Abstract
BACKGROUND
The historical approach of offering dietary advice to donors with low hemoglobin (Hb) is ineffective for preventing iron deficiency in frequent donors. Alternative approaches to maintaining donor iron status were explored.
STUDY DESIGN AND METHODS
Frequent blood donors were randomly assigned into five arms for 2 years of follow-up. Three double-blinded arms provided 60 once-daily pills after each donation (38, 19, or 0 mg of iron). Two single-blinded arms provided iron status (ferritin) or no information letters after each donation. Ferritin, soluble transferrin receptor, and complete blood count were measured at each donation.
RESULTS
There were 692 subjects enrolled and 393 completed the study. Subjects in pill groups deenrolled more than those in letter groups (39% vs. 7%). Adverse events occurred equally in subjects receiving iron or placebo pills. Of those completing the study, the prevalence of ferritin of less than 12 or less than 26 ng/mL declined by more than 50% and was statistically indistinguishable in the three intervention groups (19 or 38 mg of iron; iron status letter). Longitudinal analyses of all subjects showed improved iron status in iron pill groups and worsening iron status in control groups (placebo; no information letter). The iron pill groups experienced a net increase of approximately 0.6 g/dL Hb compared to control groups. The iron status letter group had little change in Hb.
CONCLUSION
Providing 19 or 38 mg of daily iron or iron status information were effective and mostly equivalent interventions for mitigating iron deficiency in regular donors when compared at the end of the 2-year longitudinal phase of the study. Donors without intervention had worsened iron deficiency with continued donation.
Whole blood donation is used to support numerous medical treatments that would be unavailable, if not for the generosity of volunteer blood donors. Individuals are permitted to donate whole blood every 56 days in the United States as long as their fingerstick hemoglobin (Hb) value is at least 12.5 g/dL, regardless of sex, race, or age. Each donation removes between 200 and 250 mg of iron, which in the absence of iron supplementation may take 6 months or longer to replenish in both male and female donors.1,2 Although iron is necessary to produce Hb, fingerstick Hb values do not correlate well with iron stores.3 Consequently, Hb screening does little to protect repeat donors from iron deficiency.3,4 Tissue iron stores become depleted with only one donation in many females, and two-thirds of female donors with two or more donations in the previous 12 months have iron deficiency.5 Males have larger iron stores, but one-half of males with three or more donations in the previous 12 months have iron deficiency.5 Iron deficiency is important to prevent because it is associated with adverse side effects that may occur even in the absence of anemia. These include pica,6,7 restless legs syndrome,6,7 fatigue,8,9 decreased exercise capacity,10 and decreased neurocognitive function.11,12
Blood-collecting organizations currently face challenging decisions about how best to protect against iron deficiency in their donors. Available evidence indicates that simply encouraging the donor to eat iron-rich foods is not adequate.3,13,14 Two options that appear to be effective are the use of oral iron supplements,15–17 which decreases the time for recovery of predonation iron and Hb to approximately 90 days,2 or extending the donation interval to 6 months or longer.1,2 The Strategies To Reduce Iron DEficiency (STRIDE) study was a multicenter, randomized, blinded, and placebo-controlled study performed to investigate the relative efficacy of alternate approaches for mitigating donor iron deficiency that could be readily implemented in community blood centers.18 Randomization to one of five arms representing an educational or iron supplementation intervention allowed for comparison of a broad range of strategies that are operationally feasible for most blood centers.
