Abstract
Background
Intestinal ischemia can quickly escalate to bowel necrosis and perforation. Transplantation of stem cells presents a novel treatment modality for this problem. We hypothesized that: (1) Human adipose derived stromal cells (hASCs) would increase survival and mesenteric perfusion to a greater degree compared to differentiated cellular controls following ischemic intestinal injury, (2) improved outcomes with hASC therapy would be associated with preservation of intestinal histological and tight junction architecture, and lower levels of systemic inflammation following intestinal injury.
Methods
hASCs and keratinocytes (differentiated cellular control) were cultured on polystyrene flasks at 37C in 5% CO2 in air. Adult male C57Bl6J mice were anesthetized and a midline laparotomy performed. The intestines were eviscerated, the small bowel mesenteric root identified, and intestinal ischemia was established by temporarily occluding the superior mesenteric artery for 60 minutes with a non-crushing vascular clamp. Following ischemia, the clamp was removed and the intestines were returned to the abdominal cavity. Prior to abdominal closure, two million hASCs or keratinocytes in 250 uL of PBS (carrier for cells and control solution) were infused into the peritoneum. Animals were allowed to recover for 12 or 24 hours (perfusion, histology, cytokine, and immunofluoresence studies), or 7 days (survival studies). Intestinal perfusion was assessed by laser Doppler imaging. Intestinal tissue segments were stained with H&E, as well as antibodies for the tight junction protein claudin-1. Separate aliquots of intestine, liver and lung tissue were homogenized and assessed for inflammatory cytokines via multiplex beaded assay.
Results
Animals administered hASCs following intestinal ischemia and reperfusion injury (I/R) had significantly greater 7 day survival and better post-ischemic recovery of mesenteric perfusion compared to vehicle or keratinocyte therapy. hASCs also abated intestinal mucosal destruction, facilitated preservation of intestinal tight junctions, and decreased the systemic inflammatory response to injury.
Conclusion
Human adipose derived stromal cells improved survival and mesenteric perfusion and attenuated the mucosal damage associated with intestinal ischemia and reperfusion injury. hASCs should be considered as a plausible cell source for novel cellular treatment plans following intestinal ischemia.
Keywords: Adipose stromal cells, intestinal ischemia, necrotizing enterocolitis, stem cells, perfusion
INTRODUCTION
Intestinal ischemia and necrosis affect multiple patient populations of varying ages and comorbidities. Acute mesenteric ischemia (AMI) is prevalent in the elderly population and those who undergo cardiac bypass surgery [1]. AMI affects nearly 5000 patients annually, with many requiring open or endovascular surgical intervention to lyse the clot and salvage the ischemic intestine. The mortality rate for AMI can be as high as 40% for those who progress to surgery [2]. Necrotizing enterocolitis and volvulus are two forms of intestinal ischemia that can affect the neonatal population [3]. Necrotizing enterocolitis, which ultimately manifests as intestinal ischemia and necrosis, affects the very low birth weight premature population. The mortality for the most severe cases of necrotizing enterocolitis can be quite high [4]. Midgut volvulus associated with malrotation occurs much less frequently, but carries a high mortality when a majority of the bowel is involved [5]. In any case, if injury is unrecognized or left untreated, patients can quickly decompensate and progress to shock, multi-system organ failure, and death. If patients survive these ischemic episodes, they are often faced with prolonged hospitalization and long term parenteral nutrition needs [6].
Noteworthy advancements in the medical treatment of intestinal ischemia within the last decade have been sparse, and therefore, stromal cell therapy offers a novel therapeutic option for the treatment of this disease [6]. Bone marrow-derived mesenchymal stromal cells (BMSCs), in particular, have shown the capacity to promote survival and attenuate intestinal ischemic injury [3]. These advantages are achieved, in part, through enhanced restitution and improved integrity of the intestinal mucosa, reduced translocation of bacteria from the lumen into the circulation, and a decreased inflammatory response [7, 8].
In spite of their promising potential, BMSC use may be limited secondary to lower proliferative capacity and painful isolation procedures [9] [10]. Adipose-derived mesenchymal stromal cells (ASCs), however, show greater proliferative potential than BMSCs and other mesenchymal stromal cell lines, and their ease of accessibility and limitless supply via liposuction of subcutaneous adipose tissue make them an ideal candidate for widespread therapeutic use [11-13]. ASCs have also been suggested as an alternative stem cell source for the treatment of intestinal ischemia in a rat model of intestinal I/R [14]. In this study, rat derived ASCs decreased inflammation and preserved intestinal histological architecture, but the cellular effects on survival and mesenteric perfusion were not defined.
Adipose stromal cell transplants have also been shown to improve recovery of damaged tissue in several other models including critical limb ischemia [15], acute kidney injury [16], cardiac ischemia [17], and stroke [18], but human ASCs (hASCs) have not yet been tested in preclinical models of intestinal ischemia. Therefore, the aim of the current study was to determine the efficacy of human ASC therapy in a murine model of intestinal ischemia and reperfusion injury (I/R). We hypothesized that: (1) hASCs would increase seven day survival and mesenteric perfusion compared to differentiated cellular controls following intestinal I/R; (2) improved outcomes with hASC therapy would be associated with preserved intestinal histology and tight junctional architecture following injury, and 3) hASAC therapy following intestinal I/R would limit intestinal and systemic inflammation.
