in vitro ubiquitination of Rab33b by SdeA with E1 and a series of E2s.
a, Proteins in bands corresponding to normal (upper panel) or shifted Rab33b (lower panel) were digested with trypsin and the resulting protein fragments were identified by mass spectrometry. Note that the ubiquitin tryptic fragments are present only in the shifted band of higher molecular weight. b, COS1 cells were transfected with GFP or GFP fusion of Rab33b or its mutants for 14 h. Total cell lysates resolved by SDS-PAGE were probed with a GFP-specific antibody. Tubulin was detected as a loading control. c, Reactions containing E1 and the indicated E2 proteins were allowed to proceed at 37°C for 2 h. Proteins in the reactions were resolved by SDS-PAGE followed by immunoblotting to detect ubiquitinated proteins with higher molecular weight (left panel). SdeA in the reaction was detected with specific antibodies by using 10% of the reactions (lower panel). Control reactions with wild type Legionella E3 ligase SidC1-542 and its enzymatically inactive mutant SidC1-542C46A with E1 and the E2 UbcH7 were established to monitor the activity of E1 (right panel). Note the robust self-ubiquitination of SidC1-542 (2nd lane right panel). Results in a are representative of three experiments with similar results; b and c are a representative of two and five independent experiments, respectively. b, c, Uncropped blots are shown in Supplementary Fig. 1.
Extended Data Figure 4. The activity of EDTA-dialyzed SdeA and other members of the SidE family.
a, SdeA or SdeAE/A dialyzed against a buffer containing 10 mM EDTA was used for in vitro ubiquitination of Rab33b. Reactions were allowed to proceed for 2 h at 37°C. Samples resolved by SDS-PAGE were detected by Coomassie staining (upper panel), by immunoblotting with antibodies specific for ubiquitin (middle panel) or for the Flag tag (lower panel). Note that the addition of exogenous NAD is sufficient to allow SdeA-mediated ubiquitination of Rab33b (lane 2). b, In vitro Ubiquitination of Rabs by SdeA. Reactions containing indicated proteins and NAD were allowed to proceed for 2 h at 37°C. After SDS-PAGE, ubiquitinated proteins were detected by staining 50% of the reactions resolved by SDS-PAGE with Coomassie (upper panel) or by immunoblotting with antibodies specific for ubiquitin (lower panel). Similar results were obtained from two experiments. c, In vitro ubiquitination of Rab33b by SidE, SdeB1-1751 and SdeC. Indicated testing proteins were incubated with NAD, ubiquitin and Flag-Rab33b for 2 h at 37°C. Proteins resolved by SDS-PAGE were detected by antibodies specific for Flag (upper panel) or for ubiquitin (middle panel). His6-tagged SdeA, SdeB1-1751 and SdeC and SdeAE/A used in the reactions were probed 10% of the proteins with an antibody against His (lower panel). Similar results were obtained from two independent experiments. a, b, c, Uncropped blots are shown in Supplementary Fig. 1.
Extended Data Figure 5. SdeA does not detectably ADP-ribosylate Rab33b or Rab1 and the deubiquitinase (DUB) activity of SdeA does not interfere with its ubiquitin conjugation activity.
a, 5 μg of SdeA or SdeAE/A were incubated with 5 μg of GST-Rab1, 4xFlag-Rab33b and 5 μCi of 32P-α-NAD. A reaction containing 200 ng of ExoS78-453, 2 μg of FAS and 5-μg Rab5 was established as a positive control. All reactions were allowed to proceed for 1 h at 22°C before being terminated by adding 5xSDS loading buffer. Samples resolved by SDS-PAGE were detected by Coomassie staining (upper panel) and then by autoradiography (middle and lower panels). Lane 1: 32P-α-NAD+SdeA+GST-Rab1; Lane 2: 32P-α-NAD+SdeAE/A+GST-Rab1; Lane 3: 32P-α-NAD+SdeA+4F-Rab33b; Lane 4: 32P-α-NAD+SdeAE/A+4F-Rab33b; Lane 5: no sample; Lane 6: 32P-α-NAD+ExoS78-453+FAS+Rab5. Note the strong ADP-ribosylation signals in the reaction with ExoS78-453 (lane 6). b, SdeA, its mutants SdeAC118A or SdeAC118AE/A was used for in vitro NAD-dependent ubiquitination of Rab33b. Reactions containing the indicated components were allowed to proceed for 2 h at 37°C before being terminated with SDS sample buffer. Samples resolved by SDS-PAGE were probed by Coomassie staining (upper panel) or by immunoblotting with antibody specific for ubiquitin (middle panel) or for the Flag tag (lower panel). c, Reactions containing GST-ubiquitin were similarly established to detect self-ubiquitination by SdeA. Note that SdeA and SdeAC118A exhibited similar activity in these reactions. Data in all panels are one representative of two independent experiments with similar results. a, b, c, Uncropped blots and autoradiograph images are shown in Supplementary Fig. 1.