MATERIALS AND METHODS
Recruitment, enrollment, and study design
The recruitment of blood donors, donor mailings, laboratory testing methods, and oral iron supplement formulations have been previously described.18 In brief, three blood centers enrolled donors between June 2011 and April 2012, with eligibility limited to those not taking supplemental iron. Enrollment was restricted to men with three or more, and women with two or more, red blood cell (RBC)-equivalent donations within the prior 12 months. Participants were 18 years of age or older and provided written informed consent before randomization into one of five equal-sized study arms. Subjects were asked to continue making a minimum of two (female) or three (male) RBC-equivalent donations per year during the 2-year longitudinal phase of the study. Each subject, regardless of randomization arm, was required to meet all conditions for routine blood donation. These included having fingerstick Hb level of at least 12.5 g/dL and a minimum 56-day interdonation interval at each donation during the study. A peripheral blood sample was obtained at each blood donation visit to measure complete blood count, ferritin, and soluble transferrin receptor (sTfR). Complete blood count was performed using venous blood samples collected before blood donation (Model XE2100D, Sysmex, Kobe, Japan; or Model LH 750s, Beck-man Coulter, Brea, CA). Ferritin and sTfR were performed at ARUP Laboratories (Salt Lake City, UT). Ferritin was measured using an immunoassay system (ADVIA Centaur, Siemens Healthcare Diagnostics, Deerfield, IL). sTfR was measured using an immunoassay system (Tina-quant, Roche Diagnostics, Indianapolis, IN). This study was approved by institutional review boards at all participating institutions and the data coordinating center.
Two arms were assigned to the single-blinded educational strategy and three arms assigned to the double-blinded iron supplementation strategy. Donors in the iron status letter group received a letter following each donation containing their plasma ferritin test result and instructions on how to proceed in the study based on this result (see Supplemental Information, available as supporting information in the online version of this paper, for sample letters). If plasma ferritin was less than 26 ng/mL, they were advised to either take an iron supplement or delay their next donation for 6 months. If plasma ferritin was at least 26 ng/mL, they were encouraged to continue donating blood frequently with no other recommendation. Donors in the no information letter group received a letter after each donation encouraging frequent blood donation. Donors in the iron supplementation arms received one of three strengths of oral ferrous gluconate tablets following each donation: 38, 19, or 0 mg (placebo) of elemental iron. A package distributed by mail included a childproof bottle of 60 pills (two bottles for double-RBC donors), instructions on when to take pills, and a list of possible side effects.
Randomization of subjects
A list of 1200 randomized study identification numbers (study IDs) was created. This list was divided by participating blood center (400 study IDs each) and sex (200 for females and 200 for males for each blood center). The list was subdivided in this way to ensure equal representation by blood center and gender in the study. The lists were generated using standard block randomization methods, implemented with computer software (nQuery Advisor, 2014, Statistical Solutions, Boston, MA). The randomized numbers from the algorithm correspond to one of the five study arms. While they appeared to be random numbers, they coded for the group assignment. Each of the five study arms had three to four codes so that patterns could not be identified by investigators, coordinators, and staff at study sites. When a new participant entered the study, the study management system selected the next available study ID that matched the blood center and sex of the participant from the list of IDs. Thus, the investigators and study coordinators were blinded to participant group assignment. Additionally, the pills were color coded and not identified by iron content (38, 19, or 0 mg). Only study staff at Westat knew which color was associated with which iron dosage or placebo. There was a single staff person at one blood center who did all the mailings of letters and pills and had special access to the study management system. Therefore, this individual may have had a sense of who belonged to which study arm (and who got which letter), but did not know which pills were 0, 19, or 38 mg of iron.
Sample size calculation
The baseline prevalence of iron deficiency (i.e., ferritin <26 ng/mL) was expected to be greater than 50% among eligible repeat donors. The expected outcome for each intervention was a 50% reduction in the prevalence of iron deficiency. For example, subjects in the iron status letter group, the 38 mg of iron group, and the 19 mg of iron group were expected to have a 50% reduction in the prevalence of iron deficiency by the end of study, while no change in the prevalence of iron deficiency was expected in the no information letter group and placebo group. A sample of 102 donors in each group provides 80% power in a two-sided, 0.05 level test to detect a 40% reduction in an intervention group compared to its corresponding control group (and >90% power for a 50% reduction). To allow for attrition of subjects during the study, an enrollment goal of 140 subjects per group was set.
