Abstract
Allogeneic stem cell transplantation is an effective treatment for high-risk myeloid malignancies, but relapse remains the major post-transplant cause of treatment failure. Alloreactive NK cells mediate a potent antileukemic effect and may also enhance engraftment and reduce GvHD. Haploidentical transplants provide a setting in which NK cell alloreactivity can be manipulated, but are associated with high rates of GvHD. We performed a phase I study infusing escalating doses of NK cells from an HLA haploidentical related donor – selected for alloreactivity when possible – as a component of the preparative regimen for allotransplantation from a separate HLA identical donor. The goal of infusing third party alloreactive NK cells was to augment the antileukemic effect of the transplant without worsening GvHD, and thus improve the overall outcome of hematopoietic transplantation.
Twenty-one patients with high-risk AML, MDS, or CML refractory or beyond first remission received a preparative regimen with busulfan and fludarabine followed by infusion of apheresis-derived, antibody-selected, and IL-2-activated NK cells. Doses were initially based on total nucleated cell content and later based on CD56+ cells to reduce variability. CD56+ content ranged from 0.02 to 8.32 × 106/kg. IL-2, 0.5 × 106 units/m2 SQ was administered daily for five days in the final cohort (n=10). CD3+ cells in the NK cell product were required to be <105/kg.
Median relapse-free, overall, and GvHD-free/relapse-free survival for all patients enrolled was 102, 233, and 89 days, respectively. Five patients are alive, five patients died of transplant-related causes, and eleven patients died of relapse. Despite the small sample size, survival was highly associated with CD56+ cells delivered (p = 0.022) and development of ≥ Grade 3 GvHD (p = 0.006). There were nonsignificant trends toward higher survival rates in those receiving NK cells from KIR ligand mismatched donors and KIR-B haplotype donors. There was no association with disease type, remission at time of transplant, or KIR content. GvHD was not associated with TNC, CD56+, or CD3+ cells infused in the NK cell product or the stem cell product.
This trial demonstrates a lack of major toxicity attributable to 3rd-party NK cell infusions delivered in combination with an HLA compatible allogeneic transplantation. The infusion of haploidentical alloreactive NK cells was well tolerated and did not interfere with engraftment or increase the rate of GvHD after allogeneic hematopoietic transplantation. Durable complete remissions occurred in five patients at high risk for disease recurrence. This approach is being further developed in a Phase I/II trial with ex vivo expanded NK cells to increase the NK cell dose with the objective of reducing relapse and improving the outcome of allogeneic hematopoietic transplantation for AML/MDS.
GRAPHICAL ABSTRACT
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is effective for myeloid malignancies supporting administration of high dose chemotherapy and inducing an immunologic graft-versus-leukemia (GvL) effect. However, relapse remains the major post-transplant cause of treatment failure 1.
Natural killer (NK) cells have been appreciated as contributing to the GvL effect without directly causing GvHD 2. NK cell number, as measured by the dose in the stem cell graft or recovery post-transplant, has been associated with a decreased relapse rate 3, 4. NK cells are governed by activating and inhibitory receptors. NK cells can be selected for increased alloreactivity by mismatch of licensed inhibitory receptors in a setting of missing HLA ligands (KIR receptor:ligand mismatch); these cells may have more potent GvL activity and may also enhance engraftment and reduce GvHD 5 by elimination of host T-cells and antigen presenting cells required for priming a GvHD response6. Once GvHD is established, however, NK cells may cooperate with the adaptive immune response and exacerbate GvHD 7. In addition to the release of inhibition caused by missing-self, NK cells respond to activating signals in order to trigger lysis of tumor targets. Activating ligands of NKG2D (MIC and ULBP family members) are upregulated by virus-infected and malignant cells as a consequence of stress 8, and may be further upregulated through genotoxic stress caused by radiation or chemotherapy, sensitizing tumors to NK cell lysis 9.
Haploidentical donors may be selected for the presence of KIR-ligand mismatch, thereby establishing a setting in which the donor NK cells are reactive against recipient tumor cells because of a missing KIR ligand. Haploidentical stem cell transplantation has historically been complicated by excessive GvHD, infection and treatment related mortality 10. We hypothesized that haploidentical third party NK cells could be added to an HLA identical hematopoietic transplant to increase graft-vs-leukemia effects without exacerbating GVHD. We designed a Phase I clinical trial to determine whether haploidentical NK cells could be safely administered after high dose chemotherapy and prior to an HLA matched allogeneic hematopoietic stem cell transplantation, a time of maximum stress sensitization and minimum disease burden.
MATERIALS AND METHODS
Patient Population
21 patients with high-risk myeloid malignancies were enrolled on protocol 2005-0508 (NCT00402558, phase I dose escalation) or 2010-0099 (NCT01390402, phase 2 expansion) to receive a 10/10 HLA-matched allograft between October 2006 and June 2013. High-risk disease was defined as AML past first remission or primary induction failure, MDS with intermediate or high-risk IPSS score, or CML that had failed control with tyrosine kinase inhibitor (TKI) or in accelerated or blast phase. Patients were required to meet standard institutional transplant criteria for cardiac, liver, renal, and pulmonary organ function, and initially required to be ≤ 60 years of age. The age requirement was later extended to age ≤ 70 years.
Hematopoietic progenitor cells were obtained from granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood collected by apheresis. The protocol required collecting peripheral blood stem cells of at least 6 × 106 CD34+ cells/kg recipient weight (goal 8 × 106 CD34+ cells/kg). 4 × 106 CD34+ cells/kg were infused on Day 0, retaining at least 2 × 106 CD34+ cells/kg as a backup in case of graft failure. Donors were related (13) or unrelated (8). Hematopoietic stem cells procured from unrelated donors were obtained through the National Marrow Donor Program.