MATERIALS AND METHODS
Cell Culture
Human ASCs were harvested from human subjects via liposuction and purified as previously described [19]. Briefly, human subcutaneous adipose tissue samples obtained from liposuction procedures were digested in collagenase type I (Worthington Biochemical, Lakewood, NJ) under agitation for 2 hours at 37°C. Samples were then centrifuged at 300g for 8 minutes to separate the stromal cell fraction (pellet) from adipocytes. The pellet was resuspended in DMEM/F12 containing 10% FBS (Hyclone, Logan, Utah) filtered through 250-μm Nitex filters (Sefar America Inc, Kansas City, Mo) and centrifuged at 300g for an additional 8 minutes. The cell pellet was treated with red cell lysis buffer and was resuspended in EBM-2 with 5% FBS or EGM2-MV (Lonza, Allendale, NJ). Adipose stromal cells were CD34+, CD31−, and CD144− by flow cytometry, but also expressed several mesenchymal cell lineage markers including CD10, CD13, and CD90 [19]. Cells were cultured on polystyrene flasks in Endothelial Growth Medium 2 MV with 5% FBS at 37C in 5% CO2 in air. Cells were used between passages 4-7.
Human nTERT keratinocytes were graciously donated by Dr. Jeffery Travers at the Indiana University School of Medicine. Cells were originally purchased through ATCC (Manassas, VA) and were cultured in Epilife medium with keratinocyte growth factor (Life Technologies, Grand Island, NY). Cells were used between passages 24 and 35.
Once ready for experimentation, cell disassociation was achieved using TrypLE Express (Life Technologies). Cells were then pelleted at 400g for 5 mins and resuspended in fresh media. Cells were then counted with the aid of an automated fluorescent cell counter (Luna-FL Automated Cell Counter, Logos Biosystems, Annandale, VA). Two million hASCs or keratinocytes were then resuspended in 250ul of PBS vehicle for infusion as determined by a previous stromal cell dose response curve [3].
Murine Intestinal Ischemia-Reperfusion Model
The experimental protocol and animal use were approved by the Indiana University Institutional Animal Care and Use Committee. Adult male C57Bl6J mice (8-12 weeks, 20-30g; Jackson Labs, Bar Harbor, ME) were allowed 48 hours for acclimation to the new environment prior to intervention. They had access to normal chow and were kept in 12 hour light-dark cycle housing. The animals were anesthetized at 3% isoflurane and maintained at 1.5% isoflurane for the duration of the procedure. Animals were placed on a heating pad to maintain body temperature during the procedure. The abdomen was prepped with hair removal lotion, followed by 70% ethanol and betadine. One milliliter of 0.9% normal saline was injected subcutaneously, and a midline laparotomy was performed.
The intestines were eviscerated, and the root of the superior mesenteric artery (SMA) was located. Temporary occlusion of the mesenteric root was established for 60 minutes using a non-crushing microvascular clamp. During ischemia, the abdomen was temporarily closed using silk suture to prevent evaporative heat and water loss. Following ischemia, the abdomen was reopened, the clamp was removed, and the intestines were allowed to recover. The abdominal fascia and skin were then closed in two layers using silk sutures. Prior to complete closure of the facial defect, 2 million hASCs, 2 million keratinocytes, or 250 μL of phosphate-buffered saline (PBS) vehicle were administered directly into the peritoneal cavity. Triple antibiotic ointment was applied to the abdominal incision site following complete closure, and analgesia (1 mg/kg buprenorphine and 5 mg/kg caprofen) was injected subcutaneously. Animals were recovered from anesthesia on the heating pad, placed back in their cage, and returned to animal housing.
Survival Analysis
Animals assigned to the survival protocol (n=10 I/R + hASC, 10 I/R + keratinocytes, 10 I/R + PBS vehicle) were monitored twice daily over 7 days after the surgery for death, pain, and incisional complications. End points of analysis included animal death or when Laboratory Animal Resource Center veterinarians felt that animals were suffering and needed to be euthanized. Survival curves were then created based on these end points.
Laser Doppler Imaging Analysis
Laser Doppler Imaging (LDI)(Moor Instruments, Wilmington, DE) was used to assess blood perfusion of the intestines. At each designated time point, (baseline, initiation of ischemia, initiation of reperfusion, 12 or 24 hours of reperfusion) three LDI Readings were taken for each animal and an average perfusion value was calculated based on the flux mean of the three images. The flux mean value was a unit-less numerical value, with larger numbers representing greater perfusion, and smaller numbers representing lower perfusion or ischemia. The readings for each animal were expressed as a percentage of their baseline perfusion, with baseline representing 100% perfusion.
Animals assigned to the 12 hour (N=6/group) and 24 hour (N=7/group) reperfusion group were reanesthetized at 12 or 24 hours respectively. The incision site was reopened and the intestines were eviscerated. Laser Doppler imaging was then used to assess final perfusion parameters. Animals were then euthanized by isoflurane overdose and cervical dislocation. Animals that died prior to analysis were arbitrarily assigned a perfusion value of zero.