Study withdrawal and adverse events
The methods used to elicit reasons for withdrawal were standardized across centers. For adverse events, the study coordinator, who was blinded to subject randomization, completed a standardized worksheet to capture symptoms, symptom severity, and other relevant information. These forms were first reviewed locally with protocol-associated medical staff and then reviewed by an external medical monitor. A follow-up assessment was completed and sent to an off-site study physician, who was blinded to subject randomization. Serious or unexpected adverse events were reported immediately to a local study physician. For active withdrawals not associated with an adverse event, research staff documented the information provided by the subject at the time of withdrawal.
Enrollment and final questionnaires
An enrollment questionnaire recorded information on self-administered supplements. A final questionnaire recorded actions subjects took during the study to replenish iron.
Statistical analysis
Frequency distributions of demographic characteristics and donation type by group assignment were produced to assess differences across groups at final visit. Distributional statistics were calculated for Hb, ferritin, sTfR, and (sTfR/ferritin) by demographic factors and group assignment. Statistical differences across groups at final visit for continuous variables were calculated using analysis of variance (ANOVA) and for categorical variables using chi-square analysis as specified in the protocol. Statistical differences from baseline to final visit were calculated for categorical variables using McNemar’s test and for continuous variables using paired t test also as specified in the protocol (note that the study sample size was based on the tests across groups of final visit outcomes rather than these statistically more powerful tests across groups of change in outcomes). In addition, statistical differences across groups were assessed by repeated-measures models. Linear regression repeated-measures models and logistic regression repeated-measures models were developed with compound symmetry covariance structure akin to Cable and colleagues19 for continuous and categorical variables, respectively. As in Cable and colleagues,19 covariates in the repeated-measures models included race, age, weight, smoking status, pregnancy history, menstrual status, blood center, number of donations in past 2 years, and time since last donation. Ferritin and sTfR were log transformed to better satisfy linear regression normality assumptions. The group effect was modeled as a constant effect for all visits after baseline (while other group effects are plausible—in particular, for the iron status information-only group an effect dependent on whether the post-donation informational letter indicated low iron or not seems likely—the study was not statistically powered to distinguish among other plausible group effects). The covariates in these repeated-measures models were specified in the protocol; however, the group effect was specified only loosely (i.e., mean change over time by groups). The model uses a constant effect (e.g., there is a mean change in ferritin during the 56 days after each donation that is then sustained throughout the course of the study). All statistical analyses were performed using computer software (SAS, Version 9.3, SAS Institute, Inc., Cary, NC).
RESULTS
Study completion
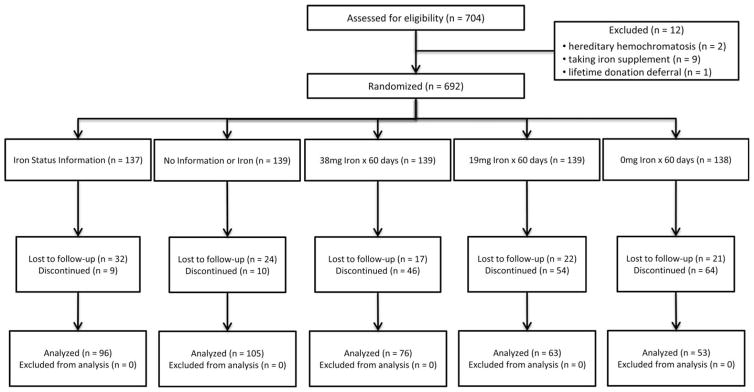
The study randomized 692 subjects, and 393 completed a final visit (Fig. 1). Of the 299 subjects discontinuing the study, 195 had one or more donation visits after enrollment, with a mean of 2.9 donation visits and mean time on study of 12 months. The number of subjects and their reasons for discontinuing the study differed by group assignment (Table 1). One-third of those not completing the study were denominated “lost to follow-up,” because they stopped donating without specific communications to research staff. An additional 13 donors reported moving from the study area. These 116 donors were denominated “passive withdrawal” and did not statistically differ by group assignment (p =0.33). In contrast, active deenrollments, where the donor reported a reason for study withdrawal, varied by group assignment. Subjects randomized to a pill arm withdrew at higher rates than those in the letter arms (39% vs. 7%; p <0.0001). The desire to stop taking pills was the main reason for active withdrawal. Subjects also withdrew for a variety of medical reasons, including physician recommendation to withdraw to ensure an iron deficient subject was taking iron instead of placebo. There were 39 adverse events recorded in subjects assigned to pill arms representing 21% of active deenrollments and 13% of subjects not completing a final visit. Adverse events did not differ between subjects receiving iron and placebo (p =0.57). Subjects in the letter arms were not monitored for adverse events. The demographics of the donors completing the study were similar across the five arms (Table 2). The 692 randomized subjects divide into two groups: 393 subjects completing a final visit and 299 subjects not completing a final visit. Comparison of these two groups revealed that ferritin at enrollment (dichotomized above or below 26 ng/mL; p =0.33), race (p =0.34), and sex (p =0.08) had no effect on the likelihood of completing the study, but age did reach significance (p =0.02) with older participants more likely to complete the study.