For the purpose of selecting haploidentical NK cell donors from available family members, NK cell alloreactivity was initially determined using the KIR ligand: ligand mismatch model, defined as the presence of a KIR-ligand (HLA Group C1, C2, or Bw4) in the donor which was missing in the recipient. KIR genotyping for the selected donor was then obtained to confirm KIR mismatch by establishing the presence of the KIR receptor gene relevant to the mismatched KIR ligand. Donors were required to have mismatch for the Phase I dose escalation study. Since many studies report that KIR: KIR ligand mismatch is not required for a response to treatment with NK cells, the Phase II expansion study preferred, but did not require, a mismatched donor when possible.
The protocols were approved by the Institutional Review Board of MD Anderson Cancer Center and conducted under Investigational New Drug applications from the Food and Drug Administration. All patients and NK cell donors provided written informed consent to the protocol.
Transplant regimen
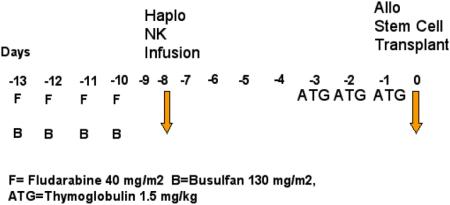
All patients received fludarabine 40 mg/m2/day and busulfan 130 mg/m2/day (adjusted for ideal body weight) for 4 days on days -13 through -10. NK cells were infused on day -8. Thymoglobulin 1.5 mg/kg/day was given on days -3 to -1 to all patients for GvHD prophylaxis and to prevent the NK cells from hindering engraftment. Tacrolimus was started on day -2 and discontinued after 3 months if there was no evidence of GvHD. Methotrexate 5 mg/m2 was given on days 1, 2, 6, and 11. All patients received granulocyte colony stimulating factor 5 mcg/kg/day starting on day 7 and until absolute neutrophil counts were > 500 × 109/L for 3 consecutive days. Standard antimicrobial prophylaxis was provided with voriconazole, pentamidine or trimethoprim-sulfamethoxazole, and acyclovir or valacyclovir for fungal, pneumocystis jiroveci, and herpes simplex, respectively.
NK cell product
The NK cell product was produced from a steady state apheresis product of up to 2 × 1010 PBMCs by first depleting T cells using the CliniMACS device and magnetic activated cell sorting (MACS) colloidal super-paramagnetic anti-CD3 monoclonal antibody (Miltenyi Biotec, Auburn, CA). A second step CD56 positive selection was performed for the first three patients, but discontinued thereafter in order to improve cell yield. The NK cell product was then cultured overnight (~16 hours) in complete media containing 1,000 IU/mL recombinant human IL-2 (Proleukin; Chiron, Emerysville, CA) and washed twice with normal saline using a COBE 2991 cell processor (COBE BCT, Lakewood, CO) prior to intravenous infusion.
Patients were treated in 4 dose levels of the NK cell enriched product based on total nucleated cell (TNC) content; 1) 106 cells/kg, 2) 5 × 106/kg, 3) 3 × 107/kg, and 4) 3 × 107/kg (or all cells collected) followed by systemic interleukin-2 (IL-2) 0.5 million units/m2 subcutaneously daily for 5 days. A subsequent phase II study in CML patients utilized a fixed dose of 5 × 106 CD56+/kg, which was the maximum dose that could be routinely obtained from a steady state apheresis from normal donors. The median number of NK cells infused at dose levels 3 and 4 was 5 × 106/kg (range 0.97 - 8.32). CD3+ cells in the NK cell product were required to be < 105/kg (median infused 0 × 105/kg, range 0 - 1.7).
Trial design
The primary objective of 2008-0508 was to assess the safety of infusing alloreactive NK cells from a haploidentical relative and to determine the maximum tolerated dose of these cells given in combination with busulfan, fludarabine, Thymoglobulin and allogeneic transplantation from a separate HLA identical related donor for treatment of AML/MDS. The secondary objectives were to determine if infusion of alloreactive haploidentical NK cells with busulfan and fludarabine/ATG will improve progression free survival post allogeneic stem cell transplantation from an HLA compatible donor compared to historical controls, and to determine the rate of engraftment, graft-vs-host disease (GvHD), immune reconstitution and survival after infusion of alloreactive haploidentical NK cells effects.
The trial was designed as a phase I dose escalation study followed by a cohort expansion at the identified MTD, or maximum feasible dose if no MTD was reached. The continual reassessment method (CRM) was used for dose-finding, with a target probability for transplant-related mortality (TRM) of .30, the baseline rate seen historically in this patient population for this regimen without NK cells.
HLA and KIR Typing and Determination of KIR content/matching
Patients and stem cell and NK cell donors were HLA typed at the intermediate resolution level for alleles at HLA-A, -B, -C, -DRB1, and -DQB1 loci by PCR amplification and oligonucleotide hybridization using commercial kits from Invitrogen (Carlsbad, CA), ELPHA, and/or One Lambda (Canoga Park, CA). The patients and selected donors were typed for the same loci by high-resolution methods using PCR amplification and nucleotide sequencing (Abbott, Abbott Park, IL, or Protrans, Hockenheim, Germany).
KIR genotyping was performed for the selected NK cell donors with reverse sequence-specific oligonucleotide (SSO) methodology using fluorescently labeled beads conjugated to oligonucleotide probes (One Lambda, Canoga Park, CA). KIR typing was performed in 17 of the 21 donors.. The revised typing kit allowing discrimination of Functional vs Deletion variants of KIR2DL4 was used for 15 donors. KIR typing was not performed for stem cell donors.