Histology
After 12 and 24 hours of reperfusion, animals were euthanized and intestinal segments harvested. Segments were placed into 4% paraformaldehyde and subsequently dehydrated in 70% ethanol. Segments were then paraffin embedded and cut using a microtome. Tissue segments were placed on slides and were stained with hematoxalin and eosin. Histologic scoring of the depth of tissue injury was performed in blinded fashion by two of the authors as we have previously described [20]: 0, no damage; 1, subepithelial space at the villous tip; 2, loss of mucosal lining of the villous tip; 3, loss of less than half of the villous structure; 4, loss of more than half of the villous structure; 5, transmural necrosis (N= 6-7 intestinal segments/group).
Immunofluorescence
Paraffin embedded small intestinal blocks (N=6-7/group) were cut in 10μm segments. Slides were deparaffinized with xylene and rehydrated in graded alcohols. Heat-induced epitope retrieval was then conducted in a standard pressure cooker and slides were placed in 10mM citrate buffer (pH 6.0) for 20 minutes and allowed to cool. Slides were then blocked with normal Goat Serum (Biogenex, Fremont, CA) diluted in PBS with 1% bovine serum albumin (Santa Cruz Biotechnology, Dallas, TX) and 0.1% Tween 20 (Sigma, St. Louis, MO) for 1 hour.
Presence of tight junctions in the intestinal tissues was then assessed by incubating slides with a 1:100 dilution of Claudin-1 primary antibody (Novus Biologicals, NBP1-67515) overnight at 4 degrees Celsius. Following washing, Alexafluor 488 goat-anti-rabbit secondary antibody (Cell Signaling Technology, Danvers, MA) was applied and incubated for 2 hours at room temperature in a humidity chamber. Slides were then washed again and DAPI (Cell Signaling Technology) was applied at 1ug/ml and allowed to incubate at room temperature for 5 minutes. Slides were then rinsed in PBS, mounted, coverslipped, and assessed using a fluorescent microscope. Staining was repeated on additional slides to ensure reproducible results.
Tissue Cytokines
Intestinal, liver, and lung tissue segments (N=6-7/group) were thawed and homogenized in RIPA buffer (Sigma) with protease and phosphatase cocktail inhibitors (1:100 dilution, Sigma). Homogenates were centrifuged at 12,000 rpm to pellet extraneous tissue, and supernatants were transferred to fresh eppendorff tubes for storage at −80C. Total protein concentration was then quantified by Bradford Assay using a spectrophotometer (Versamax microplate reader, Molecular Devices). Tissue vascular endothelial growth factor (VEGF), granulocyte colony stimulating factor (GCSF), monokine induced by interferon gamma (MIG), Interleukin-6 (IL-6), and Interleukin-1 beta (IL-1β) were quantified with a Bioplex 200 multiplex beaded assay system (Bio-Rad) using a customized multiplex kit for the designated chemokines (Millipore). Assays were performed at 1:25 dilution according to the manufacturer’s instructions.
Statistical Analysis
Survival data were compared using the Mantel-Cox log-rank test and the Gehan-Breslow-Wilcoxon test. Reperfusion data were deemed to be normally distributed based on histogram data inspection, and were compared with two way ANOVA and t test, when appropriate. Data are presented as percent change from baseline perfusion (mean% +/− SEM). Histology and multiplex cytokine data were not normally distributed, and as such, were compared using the Mann Whitney U test. Data are presented as the median with 25%-75% interquartile range. A P value of less than 0.05 was considered statistically significant.
RESULTS
hASCs Improved Survival Outcomes After Intestinal I/R Injury
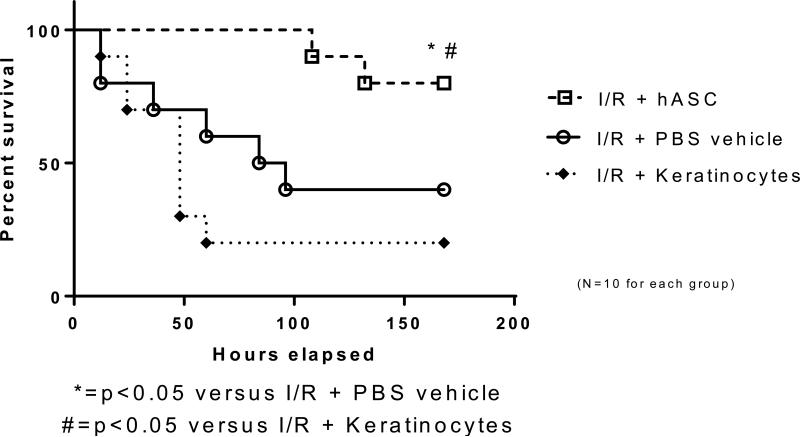
Following intestinal I/R, seven day survival was 40% in the vehicle group and 20% in those treated with keratinocytes. Seven day survival significantly increased to 80% with the post-ischemic application of hASCs (Figure 1, p<0.05). These data identify hASCs as providing significant survival advantages to mice after intestinal ischemia and reperfusion injury.
Figure 1. hASC therapy improves survival following intestinal ischemia.