Extended Data Figure 6. The reactivity of ubiquitin mutants in SdeA-mediated ubiquitination.
a, Arg42 in ubiquitin is important for SdeA-mediated ubiquitination. Ubiquitin or ubiquitinR42A was included in reactions catalyzed by SdeA or the bacterial E3 ubiquitin ligase SidC (E1 and the E2 UbcH7 were added in the latter category of reactions). After allowing the reaction to proceed for 2 h at 37°C. Samples separated by SDS-PAGE were probed with antibody against the Flag tag (on Rab33b) (middle panel) or ubiquitin (right panel). Note that ubiquitinR42A can be used by ubiquitination catalyzed by SidC but not SdeA. b, GST-ubiquitinR42A cannot be use for self-ubiquitination by SdeA. GST-ubiquitin or GST-ubiquitinR42A was used in reactions with SdeA or SdeAE/A. Self-modification was detected by the shift of SdeA detected by Coomassie staining (left panel) or by immunoblotting with a GST-specific antibody (right panel). c, the lysine residues or the carboxyl terminus of ubiquitin is not important for SdeA-catalyzed Rab33b ubiquitination. Reactions containing SdeA or SdeAE/A, NAD, Flag-Rab33b and the indicated ubiquitin mutants were allowed to proceed for 2 h at 37°C. Proteins were detected by Coomassie staining (upper panel) or probed by immunoblotting with antibody against ubiquitin. d, Utilization of the ubiquitin di-glycine mutant by different ligases. Reactions with indicated components were allowed to proceed for 2 h at 37°C. Proteins resolved by SDS-PAGE were detected by staining (upper panel) or by immunoblotting with antibodies specific to ubiquitin (lower panel). Note that the wild type but not the di-glycine Ub mutant (AA) can be conjugated to proteins in a reaction containing E1 and E2 and the bacterial E3 ligase SidC (Lanes 6 and 7). This di-glycine mutant (AA) can still be attached to Rab33b by SdeA (Lane 4). e, addition of 6 histidine residues to the carboxyl end of ubiquitin did not affect SdeA-mediated ubiquitination. Reactions containing the indicated components were established and allowed to proceed for 2 h at 37°C. SDS-PAGE resolved samples were probed by Coomassie staining (left panel) or by immunoblotting with a GST-specific antibody (right panel). The data in all panels are one representative of three independent experiments with similar results. a, b, c, d, e, Uncropped blots are shown in Supplementary Fig. 1.
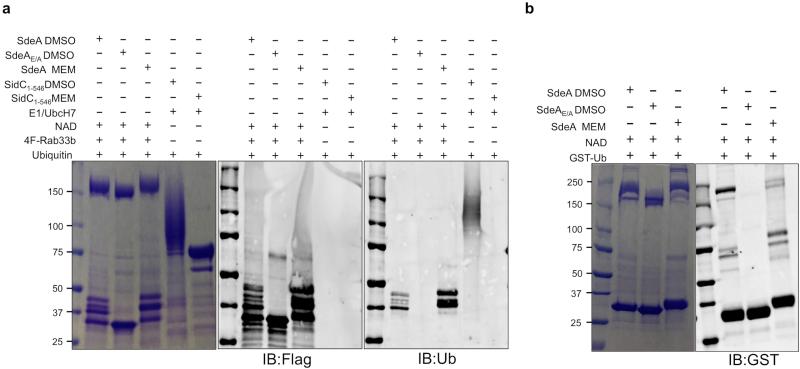
Extended Data Figure 7. Ubiquitination catalyzed by SdeA is insensitive to the cysteine modifying agent maleimide.
a, Ubiquitination reactions by SdeA or SidC together with E1 and E2 were established; maleimide was added to 50 μM to a subset of these reactions. After incubation at 37°C for 2 h, ubiquitination was detected by Coomassie staining (left panel) or by immunoblotting with the Flag-(middle panel) or ubiquitin-specific (right) antibody. Note that maleimide completely inhibits ubiquitination in the reaction catalyzed by SidC, E1 and its cognate and E2 (lane 6) but does not affect the activity of SdeA (lane 4). b, Maleimide does not affect self-ubiquitination of SdeA. Reactions containing the indicated components were established and the modification of SdeA was probed by Coomassie staining (left panel) or by immunoblotting with the GST-specific antibody (right panel). For all panels, similar results were obtained from four independent experiments. a, b, Uncropped blots are shown in Supplementary Fig. 1.