KIR-ligand:ligand mismatch was predicted using the KIR Ligand Calculator maintained by the European Bioinformatics Institute of the European Molecular Biology Labs (EMBL-EBI) (http://www.ebi.ac.uk/ipd/kir/ligand.html). KIR receptor-ligand mismatch was refined by the ligand-ligand mismatch prediction on the basis of the KIR-receptor gene being present in the donor as determined by SSO genotyping. KIR-B content was determined using the B Content Calculator maintained by EMBL-EBI (http://www.ebi.ac.uk/ipd/kir/donor_b_content.html). B content was calculated using absence/presence of KIR2DL4 regardless of the DEL/FUNC variant, or by considering the DEL variant as equivalent to being absent. Activating KIR content was determined by scoring the total number of activating KIR genes as determined by the KIR genotyping of the NK cell donor. All DS-designated KIR and KIR2DL4 were considered activating. KIR2DL4 was not counted as activating if the KIR2DL4Del variant was confirmed. Donor-recipient pairs that did not have KIR typing performed were not included in the B content or activating KIR analysis.
NK cell function/phenotype
Aliquots of NK cells produced for the six patients on protocol 2010-0099 were cryopreserved for additional testing of function and phenotype. Murine anti-human NKp30, NKp44, NKp46, CD3, CD11b, CD16, CD27, CD56, CD160, NKG2D, DNAM-1, and 2B4 and isotype control mAb were obtained from BD Biosciences (Bedford, MA). Murine anti-human KIR2DL2/3 was obtained from Miltenyi (Auburn, CA). Murine anti-human KIR2DL1 and KIR3DL1 were obtained from R&D Systems (Minneapolis, MN). For direct surface staining, cells were thawed and rested overnight in media containing 100 IU/mL IL-2, then incubated with indicated antibodies for 30 min at 4°C, washed, and resuspended in staining buffer. Data were acquired using a FACSCalibur cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
721.221 cell lines were obtained as previously described 11. The parental K562 cell line was obtained from the American Type Culture Collection (ATCC). NK cell cytotoxicity was determined using the calcein release assay as previously described 12. Briefly, target cells were labeled with 0.5 - 5 μg/mL (titrated for each target cell line) of calcein-AM (Sigma-Aldrich) for 1 h at 37 °C with occasional shaking. Cells were co-cultured at the indicated effector-to-target (E:T) ratios and incubated at 37 °C for 4 h. After incubation, 100 μL of the supernatant was harvested and transferred to a new plate. Absorbance at 570 nm was determined using a SpectraMax Plus384 spectrophotometer. The percent lysis was calculated according to the formula [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Statistics
Descriptive statistics are presented for patients, NK cell donors, and stem cell donors. The Kaplan-Meier method was used to estimate engraftment kinetics, overall survival (OS), relapse-free survival (RFS), and GvHD- and relapse-free survival (GRFS) 13. The log-rank test was used to assess differences between groups for RFS. All statistical analyses were performed using Prism 6 for Mac (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Demographics
Patient characteristics are summarized in Tables 1 and 2. Twenty-one patients with high-risk AML, MDS, or CML were enrolled. The median age was 51 years (range 2-63). Fourteen had active disease and seven were in a 1st or 2nd CR/CCR at the time of transplant. Patients with AML, MDS, and CML were enrolled, although all CML patients were enrolled on dose level 4 and accounted for seven of the 10 patients on that dose level.
Table 1.
Patient demographics and graft characteristics
| Number (%), or Median (Range) |
||
|---|---|---|
| Age | 51 (2-63) | |
| Gender | M | 15 (71) |
| F | 6 (29) | |
| Disease type | AML | 8 (38) |
| MDS | 6 (29) | |
| RAEB-1 | 3 (14) | |
| CMML | 1 (5) | |
| Unclassified/other | 2 (10) | |
| Treatment-related | 3 (14) | |
| CML | 7 (33) | |
| Accelerated phase | 2 (10) | |
| Chronic phase | 4 (19) | |
| Complex cytogenetics | 1 (5) | |
| Monosomy 7 | 1 (5) | |
| Cytogenetics (AML/MDS) | Poor | 6 (29) |
| Intermediate | 3 (14) | |
| Good | 5 (24) | |
| Remission status at transplant | NR | 14 (67) |
| CR/CCR | 7 (33) | |
| Prior Therapies | 2 (0 – 6, 1 prior transplant) | |
| SCT Donor | Matched Sibling | 13 (62) |
| Matched unrelated | 8 (38) | |
| NK Cell Donor | Parent | 6 (29) |
| Sibling | 9 (43) | |
| Child | 6 (29) | |
| NK product | TNC (×106/kg) | 29.88 (0.98 – 42.5) |
| CD3 (×106/kg) | 0 (0 – 0.17) | |
| CD3 % | 0.02 (0 – 0.62) | |
| CD14 (×106/kg) | 11.41 (0.24 – 20.75)* | |
| CD14 % | 41.77 (9.43 – 53.15)* | |
| CD19 (×106/kg) | 6.2 (0.1 – 21.99)* | |
| CD19 % | 21.84 (9.62 – 73.04)* | |
| CD56+CD3− (×106/kg) | 2.96 (0.02 – 8.32) | |
| CD56+CD3− % | 14.1 (1.5 – 27.5)* | |
| Stem Cell Product | TNC (×108/kg) | 8.07 (1.05 – 20.21) |
| CD3 (×106/kg) | 201 (11.02 – 886.7) | |
| CD34 (×106/kg) | 4.8 (3.63 – 12.47) | |
| NK Product Cell dose level/kg | 1. 106 | 6 (1) |
| 2. 5×106 | 2 (0) | |
| 3. 3×107 | 3 (1) | |
| 4. 3×107+ IL-2 | 10 (3) |
excludes CD56-selected products. The three CD56-selected product were > 90% CD56+.