The use of hASCs increased seven day survival to 80% compared to a 40% seven day survival in vehicle control following intestinal ischemia. No survival benefit was seen with the use of keratinocytes (differentiated cell control). I/R = ischemia and reperfusion; hASC = Human adipose-derived stromal cell
Post-Ischemic Mesenteric Perfusion Is Improved With The Use of hASC Therapy
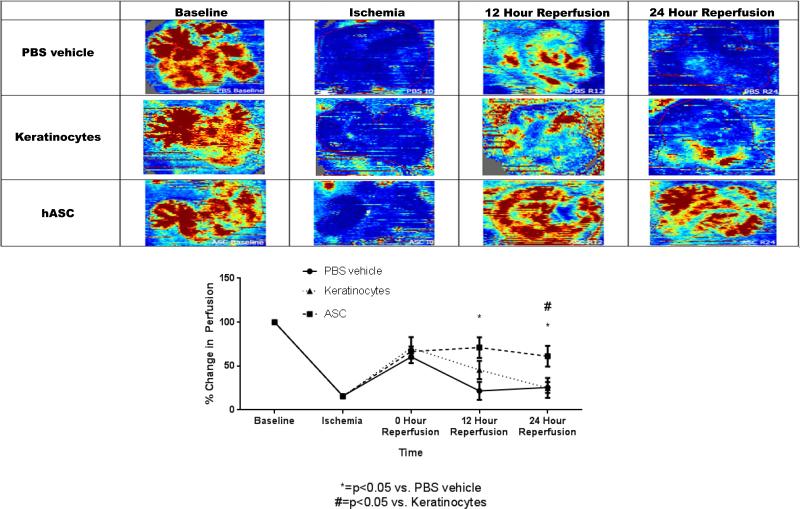
Administration of hASCs following intestinal I/R improved post-ischemic mesenteric perfusion at both 12 and 24-hours of reperfusion compared to mice administered only vehicle (12 Hour Reperfusion: PBS Vehicle: 21.67+/−10.22% vs. hASC: 71.00+/−11.76%, p<0.05; 24 Hour Reperfusion: PBS Vehicle: 25.57+/−6.07% vs. hASC: 61.14+/−11.89%, p<0.05)(Figure 2). Improved mesenteric perfusion was also appreciated at 24-hours of reperfusion for the hASC group compared to the keratinocyte group (ASC: 61.14+/−11.89% vs. Keratinocyte: 25.00+/−11.38%, p<0.05), but this was not significant at 12-hours post ischemia. These results suggest that improved survival outcomes with hASC therapy following intestinal I/R may potentially be attributed to improved mesenteric perfusion following injury.
Figure 2. hASCs increase mesenteric perfusion following ischemia.
The use of human adipose stromal cells significantly increased mesenteric perfusion above vehicle at both 12 and 24 hours following injury. hASCs also were found to promote better mesenteric perfusion compared to keratinocytes (differentiated cellular control) at 24 hours following injury. hASC = Human adipose-derived stromal cell
hASC Therapy Following Intestinal I/R Preserves Intestinal Tissue Architecture
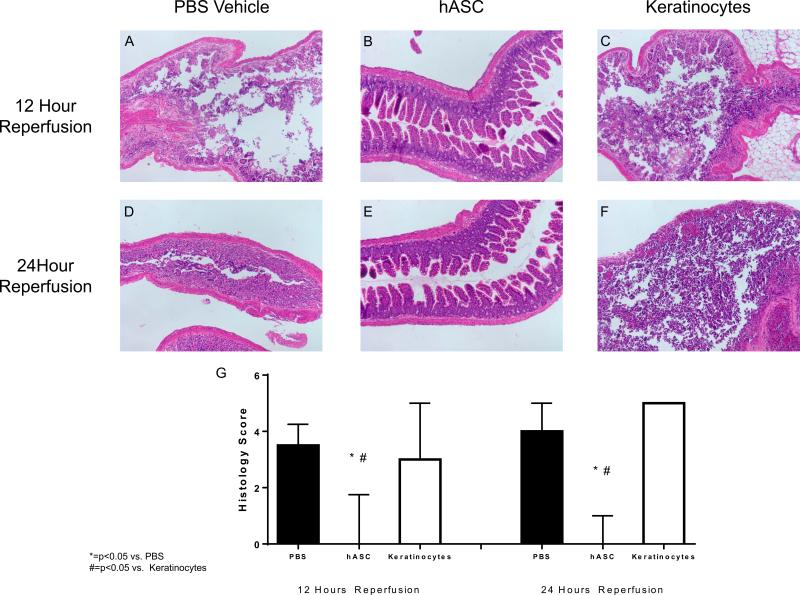
Intestinal I/R resulted in significant sloughing of the intestinal mucosa at both 12 and 24 hours following reperfusion in vehicle treated groups. This was seen as destruction of the epithelial layer in the crypt-villous architecture. Human ASC therapy following I/R abated this destruction and significantly decreased the median histology injury score (12 Hour Reperfusion: PBS Vehicle: 3.5 (25%-75%: 1.0-4.3) vs. hASC: 0 (25%-75%: 0.0-1.8), p<0.05; 24 Hour Reperfusion: PBS Vehicle: 4.0 (25%-75%: 2.0-5.0) vs. hASC: 0 (25%-75%: 0.0-1.0), p<0.05) (Figure 3). Histology scores following keratinocyte therapy were similar to vehicle and were significantly worse than hASC groups. These results suggest that hASCs have a beneficial impact to the protection of intestinal mucosal integrity following I/R.