Fig. 1.
The allocation of participants to the five study arms and the number of each group that were lost to follow-up, deenrolled, and completed the study. Analyses were performed for both the allocation cohort (n =692) and the analytic cohort (n =393). The reasons for discontinuation in the study during follow-up are presented in Table 1.
TABLE 1.
Reasons for deenrollment by group assignment
| Group assignment | Deenrollment type
|
Passive withdrawal
|
||||||
|---|---|---|---|---|---|---|---|---|
| Protocol* | Adverse event | Medical | Permanent deferral | Active withdrawal† | Total | Known‡ | Lost to follow-up | |
| Iron status letter | 5 | N/A | 1 | 0 | 3 | 9 | 5 | 27 |
| No information letter | 2 | N/A | 5 | 1 | 2 | 10 | 3 | 21 |
| 38 mg of iron | 1 | 16 | 6 | 0 | 23 | 46 | 1 | 16 |
| 19 mg of iron | 9 | 12 | 6 | 0 | 27 | 54 | 2 | 20 |
| Placebo | 5 | 11 | 14 | 0 | 34 | 64 | 2 | 19 |
| Total | 22 | 39 | 32 | 1 | 89 | 183 | 13 | 103 |
Subjects removed from study per protocol, e.g., determined to be taking vitamins with iron before enrollment or had ferritin level exceeding 300 ng/mL.
Includes those not wanting to take pills, physician recommended study withdrawal, or medical or surgical reasons unrelated to study.
Known passive refusals are those who reported moving outside the study area.
TABLE 2.
Demographics of subjects completing a final visit
| Group assignment
|
Total | |||||
|---|---|---|---|---|---|---|
| Iron status letter | No information letter | 38 mg of iron | 19 mg of iron | Placebo | ||
| Total | 96 (100) | 105 (100) | 76 (100) | 63 (100) | 53 (100) | 393 (100) |
| Sex | ||||||
| Female | 44 (45.8) | 47 (44.8) | 36 (47.4) | 29 (46.0) | 27 (50.9) | 183 (46.6) |
| Male | 52 (54.2) | 58 (55.2) | 40 (52.6) | 34 (54.0) | 26 (49.1) | 210 (53.4) |
| Race/ethnicity | ||||||
| White | 91 (94.8) | 100 (95.2) | 74 (97.4) | 62 (98.4) | 52 (98.1) | 379 (96.4) |
| Nonwhite | 5 (5.2) | 5 (4.8) | 2 (2.6) | 1 (1.6) | 1 (1.9) | 14 (3.6) |
| Age (years) | ||||||
| <30 | 2 (2.1) | 4 (3.8) | 3 (3.9) | 6 (9.5) | 6 (11.3) | 21 (5.3) |
| 30–39 | 8 (8.3) | 7 (6.7) | 3 (3.9) | 7 (11.1) | 3 (5.7) | 28 (7.1) |
| 40–49 | 18 (18.8) | 14 (13.3) | 5 (6.6) | 6 (9.5) | 7 (13.2) | 50 (12.7) |
| 50–59 | 32 (33.3) | 39 (37.1) | 28 (36.8) | 24 (38.1) | 13 (24.5) | 136 (34.6) |
| 60+ | 36 (37.5) | 41 (39.0) | 37 (48.7) | 20 (31.7) | 24 (45.3) | 158 (40.2) |
Data are reported as number (%).