Table 2.
Patient disease characteristics, survival, NK cell product characteristics, and donor classification. KIR-L MM (KIR Ligand mismatch), ND (Not Done), TNC (total nucleated cells), Func/Del (determined considering functional or deleted forms of KIR2DL4), EFS (event-free survival).
| PROTOCOL | SEX | Age at Transplant |
Diagnosis | Cytogenetic/Molecular | Prior lines of therapy |
KIR-L MM | KIR-B (Func/Del) | KIR Content |
Selection Method | Dose Level | IL-2 | TNC/kg (×10e6) | CD3-CD56+/kg (×10e6) |
CD3+/kg (×10e6) | Alive | Cause of Death | EFS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005-0508 | M | 2 | AML-M7 | 47,XY,−4,+7(q36),−9,−15,− 17,+(18)(q23),ad(19)(p13.3),+21c,+3mar; 42- 47,idem,+r,+0-4mar |
5 | C2 | Best | 7 | CD3 depletion, CD56 selection |
10e6 TNC/kg | No | 0.99 | 0.96 | 0.006 | N | Persistence of disease | 8 |
| 2005-0508 | F | 25 | AML-M4 | 46,X,−X,− 10,add(10)(q22),add(12)(p12),+14,+mar[4],i(21)(q10) add(21)(p11.2)+mar; FLT-3(+) |
3 | matched | Neutral | 2 | CD3 depletion, CD56 selection |
10e6 TNC/kg | No | 1.02 | 0.92 | 0.000 | N | Relapse | 89 |
| 2005-0508 | M | 36 | MDS/AML | 46,XY,del(3)(p12p23),del(4)(q21q23),− 7,add(17)(p13),+mar |
0 | C2 | Neutral | 4 | CD3 depletion, CD56 selection |
10e6 TNC/kg | No | 1.03 | 0.98 | 0.000 | N | Relapse | 84 |
| 2005-0508 | M | 2 | AML-M5 | 46,XY,der(5)t(5;8)(q35;q13),del(6)(q23q25)[14]; MLL(+) |
2 | C1 | Better (Better) | 6 | CD3 depletion | 10e6 TNC/kg | No | 1.02 | 0.02 | 0.000 | N | Relapse | 131 |
| 2005-0508 | M | 21 | AML-M2 | 46,XY | 2 | C2 | ND | ND | CD3 depletion | 10e6 TNC/kg | No | 0.98 | 0.23 | 0.000 | Y | 2251 | |
| 2005-0508 | F | 31 | AML-M5 | 46,XX; RAS(+) | 5 | C2 | Better | 5 | CD3 depletion | 10e6 TNC/kg | No | 0.99 | 0.09 | 0.000 | N | Relapse | 38 |
| 2005-0508 | M | 53 | tMDS- RAEB-1 |
46,XY | 2 | Bw4 | ND | ND | CD3 depletion | 5 × 10e6 TNC/kg |
No | 5.02 | 0.44 | 0.011 | N | Relapse | 60 |
| 2005-0508 | M | 53 | tMDS | 45,XY,del(1)(q21),der(3)t(1;3)(q21;p21)ins(3;5)(p13;? ), der(5)t(3;5)(p21;q13),del(7)(q22q34),−10,−12 |
1 | Bw4 | Neutral | 2 | CD3 depletion | 5 × 10e6 TNC/kg |
No | 5.04 | 0.97 | 0.004 | N | Persistence of disease | 51 |
| 2005-0508 | M | 57 | CMML | 46,XY[20]; RAS(+); FLT-3(+) | 1 | C2 | Neutral (Neutral) |
2 | CD3 depletion | 3 × 10e7 TNC/kg |
No | 30.2 | 5.15 | 0.000 | Y | 1812 | |
| 2005-0508 | M | 53 | tMDS | 45,Y,− X,add(3)(p13),add(5)(q31),del(6)(q13),del(7)(q22q34) ,−12,−16,+mar1,+mar2 |
0 | Bw4 | Neutral | 4 | CD3 depletion | 3 × 10e7 TNC/kg |
No | 30.1 | 4.77 | 0.000 | N | Persistence of disease | 33 |
| 2005-0508 | F | 51 | AML-M4 | 46,XX, inv(16)(p13.1q22) | 2 | C2 | Neutral | 5 | CD3 depletion | 3 × 10e7 TNC/kg |
No | 30.0 | 0.97 | 0.000 | N | Sepsis | 84 |
| 2005-0508 | M | 57 | MDS- RAEB-1 |
46,XY | 2 | C1 | ND | ND | CD3 depletion | 3 × 10e7 TNC/kg |
Yes | 30.3 | 8.32 | 0.173 | N | Relapse | 997 |
| 2005-0508 | M | 60 | MDS- RAEB-1 |
46 ,XY | 1 | C2, Bw4 | Neutral (Better) | 1 | CD3 depletion | 3 × 10e7 TNC/kg |
Yes | 30.0 | 4.04 | 0.000 | Y | 1473 | |
| 2005-0508 | F | 55 | CML | 46,XX,t(9;22)(q34;q11.2); 46,sl,inv(3)(q21q26.2); 46,sl,del(11)(q23q25); 47,sl,+8 |
3 | C2 | ND | ND | CD3 depletion | 3 × 10e7 TNC/kg |
Yes | 29.8 | 1.87 | 0.000 | N | Relapse | 42 |
| 2005-0508 | M | 63 | MDS/AML- M0 |
46,XY | 2 | C1 | Neutral (Better) | 4 | CD3 depletion | 3 × 10e7 TNC/kg |
Yes | 30.0 | 6.95 | 0.024 | Y | 343 | |
| 2010-0099 | F | 50 | CML | 46,XX,t(9;22)(q34;q11.2) | 5 | matched | Neutral (Neutral) |
2 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 35.6 | 5.03 | 0.068 | N | Chronic GvHD, Cirrhosis, Liver failure |
144 |
| 2010-0099 | F | 42 | CML | 46,XX,t(9;22)(q34;q11.2); BCR-ABL(+) | 2 | Bw4 | Neutral (Better) | 4 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 27.2 | 4.97 | 0.027 | Y | 767 | |
| 2010-0099 | M | 55 | CML | BCR-ABL(+) | 2 | matched | Better (Better) | 6 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 34.5 | 5.01 | 0.014 | N | Relapsed, developed Acute GvHD post DLI off-study |