Figure 3. hASCs improve intestinal histology following ischemic injury.
Infusion of PBS vehicle (A, D) and keratinocytes (C,F) were associated with sloughing of the mucosa and transmural necrosis. hASC therapy (B,E) following intestinal injury inhibited this damage. Intestinal segments were scored according to Watkins, et al [36] and hASC therapy had significantly lower histology scores at both 12 and 24 hours of reperfusion. hASC = Human adipose-derived stromal cell
hASC Therapy Preserves Intestinal Tight Junction Architecture Following Injury
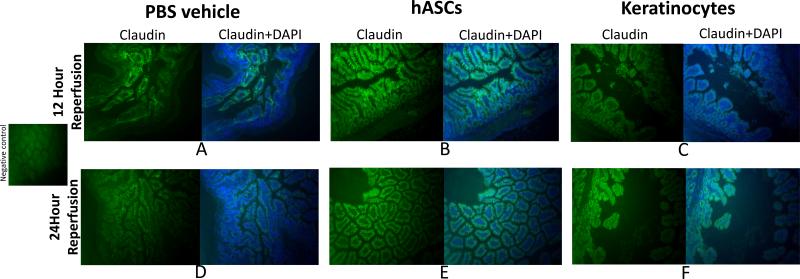
Animals exposed to hASCs following intestinal I/R injury demonstrated preservation of Claudin-1 tight junctional proteins in the expected boarder zones between intestinal epithelial cells at 12 and 24 hours of reperfusion. Cell junctions stained brightly with Claudin-1 and the cell borders were crisply and clearly stained. Epithelial cells did not stain as strongly or as uniformly in PBS treated groups. Keratinocyte treated groups had decreased staining at 12 hours, and evidence of hazy boarders between cells at 24 hours, thereby suggesting disrupted gap junctions in these treated groups (Figure 4).
Figure 4. Human adipose derived stromal cell therapy during intestinal ischemia preserves claudin-1 tight junction proteins.
Infusion of PBS vehicle (A,D) and keratinocytes (C,F) were associated with less immunofluorescence staining of Claudin-1 in intestinal tissue segments. Infusion of hASCs (B,E) preserved claudin-1 integrity following 12 and 24 hours of reperfusion as noted by more uniform, robust, and crisp staining of claudin-1 at the epithelial cell boarders. hASC = Human adipose-derived stromal cell
hASC therapy affects intestinal, hepatic, and pulmonary inflammatory tissue cytokine production
Intestinal tissue levels of GCSF were significantly decreased at 24 hours with the use of hASC therapy compared to PBS. Intestinal tissue levels of VEGF, MIG, IL-6, and IL-1β were not different between groups in intestinal tissues. Liver levels of GCSF were also significantly decreased in hASC groups at 24 hours of reperfusion compared to PBS or keratinocyte groups. Liver levels of IL-1β were significantly lower in hASC groups compared to keratinocytes at 12 hours of reperfusion, but no difference was seen at 24 hours (Table 1).
Table 1.
Assayed Chemokines in Intestinal, Hepatic, and Pulmonary Tissues Following Cellular Therapy for Intestinal I/R
| 12 hour Reperfusion | 24 hour Reperfusion | ||||||
|---|---|---|---|---|---|---|---|
| PBS | hASC | Keratinocytes | PBS | hASC | Keratinocytes | ||
| Intestine | VEGF | 49.31 | 48.5 | 40.1 | 47.5 | 52.7 | 52.1 |
| 34.2-65.8 | 20.6-66.3 | 0-49.6 | 29.2-71.2 | 27.8-80.8 | 0-106.6 | ||
| GCSF | 291.9 | 300.8 | 396.7 | 1609 | 201.4 * | 487.2 | |
| 32.3-958.3 | 77.4-1477 | 97.3-1700 | 1379-3533 | 178.7-410.2 | 210.3-2557 | ||
| MIG | 523.4 | 316.9 | 453.8 | 399.8 | 534.5 | 604.4 | |
| 314.6-707.5 | 245.2-389 | 373.5-487.8 | 345.6 | 568.1 | 396.0-738.0 | ||
| IL6 | 961.1 | 210.5 | 716.7 | 235.7 | 91.56 | 164.9 | |
| 52.3-2860 | 122.6-1004 | 70.0-1413 | 136.5-582.2 | 51.03-144.8 | 81.2-1169 | ||
| IL1β | 49.3 | 48.5 | 40.4 | 47.5 | 52.7 | 52.1 | |
| 34.2-65.8 | 20.6-66.3 | 0-49.6 | 29.2-71.2 | 27.8-80.8 | 0-106.6 | ||
| Liver | VEGF | 22.8 | 21 | 19.5 | 27.7 | 36.1 | 22.1 |
| 19.4-27.6 | 18.0-30.0 | 15-24.5 | 17.6-39.3 | 19.7-42.8 | 12.1-27.8 | ||
| GCSF | 17.1 | 42.1 | 239 | 197 | 25.2 *# | 157.8 | |
| 11.8-158.5 | 8.2-216.3 | 29.0-296.0 | 112.2-343.1 | 23.4-46.1 | 79.6-370.1 | ||
| MIG | 183.7 | 133.1 | 571.8 | 142 | 163.5 | 351.8 | |
| 108.2-281.6 | 113.7-228.9 | 326.6-654.7 | 118.0-205.6 | 93.2-183.8 | 106.0-396.7 | ||
| IL6 | 85.2 | 92.1 | 121.4 | 122.2 | 76.1 | 111.5 | |
| 67.1-195.6 | 78.2-155.5 | 113.6-137.7 | 69.1-131.5 | 69.6-95.6 | 71.2-166.1 | ||
| IL1β | 30.6 | 42.8 *# | 21.7 | 43.2 | 46.2 | 30 | |
| 23.0-33.1 | 30.8-56.5 | 18.1-31.6 | 26.7-64.2 | 20.6-60.5 | 21.1-30.1 | ||
| Lung | VEGF | 77.8 | 70.1 | 49.4 | 23 | 128.1 * | 75.1 |
| 46.5-91.5 | 35.1-75.3 | 28.0-83.8 | 20.7-73.1 | 81.0-145.7 | 37.8-83.5 | ||
| GCSF | 104.3 | 94.1 | 785.2 | 958 | 83.1 *# | 542.5 | |