Extended Data Figure 8. SdeA-mediated ubiquitination affects the activity but not stability of Rab33b and SdeA ubiquitinates Rab33b independently of its nucleotide binding status.
a, Evaluation of the ubiquitinated Rab33b. 4xFlag-Rab33b was loaded with unlabeled GDP (5 mM) before ubiquitination reaction. GDP loaded Rab33b was subjected to ubiquitination by SdeA or SdeAE/A for 2 h at 37°C; 20% of the samples were withdrawn to determine the extent of ubiquitination by Coomassie staining. b, Ubiquitination affected the GTP loading activity of Rab33b. Ubiquitinated or non-ubiquitinated 4xFlag-Rab33b was incubated in 50 μL nucleotide exchange buffer containing 5-μCi 35SγGTP at 22°C. Aliquots of reactions were withdrawn at indicated time points and passed through nitrocellulose membrane filters. Membranes were washed for three times using exchange buffer before being transferred into scintillation vials containing scintillation fluid to detect incorporated 35SγGTP with a scintillation counter. c, Ubiquitination affected the GTPase activity of Rab33b. Samples withdrawn from Ub-Rab33b or Rab33b loaded with 32PγGTP were measured for the associated radioactivity to set as the starting point. Equal volumes of samples were withdrawn at the indicated time points to monitor intrinsic GTP hydrolysis. The GTP hydrolysis index was calculated by dividing the readings obtained in later time points by the values of the starting point. Similar results (a-c) were obtained in three independent experiments and the data shown were from one representative experiment. d, SdeA-mediated ubiquitination does not lead to degradation of Rab33b. GFP fusion of SdeA or SdeAE/A was co-transfected with Rab33b for 14 h. The proteasome inhibitor MG132 (10 μM) was added to one of the SdeA samples. The levels of Rab33b were detected by immunoblotting following immunoprecipitation with the Flag-specific antibody. Note that the addition of MG132 does not affect the level of modified Rab33b in samples co-transfected with SdeA. Similar results were obtained from two independent experiments. e, The nucleotide binding status of Rab33b does not affect its suitability as substrate in SdeA-mediated ubiquitination. Equal amounts of Rab33b, its dominant negative mutant Rab33bT47N, or the dominant positive mutant Rab33bQ92L was incubated with SdeA. Samples withdrawn at the indicated time points were detected for ubiquitination by Coomassie staining (upper panel); 293T cells transfected to express these mutants were infected the indicated L. pneumophila strains and ubiquitinated Rab33b or its mutants were probed by molecular weight shift in Rab33b obtained by immunoprecipitation (lower panel). Data in this panel are one representative of two independent experiments with similar results. a, d, e, Uncropped blots and gel images are shown in Supplementary Fig. 1.
Extended Data Figure 9. Detection of the reaction intermediates in SdeA-catalyzed ubiquitination.
a, Controls were analyzed by HPLC of NAD alone and in the presence of SdeA, Ub, and SdeA and Ub. In these reactions, AMP and NAD were identified with retention times of 3.6 and 6.8 min, respectively. b, Both AMP (left) and NAD (right) were additionally identified by ESI mass spectrometry. Both NAD and a product in which the nicotinamide group has been lost were observed in these experiments. c, To determine whether other fragments are generated in this reaction, retention time for nicotinamide mononucleotide (NMN, left) and nicotinamide (Nic, right) was determined by HPLC to be 5.6 and 2.6 min respectively. d, To identify additional components, a reaction was set up and the individual components were identified by HPLC. In the reaction mixture, AMP (3.5 min), nicotinamide (Nic 5.5 min), and NAD (6.5 min) were observed. An additional component to the reaction mixture (labeled X) was observed (6.1 min), but could not be further identified by mass spectrometry. Data in all panels are one representative from three independent experiments with similar results.
Extended Data Figure 10. Detection of the ubiquitination intermediate by using SdeA519-1100.