102 |
| 2010-0099 | M | 27 | CML | Monosomy 7; BCR-ABL(+) | 2 | matched | Neutral (Indeterminant) |
1 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 37.8 | 5.00 | 0.034 | N | Relapse | 76 |
| 2010-0099 | M | 25 | CML | 46,XY,t(9;22)(q34;q11.2) | 2 | matched | Better (Better) | 5 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 42.5 | 5.03 | 0.000 | N | Relapsed, developed Acute GvHD post DLI off study |
233 |
| 2010-0099 | M | 53 | CML | 46,XY,t(9;22)(q34;q11.2); BCR-ABL(+) | 6 | C2 | Neutral | 2 | CD3 depletion | 5 × 10e6 NK/kg | Yes | 22.0 | 5.00 | 0.000 | N | Relapse | 180 |
All stem cell donors were matched for 10/10 HLA A, B, C DRB1, and DQB1 alleles. Eight stem cell donors were unrelated and 13 were siblings.
NK cell donors were all haploidentical related donors. Six were parents, nine were siblings, and six were children. Because of protocol requirements during the dose-escalation phase, 16 NK donor-recipient pairs had KIR mismatch and five did not. Three were mismatched for Group C1, eight for Group C2, four for Bw4, and one for both Group C1 and Bw4.
Cell products
The stem cell product infused contained a relatively high total nucleated cell (TNC) dose and CD34+ cell dose, with a median of 8.07 × 108/kg (range 1.05 – 20.21) and 4.8 × 106/kg (range 3.63 – 12.47), respectively. The T cell dose was similarly high, with a mean 201 × 106/kg (range 11.02 – 886.7) (Tables 1 and 2).
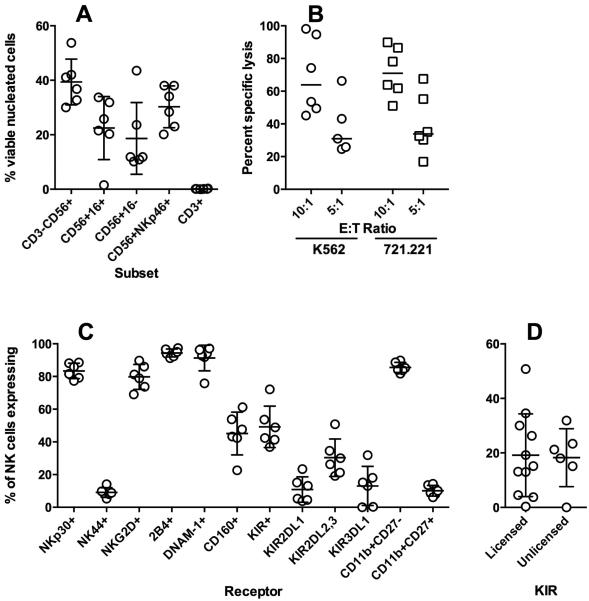
The NK cell products were delivered at the doses prescribed by the protocol according to TNC in the Phase I trial, and according to CD56+ in the Phase 2 trial. The median CD56+CD3− content of the NK cell products was 15.2%, with a wide variation in NK cell content as might be expected from the wide variability in peripheral blood NK cells in the normal population. The T cell depletion of the apheresis product was 99.8% efficient, reducing the median CD3+ cell content from 53.7% to 0.015%, resulting in a mean T cell dose of the NK cell products that was 1/10,000 that of the stem cell products (0.018 vs. 233 × 106/kg). The NK cell products also varied widely in distribution of CD16+ vs CD16− subsets (Fig. 1A), cytotoxic function (Fig. 1B), and KIR and CD160 expression. There was minimal variability in the expression of activating receptors or the relative proportions of immature vs. mature subsets as defined by CD11b and CD27 (Fig. 1C). Surprisingly, the percentage of NK cells expressing a given inhibitory receptor was not associated with whether that KIR was licensed (as predicted by the donor HLA/KIR-ligand genotype) (Fig. 1D).
Figure 1.
Product characteristics. A) NK cell and T cell content of the NK cell product as determined by flow cytometry. Total NK cell content was determined by CD3-CD56+ or as NK cell subsets (CD16+/−) or co-expressing NKp46. B) Cytotoxicity of the product after overnight activation with IL-2 as measured against HLA Class I-negative targets K562 and 721.221. C) Percent of NK cells expressing NK cell activating and inhibitory receptors and maturation markers. D) Percent of NK cells expressing KIR, grouped according to predicted licensing by donor HLA.
NK cell infusions
Only mild toxicities occurred with the NK cell infusions. Two patients experienced Grade 2 fever, two patients experienced Grade 1 rigor/chills, and one patient experienced Grade 2 allergic reaction for which the infusion was held and diphenhydramine given before completing the infusion. The ten patients at dose level 4 tolerated IL-2 systemic treatment, 0.5 million units/m2 subcutaneously daily for 5 days without major toxicity. Four patients experienced Grade 1-2 fevers from IL-2. Other toxicities documented during the transplant and post-transplant follow-up were similar to that reported for this preparative regimen without NK cells.