| 43.3-1639 | 34.2-906.9 | 518.1-1339 | 512.7-1782 | 50.1-167.8 | 430.8-2154 | ||
| MIG | 66.5 | 69.7 # | 266.7 | 89.7 | 59.9 # | 128.6 | |
| 59.7-83.4 | 40.2-124.3 | 149.4-277.6 | 63.8-132.7 | 49.3-82.0 | 97.1-138.1 | ||
| IL6 | 69.3 | 26.5 | 361.3 | 68.8 | 0 *# | 153.6 | |
| 14.5-3661.0 | 14.02-210.7 | 78.1-1105 | 18.4-103.7 | 0-11.6 | 84.2-1382.0 | ||
| IL1β | 56.8 | 36.9 | 59.2 | 40.6 | 33.1 *# | 52.6 | |
| 38.6-100.9 | 22.6-71.1 | 54.3-63.2 | 33.7-58.6 | 29.6-56.2 | 39.9-67.8 | ||
p<0.05 versus PBS
p<0.05 versus keratinocytes
hASC therapy seemed to have the most significant impact on pulmonary inflammation following intestinal ischemia and reperfusion injury. Levels of GCSF, MIG, IL-6, and IL-1β were all significantly decreased in hASC groups at 24 hours of reperfusion compared to PBS or keratinocyte groups. Levels of pulmonary VEGF were also significantly elevated in hASC groups at 24 hours of therapy compared to PBS control, but were not significantly different from keratinocyte groups (Table 1).
DISCUSSION
Intestinal ischemia originates from multiple etiologies and can affect diverse patient demographics. No definitive medical advancements have been made in the treatment of intestinal ischemia in the last decade and therefore the development of novel treatment modalities is paramount. The ultimate therapeutic goal in patients with intestinal ischemia is to restore blood flow to ischemic tissues prior to the development of necrosis and bowel wall perforation. In this study, we observed that hASCs significantly increased survival and mesenteric perfusion while simultaneously preserving intestinal histological and tight junction architecture compared to keratinocytes or vehicle in a murine model of intestinal ischemia and reperfusion injury. In addition, hASCs abated the systemic inflammatory response, as seen predominantly by a decrease in lung tissue inflammatory markers.
While previous studies have demonstrated the therapeutic benefits of hASCs in attenuation of other animal models of I/R injury [21], this is the first study to demonstrate the efficacy of human ASCs in rescuing murine intestinal ischemia. Previous studies have suggested that ASCs provide their benefit at least in part, by the release of paracrine factors [22, 23]. There are likely multiple growth factors and immune modulators that play a role in end organ protection, and current studies by our group are attempting to define which of these factors may be the most efficacious in acute organ protection following injury.
Decreased mortality with hASC therapy was likely related to improved mesenteric perfusion and mucosal integrity. Improved perfusion likely restored blood flow and tissue oxygen levels to physiologic levels, which then prevented intestinal mucosal injury, sloughing, and the impending bacterial translocation and sepsis that would have ensued due to cell junction degradation. This concept was seen in this study by preservation of histological architecture and the tight junction protein claudin-1 in those animals treated with hASCs. It is unclear though, how the hASCs promoted improved mesenteric perfusion, but it may be in part to the release of specific vasodilators such as nitric oxide or hydrogen sulfide [24, 25] from these cells. Future studies aim to determine the specific properties of the hASCs that promote improved vascular perfusion and survival.
GCSF and MIG are two chemokines that are associated with inflammation and leukocyte infiltration, while IL-1β and IL-6 are notable proinflammatory cytokines associated with the acute phase response [26-29]. hASC treated groups exhibited a reduced systemic inflammatory response as noted by a decrease in these pulmonary inflammatory markers assessed at 24 hours of reperfusion. It is unclear though as to why levels of these markers were not significantly altered in intestinal and hepatic tissues over the same time periods. Preservation of tight junctions and intestinal integrity with hASC therapy may have ameliorated the systemic inflammatory response and likely contributed to improvements in animal survival.