Iron status at the end of the study
The three biochemical measures, ferritin, sTfR, and log(sTfR/ferritin), used to assess iron status, as well as Hb, were statistically equivalent across the five arms at baseline.18 In addition, there was no difference in the number of donation visits during the previous 2 years among subjects in the five study arms at baseline with the mean number of previous donations by subjects across groups ranging from 7.0 to 7.1. Ferritin is an indicator of iron stores with increasing values representing greater stores. sTfR is an indicator of cellular need for iron with higher values representing increased cellular need for iron. The log(sTfR/ferritin) ratio is a sensitive and specific indicator of iron deficiency with increasing values representing worsening iron deficiency. Iron status among the 393 subjects completing the study is presented in Table 3. The iron status letter, 19 mg of iron, and 38 mg of iron groups had statistically equivalent iron status as assessed by all three biochemical measures at the end of the study (p =0.56, p =0.62, and p =0.78, for ferritin, sTfR, and log(sTfR/ferritin), respectively). Iron status in these three groups, as assessed by all three iron variables, was significantly better than that of control subjects in the placebo or no information letter groups at the end of the study (p <0.0001, p =0.03, and p <0.0001, for ferritin, sTfR, and log(sTfR/ferritin), respectively). Iron status of the two control groups, as assessed by all three iron variables, was statistically equivalent at the end of the study (p =0.39, p =0.68, p =0.62, for ferritin, sTfR, and log(sTfR/ferritin), respectively). These patterns remain statistically consistent after stratification by sex (Table 3).
TABLE 3.
Iron and Hb status at final visit by sex and group assignment
| Number | Ferritin (ng/mL)* | sTfR (mg/L)* | log(sTfR/ferritin)† | Number | Venous Hb (g/dL)†‡ | |
|---|---|---|---|---|---|---|
| All participants | 382 | 26.2 (24.2–28.4) | 3.6 (3.5–3.8) | 2.14 (2.10–2.18) | 370 | 14.0 (13.9–14.1) |
| Group assignment | ||||||
| Iron status letter | 92 | 31.2 (27.2–35.8) | 3.5 (3.3–3.7) | 2.05 (1.98–2.12) | 89 | 14.1 (13.9–14.4) |
| No information letter | 102 | 20.6 (17.3–24.5) | 3.9 (3.7–4.2) | 2.28 (2.19–2.37) | 98 | 13.8 (13.5–14.0) |
| 38 mg of iron | 75 | 33.0 (28.1–38.9) | 3.5 (3.2–3.7) | 2.02 (1.94–2.11) | 73 | 14.2 (14.0–14.5) |
| 19 mg of iron | 61 | 30.8 (25.7–36.8) | 3.4 (3.1–3.7) | 2.04 (1.94–2.14) | 59 | 14.1 (13.8–14.4) |
| Placebo | 52 | 18.2 (14.6–22.7) | 3.8 (3.4–4.3) | 2.32 (2.19–2.45) | 51 | 13.8 (13.4–14.1) |
| p value§ | < 0.0001 | 0.02 | < 0.0001 | 0.06 | ||
| All males | 202 | 31.2 (27.9–34.8) | 3.6 (3.5–3.8) | 2.06 (2.00–2.12) | 174 | 14.6 (14.4–14.7) |
| Group assignment | ||||||