Transplant Outcomes
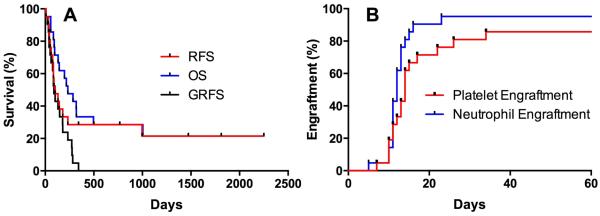
Transplant outcomes are summarized in Table 3 and Figure 2. Overall survival was low for this cohort (Fig. 2A). GvHD was not increased, with only five patients developing a maximum acute GvHD of Grade 2, and two patients with Grade 3. No patients developed Grade 4 GvHD. Six patients developed chronic GvHD, five of which eventually met criteria for chronic extensive. Eleven of the 14 patients with active disease at the time of transplant achieved a complete remission. Neutrophil engraftment occurred in all patients (Fig. 2B, median 12 days), with the exception of one early death prior to engraftment. None had graft failure. Median platelet engraftment occurred by day 14 (Fig. 2B). Two patients failed to achieve platelet engraftment, and in both patients failure was due to relapse prior to day 100.
Table 3.
Patient Outcomes
| Number (%), or Median (Range) |
||
|---|---|---|
| Engraftment (%) | 20 (100) | |
| Chimerism (%) | Full donor | 11 (55) |
| Mixed | 9 (45) | |
| Time (days) | Neutrophil | 12 (5 – 23) |
| Plt | 13.5 (7 – 34) | |
| aGVH (max) | Gr 1 | 3 (15) |
| Gr 2 | 5 (25) | |
| Gr 3 | 2 (10) | |
| cGVH | 6 (30) | |
| Final cause of death | Sepsis | 1 (5) |
| Relapse | 12 (60) | |
| GVH | 3 (15) | |
| Survival (days) | Overall | 233 (8-2,251) |
| Relapse-free | 102 (8-2,251) | |
| GVH- and Relapse-free | 89 (8-343) | |
Reported as % of 20 evaluable patients
Figure 2.
Clinical outcomes-survival and engraftment. A) Survival curves showing relapse-free, overall, and GvHD-free/relapse-free survival for all patients enrolled. GFRS was determined described in Holtan et al. B) Cumulative incidence of neutrophil engraftment and platelet engraftment for all patients enrolled.
There was one early death prior to engraftment at Day +8 due to resistant acute leukemia. One patient died from infection two months post-transplant. Two patients who relapsed without developing GvHD were taken off study and subsequently died of acute GvHD after receiving a donor lymphocyte infusion. One patient with pre-existing cirrhosis died of chronic liver GvHD. The remaining 10 deaths were due to relapse. The day 100 transplant-related mortality (TRM) was 9%, similar to expectations for conventional hematopoietic transplants in this high-risk patient population.
Predictive models
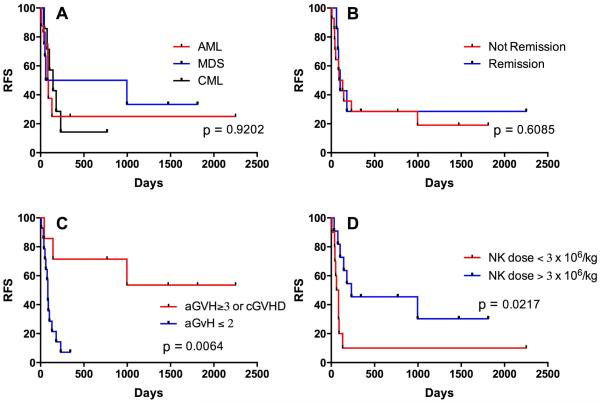
In this group of patients with very high-risk myeloid malignancies with refractory disease, there was no difference in survival according to the pre-transplant diagnosis (Fig. 3A) or remission status at the time of transplant (Fig. 3B). Despite the fact that patients with CML were overrepresented among those treated at higher dose levels, these patients did most poorly in the study (Fig. 3A). Diseases of the five survivors consisted of one each of AML, MDS, MDS transformed to AML, CMML, and CML (Table 2). There were no survivors who did not develop grade 3 or greater GvHD or chronic GvHD (GRFS, Fig. 2A), resulting in a strong statistical association (p = 0.006) between relapse-free survival and GvHD (Fig. 3C) as has been reported in many studies.
Figure 3.
Relapse-free survival as a function of disease type, status, GvHD, and NK cell dose. A) Survival plotted according to malignant diagnosis at the time of transplant (e.g., patients with MDS-related AML are included in the AML cohort). B) Survival according to whether patients were in remission at the time of starting transplant conditioning. C) Survival according to the development of GvHD. Patients who developed ≥ grade 3 acute GvHD, chronic GvHD, or both were considered together as one cohort (as in Fig. 2). D) Survival plotted according to dose of NK cells (CD56+) received.
Survival was associated with higher CD3-CD56+ NK cell dose (p = 0.0217, Fig. 3D), but because NK cell content varied in the NK cell product survival was not associated with assigned TNC dose level (p = 0.34). Monocyte dose correlated closely with TNC, and similarly did not correlate with survival (p = 0.38). Despite a positive association of survival with both GvHD and NK cell dose, GvHD was not independently associated with TNC (p = 0.24), NK (p = 0.40), or T cell content (p = 0.31) of the NK cell infusion, nor with T cell content of the stem cell infusion (p = 0.28).