Clinical Considerations to Stromal Cell Therapy
The clinical use of hASCs in patients with intestinal ischemia provides a novel treatment option for a disease process that has not had a sound medical advancement in many years. Initial ASC clinical trials in other organ systems have been quite promising. Transendocardial injection of ASCs in patients with no-option ischemic cardiomyopathy indicated that autologous transplantation of ASCs was not only safe, but also preserved ventricular function, myocardial perfusion, and functional capacity [30]. Another study using autologous ASCs in patients with non-revascularizable critical limb ischemia demonstrated that ASCs improved wound healing, decreased pain, and expedited recovery time [31]. Trials in patients with Crohn’s disease and complex perianal fistulas showed reduced fistula size and improved recovery when patients were administered ASCs [32].
Multiple animal studies and now several clinical trials have demonstrated extremely promising results with the use of ASC therapy. Challenges that face initiating clinical trials in human subjects with intestinal ischemia include identifying the most appropriate patients for whom to offer therapy. Many previous initial clinical trials have utilized patients in whom surgical revascularization was not an option. In the case of intestinal ischemia, patients who undergo surgical resection of necrotic bowel, but who have marginally ischemic boarder zones of intestine may be the best candidates for initial therapeutic trials. With the aid of second look laparotomy, surgeons could apply the cells on the day of intestinal resection, temporarily close the abdomen, and return to the operating room in 24-48 hours to assess the viability of the remaining intestines.
An additional challenge to therapy is identifying the optimal stromal cell source (bone marrow, adipose tissue, umbilical cord), as well as determining the donor type for therapy (allogenic versus autologous). Risks to allogenic therapy include potential immunogenic reactions to foreign cells, although this is less likely given the ability of mesenchymal stromal cells to down-regulate T-cell proliferation [33]. Finally, risks of malignancy are always considered with stromal cell therapy given the highly proliferative potential of these cells. The risks of malignancy in the future would need to be balanced with the risks of mortality and morbidity at the time of therapeutic use.
Limitations to this study
This study has several limitations that may affect the impact of the results. The first limitation is that human cells were utilized as a preclinical assessment in a mouse model of intestinal ischemia and reperfusion injury. While xenotransplantation may be less desirable, the use of stromal cells from animals is certainly feasible. Mesenchymal stromal cells have unique immunomodulatory properties that suppress T-lymphocyte proliferation and allow them to be used in different [33]. There have been over 25 different studies where human mesenchymal stromal cells have been placed into immunocompetent hosts of different species [3, 34]. Interestingly, in the majority of these cases, the cells survived several weeks and engrafted into the host tissue [35].
A subsequent limitation to the study is that the SMA ligation model of intestinal I/R does not model clinical intestinal ischemia to its fullest. Although complete small bowel ischemia is possible secondary to SMA thrombus or embolus, the majority of intestinal ischemic episodes are due to segmental intestinal ischemia, such as may be seen with adhesive bowel obstructions or incarcerated hernias. Nonetheless, the SMA ligation model mimics the most severe form of intestinal ischemia, and therefore, is likely the best animal model available to test the effectiveness of new therapies.
Additionally, a limitation exists in the assessment of tissue cytokines. Despite normalizing for total protein concentration, a wide variation of levels was observed between group samples. Although the same relative area of intestine, liver, and lung was procured from each subject, it is likely that tissue levels of cytokines are not exactly equal throughout even small segments of tissue.
CONCLUSION
Adipose-derived stromal cells have shown promise as a potential novel treatment option for patients with intestinal ischemia. More specifically, we observed that human ASCs improve survival outcomes, improve mesenteric perfusion and intestinal histologic architecture, and decrease systemic inflammation. Clinical trials that utilize hASCs for the treatment of intestinal ischemia are certainly on the horizon, but a clear understanding of the mechanism of action must be addressed to ensure the safety and efficacy of therapy prior to widespread clinical use.