a, Full-length SdeA cannot produce 32P-labeled product in reactions using 32P-α-NAD. Reaction samples resolved by SDS-PAGE were detected by Coomassie staining (left panel) and then by autoradiography (right panel). Note the 32P-α-AMP-GST-ubiquitin complex can be detected in the reaction containing E1 but not SdeA. b-c, SdeA519-1100 is defective in auto-ubiquitination. Reactions containing the indicated components were allowed to proceed for the indicated time duration and the production of ubiquitinated Rab33b (b) or SdeA519-1100 was detected by immunoblotting. d, SdeA519-1100 induces the production of nicotinamide from NAD and ubiquitin. Retention time for nicotinamide and NAD was first determined by HPLC and nicotinamide can only be detected in the reaction containing SdeA519-1100, NAD and ubiquitin. e, SdeA519-1100 induces the production of 32P-ADPR-labeled ubiquitin. GST-ubiquitin or GST-ubiquitinR42A was incubated with 32P-α-NAD and SdeA519-1100 for 6 h. Classical E1 incubated with GST-ubiquitin was included as a control. Samples resolved by SDS-PAGE before autoradiography (20 min) (right panel). Note that GST-ubiquitinR42A cannot be labeled by 32P. Data in panels a to e are one representative from two independent experiments with similar results. f, The detection of a peptide with m/z 737.33 corresponding to the tryptic peptide -E34GIPPDQQRLIFAGK48- containing one ADP-ribosylation site was detected only after ubiquitin was incubated with SdeA519-1100. As a loading control, another unmodified ubiquitin peptide -T55LSDYNIQK63- was detected in both control and treated samples. g, Tandem mass analysis revealed that ADP-ribosylation occurred on Arg42 evidenced by the extensive fragmentation of the ADP-ribosylation into adenine, adenosine, AMP and ADP ions. Although not as extensive, the fragmentation of the peptide backbone helps confirm the peptide sequence. Data shown in all panels are one representative from two independent experiments with similar results. a, b, c, e, Uncropped blots and autoradiograph images are shown in Supplementary Fig. 1.
Supplementary Material
Acknowledgements
We thank Dr. Peter Hollenbeck (Purdue University, West Lafayette, IN USA) for critical reading of the manuscript. Dr. Joseph Barbieri (Medical College of Wisconsin, Milwaukee, WI) for plasmids. This work was supported by National Institutes of Health grants R56AI103168, K02AI085403 and R21AI105714 (ZQL), 2R01GM103401 (CD) and National Natural Science Foundation of China grants 21305006 and 21475005 (XL).
Footnotes
Author contributions J.Q. and Z.-Q.L. conceived the general ideas for this work. J.Q. and Z.-Q.L. planned, performed and interpreted experiments. Y.T. performed the bioinformatics analysis and determined the importance of the predicted mART motif in yeast toxicity. M.S. E.S.N., J.Q. and C.D. determined the reaction intermediates. K.Y., X.L. and E.S.N. performed mass spectrometric analyses. J.Q. and Z.-Q.L. wrote the manuscript and all authors provided editorial input.
Author Information The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. doi:10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 2.Zinngrebe J, Montinaro A, Peltzer N, Walczak H. Ubiquitin in the immune system. EMBO Rep. 2014;15:28–45. doi: 10.1002/embr.201338025. doi:10.1002/embr.201338025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Zhu Y. Diversity of bacterial manipulation of the host ubiquitin pathways. Cell Microbiol. 2015;17:26–34. doi: 10.1111/cmi.12384. doi:10.1111/cmi.12384. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, et al. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science. 2010;329:1215–1218. doi: 10.1126/science.1193844. doi:10.1126/science.1193844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Luo ZQ. Cell biology of infection by Legionella pneumophila. Microbes Infect. 2013;15:157–167. doi: 10.1016/j.micinf.2012.11.001. doi:S1286-4579(12)00271-7 [pii]10.1016/j.micinf.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci U S A. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, et al. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 2010;13:227–245. doi: 10.1111/j.1462-5822.2010.01531.x. doi:10.1111/j.1462-5822.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lifshitz Z, et al. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc Natl Acad Sci U S A. 2013;110:E707–715. doi: 10.1073/pnas.1215278110. doi:1215278110 [pii]10.1073/pnas.1215278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana MF, et al. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 2011;7:e1001289. doi: 10.1371/journal.ppat.1001289. doi:10.1371/journal.ppat.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy A, et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. doi:10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon S, Wagner MA, Rothmeier E, Muller-Taubenberger A, Hilbi H. Icm/Dot- dependent inhibition of phagocyte migration by Legionella is antagonized by a translocated Ran GTPase activator. Cell Microbiol. 2014;16:977–992. doi: 10.1111/cmi.12258. doi:10.1111/cmi.12258. [DOI] [PubMed] [Google Scholar]