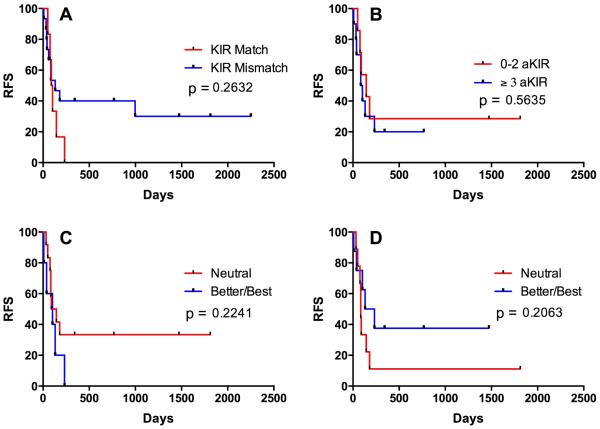
There was a trend towards improved survival in those who received KIR mismatched NK cells, but the small number of patients with KIR matched donors in this study (n = 5) prevented a strong association (p = 0.26, Fig. 4A; one-sided Fisher’s exact test for survival, p = 0.15). Results were not different when based on the ligand:ligand or KIR:ligand models, as in no cases did the KIR genotyping results change the mismatch predicted by the ligand-ligand model. No donors lacked KIR2DL1 or both KIR2DL2 and KIR2DL3. One donor lacked KIR3DL1, but was not mismatched for Bw4 with the recipient. There was no association of survival with the number of activating KIR genes in the NK cell donor (Fig. 3B). The association with KIR B content as determined by the Minnesota model 14 depended on whether KIR2DS4 Func/Del variants were considered. Without this consideration, there was a non-significant trend in patients receiving NK cells from Better/Best donors having lower survival (Fig. 4C), whereas this association was reversed when the Del variants were re-categorized as though they were KIR2DS4-negative (Fig. 4D), which is more consistent with previously reported clinical associations and functional models.
Figure 4.
Correlation of relapse-free survival with NK cell-related donor:recipient characteristics. A) Survival plotted according to the existence of KIR ligand-ligand mismatch between NK cell donor and patient. B) Survival as stratified according to the number of activating KIR genes in the NK cell donor. C) Survival stratified according to KIR B content without respect to Func/Del variants of KIR2DL4. D) Survival stratified according to KIR B content when considering KIR2DL4Del variant as absence of KIR2DL4. N=17 for B, C, D.
DISCUSSION
We treated 21 patients with escalating doses of third party NK cells from a haploidentical related donor after conditioning chemotherapy and before stem cell infusion from an HLA matched donor with the goal of augmenting graft-vs-leukemia effects without exacerbating GvHD. We did not identify a maximum tolerated dose of NK cells up to the maximum feasible dose attainable by apheresis and CD3 depletion of the product. This resulted in approximately 3 × 107 TNC/kg containing approximately 3 × 106 CD56+ CD3neg cells/kg, consistent with other reported NK cell studies utilizing apheresis-derived products 15-18. This approach was associated with 100% engraftment and a relatively low rate of ≥ grade 3 aGvHD (10%), similar to that observed with conventional hematopoietic transplants.
The association of high NK cell content in the stem cell graft 3, 19 and early NK cell reconstitution 4, 20 with improved relapse-free survival in HSCT suggest that the early anti-leukemic effect of NK cells is important, and to our knowledge this is the first report describing NK cell adoptive immunotherapy after myeloablative chemotherapy and prior to stem cell infusion. This study demonstrates the safety and feasibility of this approach, with no increase in adverse events. Other studies have demonstrated that NK cells derived from the stem cell donor can be delivered after engraftment. NK cells have been infused 2 months after related donor (matched or mismatched) HSCT 21, 2 and 3 weeks after haploidentical HSCT 22, or 3, 40, and 100 days after haploidentical HSCT 23. 3rd-party haploidentical NK cells have also been delivered approximately 3 months after autologous HSCT 15 or matched allogeneic HSCT 24.
Allogeneic NK cells in the non-transplant setting have been associated with prolonged neutropenia presumably from a direct effect against normal hematopoietic progenitors 25, 26. In this study delivering NK cells prior to transplant, we chose to give thymoglobulin 5 days after the NK cells and prior to stem cell infusion to reduce the likelihood that the 3rd party NK cells would adversely affect engraftment. Graft failure or delayed engraftment did not occur. However, the administration of thymoglobulin also limited the duration of effect for the NK cells. Given that we saw no graft failure and relapse continued to be the primary cause of treatment failure, elimination of the thymoglobulin should be studied to favor a more sustained anti-leukemia response.
Despite the low rates of aGvHD, there was still a strong overall association with aGVhD/cGvHD and relapse free survival, as has been seen in many studies of hematopoietic transplantation for myeloid malignancies. There was also a non-statistical trend toward survival association with both NK cell dose and KIR mismatch, raising the prospect for improved outcomes if higher cell doses can be achieved, and if KIR-MM can be accomplished for a greater proportion of patients such as with the use of KIR-blocking antibodies 27. The beneficial effect of KIR mismatch has been more consistently reported in the context of haploidentical HSCT. There is a proposed mechanism of alloreactive NK cells to reduce GvHD 28-30, but there are also reports of KIR mismatch and increased risk of GvHD in some settings 31, 32. The lack of a KIR ligand has also been associated with increased GvHD in matched allotransplants 33. Thus, the relationship of NK cell alloreactivity, GvHD, and survival is complex and it is not possible in this study to determine whether the impact of NK cells on survival is mediated through GvHD or independent of it. Given the competing impact of GvHD on relapse and quality of life, a modified criteria for assessing GvHD-free and relapse-free survival (GRFS) has been proposed 13 and should be used to assess treatments designed to augment GVL effects without GvHD.