Acknowledgments
This publication was made possible with support from:
1) KL2TR001106, and UL1TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award
2) Indiana University Health, Indianapolis, IN
3) The Thrasher Research Fund
4) The Summer Research Program in Academic Medicine
5) The Showalter Research Trust Fund
Footnotes
No disclosures to report
REFERENCES
- 1.Paladino NC, Inviati A, Di Paola V, Busuito G, Amodio EA, Bonventre S, Scerrino G. Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study. Ann Ital Chir. 2013:84. [PubMed] [Google Scholar]
- 2.Roussel A, Castier Y, Nuzzo A, Pellenc Q, Sibert A, Panis Y, Bouhnik Y, Corcos O. Revascularization of acute mesenteric ischemia after creation of a dedicated multidisciplinary center. Journal of vascular surgery. 2015 doi: 10.1016/j.jvs.2015.06.204. [DOI] [PubMed] [Google Scholar]
- 3.Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. The Journal of surgical research. 2015 doi: 10.1016/j.jss.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 4.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25(4):329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 5.Mehall JR, Chandler JC, Mehall RL, Jackson RJ, Wagner CW, Smith SD. Management of typical and atypical intestinal malrotation. Journal of pediatric surgery. 2002;37(8):1169–1172. doi: 10.1053/jpsu.2002.34465. [DOI] [PubMed] [Google Scholar]
- 6.Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector J, Meldrum DR. Stem cells as a potential future treatment of pediatric intestinal disorders. Journal of pediatric surgery. 2008;43(11):1953–1963. doi: 10.1016/j.jpedsurg.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Qu L, Dou R, Lu L, Bian S, Zhu W. Potential Role of Mesenchymal Stem Cells in Alleviating Intestinal Ischemia/Reperfusion Impairment. PLoS ONE. 2013;8(9):e74468. doi: 10.1371/journal.pone.0074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone Marrow Mesenchymal Stem Cells Reduce Intestinal Ischemia/Reperfusion Injuries in Rats. Journal of Surgical Research. 2011;168(1):127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology. 2006;24(4):150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. International Journal of Molecular Medicine. 2014;34(3):695–704. doi: 10.3892/ijmm.2014.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casteilla L, Planat-Benard V, Bourin P, Laharrague P, Cousin B. Tissu adipeux et médecine régénératrice. Transfusion Clinique et Biologique. 2011;18(2):124–128. doi: 10.1016/j.tracli.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno H. Adipose-derived Stem Cells for Tissue Repair and Regeneration: Ten Years of Research and a Literature Review. Journal of Nippon Medical School. 2009;76(2):56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 13.Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012;347(2):419–427. doi: 10.1007/s00441-011-1306-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang C-L, Sung P-H, Sun C-K, Chen C-H, Chiang H-J, Huang T-H, Chen Y-L, Zhen YY, Chai H-T, Chung S-Y, et al. Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. Journal of Pineal Research. 2015;59(2):206–220. doi: 10.1111/jpi.12251. [DOI] [PubMed] [Google Scholar]
- 15.Zhi K, Gao Z, Bai J, Wu Y, Zhou S, Li M, Qu L. Application of adipose-derived stem cells in critical limb ischemia. Frontiers in bioscience (Landmark edition) 2014;19:768–776. doi: 10.2741/4243. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. BioMed research international. 2014;2014:462472. doi: 10.1155/2014/462472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Chang P, Pei Y, Li B, Liu Y, Zhang Z, Yu J, Zhu D, Liu X. Intramyocardial injection of hypoxia-preconditioned adipose-derived stromal cells treats acute myocardial infarction: an in vivo study in swine. Cell and tissue research. 2014;358(2):417–432. doi: 10.1007/s00441-014-1975-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Yoon JG. Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2008;15(8):907–912. doi: 10.1016/j.jocn.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation research. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 20.Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. Journal of Surgical Research. doi: 10.1016/j.jss.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 21.Lee SC, Kim JO, Kim SJ. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery. 2015;157(5):934–943. doi: 10.1016/j.surg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Carceller MC, Guillen MI, Ferrandiz ML, Alcaraz MJ. Paracrine in vivo inhibitory effects of adipose tissue-derived mesenchymal stromal cells in the early stages of the acute inflammatory response. Cytotherapy. 2015;17(9):1230–1239. doi: 10.1016/j.jcyt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Davis TA, Anam K, Lazdun Y, Gimble JM, Elster EA. Adipose-derived stromal cells promote allograft tolerance induction. Stem cells translational medicine. 2014;3(12):1444–1450. doi: 10.5966/sctm.2014-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikami S, Nakashima A, Nakagawa K, Maruhashi T, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, et al. Autologous bone-marrow mesenchymal stem cell implantation and endothelial function in a rabbit ischemic limb model. PloS one. 2013;8(7):e67739. doi: 10.1371/journal.pone.0067739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltowski J, Jamroz-Wisniewska A. Hydrogen Sulfide and Endothelium-Dependent Vasorelaxation. Molecules. 2014;19(12):21183–21199. doi: 10.3390/molecules191221183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- 27.Tensen CP, Flier J, Van Der Raaij-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, Scheper RJ, Boorsma DM, Willemze R. Human IP-9: A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). The Journal of investigative dermatology. 1999;112(5):716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. The Journal of infectious diseases. 1997;176(2):439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 29.Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. European cytokine network. 2002;13(2):161–172. [PubMed] [Google Scholar]
- 30.Perin EC, Sanz-Ruiz R, Sánchez PL, Lasso J, Pérez-Cano R, Alonso-Farto JC, Pérez-David E, Fernández-Santos ME, Serruys PW, Duckers HJ, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. American Heart Journal. 2014;168(1):88–95. e82. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with nonrevascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 32.de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28(3):313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 33.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 34.Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem cells and development. 2012;21(15):2770–2778. doi: 10.1089/scd.2012.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Experimental hematology. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 36.Watkins DJ, Yang J, Matthews MA, Besner GE. Synergistic effects of HB-EGF and mesenchymal stem cells in a murine model of intestinal ischemia/reperfusion injury. Journal of pediatric surgery. 2013;48(6):1323–1329. doi: 10.1016/j.jpedsurg.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]