| Iron status letter | 50 | 36.2 (30.1–43.5) | 3.6 (3.3–3.9) | 2.00 (1.90–2.10) | 40 | 14.8 (14.4–15.1) |
| No information letter | 56 | 26.2 (20.6–33.2) | 3.8 (3.4–4.1) | 2.16 (2.03–2.28) | 45 | 14.3 (13.9–14.7) |
| 38 mg of iron | 39 | 42.4 (34.1–52.7) | 3.4 (3.1–3.8) | 1.91 (1.80–2.02) | 36 | 14.9 (14.5–15.2) |
| 19 mg of iron | 32 | 34.6 (26.4–45.3) | 3.3 (3.0–3.7) | 1.98 (1.83–2.14) | 27 | 14.7 (14.3–15.1) |
| Placebo | 25 | 18.6 (13.4–25.8) | 4.0 (3.3–4.8) | 2.33 (2.13–2.54) | 26 | 14.3 (13.9–14.7) |
| p value§ | 0.0002 | 0.22 | 0.0005 | 0.09 | ||
| All females | 180 | 21.5 (19.3–24.0) | 3.6 (3.5–3.8) | 2.23 (2.17–2.29) | 196 | 13.3 (13.2–13.5) |
| Group assignment | ||||||
| Iron status letter | 42 | 26.1 (21.4–31.9) | 3.4 (3.2–3.7) | 2.12 (2.01–2.22) | 49 | 13.4 (13.0–13.7) |
| No information letter | 46 | 15.4 (12.1–19.4) | 4.1 (3.7–4.6) | 2.43 (2.31–2.56) | 53 | 13.2 (12.9–13.4) |
| 38 mg of iron | 36 | 25.2 (20.2–31.5) | 3.5 (3.1–3.9) | 2.14 (2.01–2.27) | 37 | 13.6 (13.3–13.9) |
| 19 mg of iron | 29 | 27.1 (21.3–34.5) | 3.4 (3.0–3.9) | 2.10 (1.96–2.24) | 32 | 13.4 (13.0–13.8) |
| Placebo | 27 | 17.9 (13.0–24.6) | 3.7 (3.1–4.3) | 2.31 (2.13–2.49) | 25 | 13.3 (12.9–13.7) |
| p value§ | 0.0007 | 0.06 | 0.0004 | 0.36 | ||
Data are reported as geometric mean (95% CI).
Data are reported as mean (95% CI).
Number varies due to missing data.
p value testing for differences across the five group assignments using ANOVA.
Changes in iron status between enrollment and final visit
Examination of changes in iron status by group assignment between enrollment and final visit among the 393 subjects completing the study revealed broad improvement in most iron variables among subjects receiving iron pills or iron status information, while subjects receiving placebo or no iron status information had unchanged or worsening iron status, depending on the variable examined (Table 4). At the end of the study, the mean ferritin had increased by 10.3 ng/mL in the iron status letter group, by 18.3 ng/mL in the 19 mg of iron group, and by 16.7 ng/mL in the 38 mg of iron group (p <0.0001 for all) but had not changed in the no information letter group or the placebo group (p =0.69 and p =0.77, respectively). The mean log(sTfR/ferritin) decreased by 0.15 in the iron status letter group, by 0.32 in the 19 mg of iron group, and by 0.25 in the 38 mg of iron group (p <0.0001 for all) but did not change in the no information letter group or in the placebo group (p =0.29 and p =0.52, respectively). sTfR did not change in the iron status letter, the 19 mg of iron, or the 38 mg of iron groups but had significant increases (worsening status) in the no information letter group of 0.57 mg/L (p <0.0001) and of 0.60 mg/L in the placebo group (p =0.01).
TABLE 4.