This study prioritized the identification of KIR-mismatched donors on the basis HLA and KIR genotyping. Surprisingly, in the samples available for testing we found no correlation with licensing on the size of the alloreactive NK cell subset. Thus, despite the hypothesized benefit of enhanced alloreactivity with KIR-mismatched donors, the relative frequency of alloreactive NK cells may need to be considered as part of the donor selection criteria in future studies.
In conclusion, we demonstrate the safety and feasibility of early pre-transplant adoptive transfer of NK cells with a goal to improve the anti-leukemic potency of a well-established transplant regimen. No toxicity occurred with the maximal cell doses that can be obtained by apheresis from normal donors. Efficacy was potentially limited by the relatively low dose of NK cells that could be obtained. We are undertaking a follow-up clinical trial which aims to dramatically increase the number if NK cells available for infusion through ex vivo propagation 34, 35, utilize cord blood and HLA-matched sources for the NK cells for those without mismatched haploidentical donors, and eliminate thymoglobulin to increase NK cell persistence. Larger controlled studies are required to determine if the addition of NK cells to stem cell transplantation will improve leukemia free survival for patients with hematologic malignancies.
HIGHLIGHTS.
3rd party haploidentical NK cells can be safely administered in the peritransplant period without impacting engraftment.
NK cell immunotherapy products derived by steady-state leukapheresis are highly variable in cell content but can deliver single infusions containing a mean of approximately 3 × 106 CD56+CD3− cells/kg.
Survival following this regimen correlates with number of NK cells infused.
ACKNOWLEDGEMENTS
Funding for this study was provided in part by the National Institutes of Health, awards P01-CA49639 and the Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors declare no relevant conflicts of interest.
REFERENCES
- 1.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert review of hematology. 2010;3:429–441. doi: 10.1586/ehm.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farhan S, Lee DA, Champlin RE, Ciurea SO. NK cell therapy: targeting disease relapse after hematopoietic stem cell transplantation. Immunotherapy. 2012;4:305–313. doi: 10.2217/imt.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DH, Sohn SK, Lee NY, et al. Transplantation with higher dose of natural killer cells associated with better outcomes in terms of non-relapse mortality and infectious events after allogeneic peripheral blood stem cell transplantation from HLA-matched sibling donors. Eur J Haematol. 2005;75:299–308. doi: 10.1111/j.1600-0609.2005.00514.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang YJ, Zhao XY, Xu LP, et al. Early lymphocyte recovery predicts superior overall survival after unmanipulated haploidentical blood and marrow transplant for myelodysplastic syndrome and acute myeloid leukemia evolving from myelodysplastic syndrome. Leuk Lymphoma. 2013;54:2671–2677. doi: 10.3109/10428194.2013.783912. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 6.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (New York, N.Y. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 7.Shah NN, Baird K, Delbrook CP, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–792. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara M, Yoshida S. Immunogenetics of the NKG2D ligand gene family. Immunogenetics. 2012;64:855–867. doi: 10.1007/s00251-012-0638-9. [DOI] [PubMed] [Google Scholar]
- 9.Seyedin SN, Schoenhals JE, Lee DA, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy. 2015 doi: 10.2217/imt.15.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kongtim P, Lee DA, Cooper LJ, Kebriaei P, Champlin RE, Ciurea SO. Haploidentical Hematopoietic Stem Cell Transplantation as a Platform for Post-Transplantation Cellular Therapy. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurton LV, Siddik RI, Singh H, et al. Identifying candidate allogeneic NK-cell donors for hematopoietic stem-cell transplantation based on functional phenotype. Leukemia. 2010;24:1059–1062. doi: 10.1038/leu.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp. 2011 doi: 10.3791/2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingemann H, Grodman C, Cutler E, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53:412–418. doi: 10.1111/j.1537-2995.2012.03764.x. quiz 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer-Monard S, Passweg J, Siegler U, et al. Clinical-grade purification of natural killer cells in haploidentical hematopoietic stem cell transplantation. Transfusion. 2009;49:362–371. doi: 10.1111/j.1537-2995.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 18.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki S, Henzan H, Ohno Y, et al. Influence of transplanted dose of CD56+ cells on development of graft-versus-host disease in patients receiving G-CSF-mobilized peripheral blood progenitor cells from HLA-identical sibling donors. Bone Marrow Transplant. 2003;32:505–510. doi: 10.1038/sj.bmt.1704165. [DOI] [PubMed] [Google Scholar]
- 20.Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 21.Rizzieri DA, Storms R, Chen DF, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi I, Yoon SR, Park SY, et al. Donor-derived natural killer cells infused after human leukocyte antigen-haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant. 2014;20:696–704. doi: 10.1016/j.bbmt.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Stern M, Passweg JR, Meyer-Monard S, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48:433–438. doi: 10.1038/bmt.2012.162. [DOI] [PubMed] [Google Scholar]
- 24.Rubnitz JE, Inaba H, Kang G, et al. Natural killer cell therapy in children with relapsed leukemia. Pediatr Blood Cancer. 2015;62:1468–1472. doi: 10.1002/pbc.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 26.Szmania S, Lapteva N, Garg T, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother. 2015;38:24–36. doi: 10.1097/CJI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locatelli F, Pende D, Mingari MC, et al. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Frontiers in immunology. 2013;4:15. doi: 10.3389/fimmu.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 30.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunstein CG, Wagner JE, Weisdorf DJ, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113:5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JS, Cooley S, Parham P, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobecks RM, Wang T, Askar M, et al. Impact of KIR and HLA Genotypes on Outcomes after Reduced-Intensity Conditioning Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1589–1596. doi: 10.1016/j.bbmt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah N, Martin-Antonio B, Yang H, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8:e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]