- 12.Rolando M, et al. Legionella pneumophila Effector RomA Uniquely Modifies Host Chromatin to Repress Gene Expression and Promote Intracellular Bacterial Replication. Cell Host Microbe. 2013;13:395–405. doi: 10.1016/j.chom.2013.03.004. doi:S1931-3128(13)00114-5 [pii]10.1016/j.chom.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Hsu F, et al. Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase. Proc Natl Acad Sci U S A. 2012;109:13567–13572. doi: 10.1073/pnas.1207903109. doi:1207903109 [pii]10.1073/pnas.1207903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W, Luo ZQ. Cell biology and immunology lessons taught by Legionella pneumophila. Sci China Life Sci. 2016;59:3–10. doi: 10.1007/s11427-015-4945-x. doi:10.1007/s11427-015-4945-x. [DOI] [PubMed] [Google Scholar]
- 15.Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai J, Nagahama M, Oda M, Tsuge H, Kobayashi K. Clostridium perfringens iota-toxin: structure and function. Toxins (Basel) 2009;1:208–228. doi: 10.3390/toxins1020208. doi:10.3390/toxins1020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilde C, Aktories K. The Rho-ADP-ribosylating C3 exoenzyme from Clostridium botulinum and related C3-like transferases. Toxicon. 2001;39:1647–1660. doi: 10.1016/s0041-0101(01)00152-0. [DOI] [PubMed] [Google Scholar]
- 18.Ganesan AK, Frank DW, Misra RP, Schmidt G, Barbieri JT. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem. 1998;273:7332–7337. doi: 10.1074/jbc.273.13.7332. [DOI] [PubMed] [Google Scholar]
- 19.Simon NC, Aktories K, Barbieri JT. Novel bacterial ADP-ribosylating toxins: structure and function. Nat Rev Microbiol. 2014;12:599–611. doi: 10.1038/nrmicro3310. doi:10.1038/nrmicro3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havey JC, Roy CR. Toxicity and SidJ-Mediated Suppression of Toxicity Require Distinct Regions in the SidE Family of Legionella pneumophila Effectors. Infection and Immunity. 2015;83:3506–3514. doi: 10.1128/IAI.00497-15. doi:10.1128/IAI.00497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong KC, Sexton JA, Vogel JP. Spatiotemporal Regulation of a Legionella pneumophila T4SS Substrate by the Metaeffector SidJ. Plos Pathogens. 2015;11 doi: 10.1371/journal.ppat.1004695. doi:UNSP e100469510.1371/journal.ppat.1004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y, Arnold RJ, Luo ZQ. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proc Natl Acad Sci U S A. 2011;108:21212–21217. doi: 10.1073/pnas.1114023109. doi:1114023109 [pii]10.1073/pnas.1114023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Luo ZQ. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect Immun. 2007;75:592–603. doi: 10.1128/IAI.01278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwood RK, Roy CR. A Rab-Centric Perspective of Bacterial Pathogen- Occupied Vacuoles. Cell Host & Microbe. 2013;14:256–268. doi: 10.1016/j.chom.2013.08.010. doi:10.1016/j.chom.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz Sandoval C, Simmen T. Rab proteins of the endoplasmic reticulum: functions and interactors. Biochem Soc Trans. 2012;40:1426–1432. doi: 10.1042/BST20120158. doi:10.1042/BST20120158. [DOI] [PubMed] [Google Scholar]
- 27.Itoh T, et al. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell. 2008;19:2916–2925. doi: 10.1091/mbc.E07-12-1231. doi:10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheedlo MJ, et al. Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1514568112. doi:10.1073/pnas.1514568112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herhaus L, Dikic I. Expanding the ubiquitin code through post-translational modification. EMBO Rep. 2015;16:1071–1083. doi: 10.15252/embr.201540891. doi:10.15252/embr.201540891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glowacki G, et al. The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. doi:10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, et al. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 2010;6:e1000822. doi: 10.1371/journal.ppat.1000822. doi:10.1371/journal.ppat.1000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci. 2001;114:4637–4650. doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Solomon JM, Isberg RR. Dictyostelium discoideum strains lacking the RtoA protein are defective for maturation of the Legionella pneumophila replication vacuole. Cell Microbiol. 2005;7:431–442. doi: 10.1111/j.1462-5822.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 35.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan HY, Cheng KK, Klein HL. Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1 delta of Saccharomyces cerevisiae. Genetics. 1996;142:749–759. doi: 10.1093/genetics/142.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 38.Fazzio TG, Tsukiyama T. Chromatin remodeling in vivo: evidence for a nucleosome sliding mechanism. Mol Cell. 2003;12:1333–1340. doi: 10.1016/s1097-2765(03)00436-2. [DOI] [PubMed] [Google Scholar]
- 39.Dumenil G, Isberg RR. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol Microbiol. 2001;40:1113–1127. doi: 10.1046/j.1365-2958.2001.02454.x. [DOI] [PubMed] [Google Scholar]
- 40.Conover GM, Derre I, Vogel JP, Isberg RR. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.