Change in the mean (Δ) from enrollment to final visit for measures of iron status and venous Hb
| Group assignment† | Ferritin (ng/mL)
|
sTfR (mg/L)
|
log (sTfR/ferritin)
|
Venous Hb (g/dL)
|
||||
|---|---|---|---|---|---|---|---|---|
| Δ | p value* | Δ | p value* | Δ | p value | Δ | p value | |
| Iron status letter | 10.3 | <0.0001 | 0.13 | 0.10 | −0.15 | <0.0001 | 0.08 | 0.38 |
| No information letter | 0.3 | 0.69 | 0.57 | <0.0001 | 0.04 | 0.29 | −0.13 | 0.23 |
| 38 mg of iron | 16.7 | <0.0001 | −0.08 | 0.99 | −0.25 | <0.0001 | 0.28 | 0.04 |
| 19 mg of iron | 18.3 | <0.0001 | −0.21 | 0.18 | −0.32 | <0.0001 | 0.39 | 0.01 |
| Placebo | 0.8 | 0.77 | 0.60 | 0.01 | 0.04 | 0.52 | −0.07 | 0.59 |
As ferritin and sTfR are log-normally distributed, the p values were calculated using SAS procedure t test on the log-transformed values.
Sample sizes differ due to missing data. The numbers for each group, in table order, are 89, 98, 73, 59, and 50.
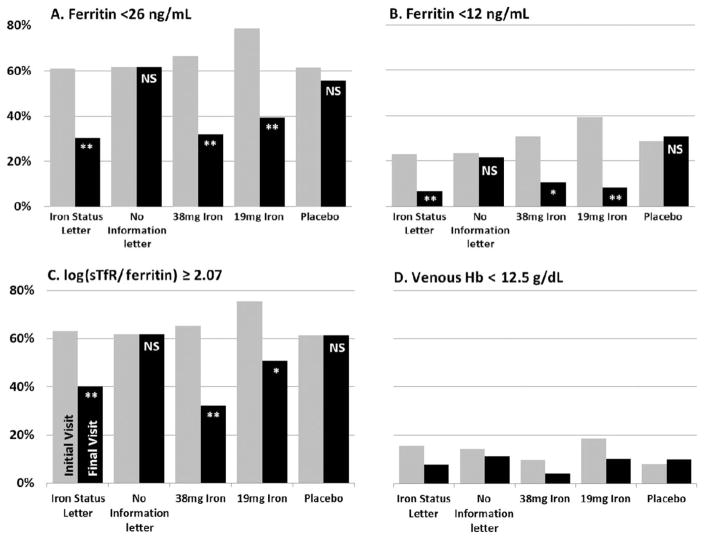
All subjects were frequent donors, such that many had iron deficiency at enrollment.18 The changes in iron status of those randomly assigned to the iron status letter, the 19 mg of iron, or the 38 mg of iron groups translated into marked decreases in the prevalence of iron deficiency as assessed using diagnostic cutoff values for different laboratory tests. At study end, the proportion of subjects with ferritin level of less than 26 ng/mL declined by 50% in each of the three intervention groups (p <0.0001 for all), but was unchanged in the no information letter group and the placebo group (p =1.00 and p =0.58, respectively; Fig. 2 and Table S1, available as supporting information in the online version of this paper). The proportion of subjects with ferritin levels of less than 12 ng/mL declined by 70% or more in the iron status letter, the 19 mg of iron, or the 38 mg of iron groups (p ≤0.002 for all), while not improving for the two control groups (p =0.83 and p =1.00; Fig. 2 and Table S1). The proportion of subjects with log(sTfR/ferritin) of not less than 2.07 decreased by 30% to 50% in the iron status letter, the 19 mg of iron, or the 38 mg of iron groups (p ≤0.003 for all), but did not change in the control groups (p =1.00 for both).
Fig. 2.
Percentage of subjects who completed the study with laboratory measures of iron status or Hb beyond clinical cutoff values for iron deficiency or anemia. p values for differences between initial and final visits are indicated with **p < 0.0001, *p < 0.01, NSp > 0.05. For D, no differences were significant at p < 0.05. (■) Initial visit values; (
 ) final visit values.
) final visit values.
Iron status in longitudinal analyses of all visits by all enrolled subjects
While the 393 subjects completing the study were found to be similar to the 299 subjects not completing the study, the paired analyses presented in the previous section may be subject to biases due to a possible differential response among those not completing the study. Therefore, longitudinal analyses were performed using data from all 692 subjects including all interim study donations. Repeated-measures regression models were developed to quantify the impact of group assignment over the longitudinal phase of the study on three continuous outcome measures of iron status: 1) log ferritin, 2) log sTfR, and 3) log(sTfR/ferritin) (Table 5). Iron status uniformly improved in subjects in the 19 and 38 mg of iron groups with no differences between the two groups (p values for differences ranged from 0.67 to 0.76 for the three models). In contrast, subjects in the placebo group or the no information letter group had uniformly worse iron status with no differences between these two groups (p values for differences ranged from 0.76 to 0.92 for the three models). Subjects in the iron status letter group had intermediate iron status with significant improvement in ferritin and log(sTfR/ferritin) (p ≤0.005 for both), but the magnitude of the improvement was less than that observed in the iron pill groups.
TABLE 5.
Longitudinal regression* results: change after enrollment (Δ) for measures of iron status and venous Hb, by group assignment
| Group assignment | log Ferritin
|
log sTfR
|
log (sTfR/ferritin)
|
Venous Hb
|
||||
|---|---|---|---|---|---|---|---|---|
| Δ | p value | Δ | p value* | Δ | p value | Δ | p value | |
| Iron status letter | 0.09 | <0.0001 | 0.01 | 0.33 | −0.08 | 0.005 | −0.04 | 0.60 |
| No information letter | −0.05 | 0.007 | 0.04 | <0.0001 | 0.09 | <0.0001 | −0.34 | <0.0001 |
| 38 mg of iron | 0.24 | <0.0001 | −0.04 | 0.0003 | −0.28 | <0.0001 | 0.31 | 0.0001 |
| 19 mg of iron | 0.23 | <0.0001 | −0.03 | 0.0005 | −0.26 | <0.0001 | 0.32 | 0.0002 |
| Placebo | −0.04 | 0.01 | 0.04 | <0.0001 | 0.09 | <0.0001 | −0.33 | <0.0001 |
Longitudinal regression adjusting for race, sex, age, center, weight, smoking status, pregnancy history, menstrual status, number of donations in previous 2 years, and time since last donation.
Repeated-measures longitudinal logistic regression models were developed to determine the impact of group assignment over the longitudinal phase of the study on three binary outcome measures of iron status: 1) ferritin of less than 26 ng/mL, 2) ferritin of less than 12 ng/mL, and 3) log(sTfR/ferritin) of 2.07 or more (Table 6). Again, there were no differences between those in the 19 mg of iron and 38 mg of iron groups with either pill reducing the odds for ferritin of less than 12 or of less than 26 ng/mL by more than 80%. Subjects in the iron status letter group exhibited improved iron status. However, the improvement was less dramatic than that observed in subjects receiving iron pills, with risk for ferritin of less than 12 or of less than 26 g/dL decreased by approximately 50%. The improvements in the pill groups were statistically better than those in the iron status letter group (p ≤0.004 for both levels of ferritin), and all three intervention groups were different from the two control groups, which had worsening iron status over the course of follow-up with no differences between the two groups. In the two control groups, the largest impact on iron status was on the risk for having ferritin of less than 12 ng/mL, which increased by 48% to 76% for the placebo and no information letter groups (p =0.04 and p =0.004, respectively).
TABLE 6.
Longitudinal logistic regression* results: ORs (change after enrollment) for measures of iron status and venous Hb
| Group assignment | Ferritin (<26 ng/mL)
|
Ferritin (<12 ng/mL)
|
log (sTfR/ferritin) (≥2.07)
|
Venous Hb (<12.5 g/dL)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | OR | 95%CI | p value | OR | 95%CI | p value | |
| Iron status letter | 0.50 | (0.34–0.73) | 0.0004 | 0.43 | (0.33–0.73) | 0.0004 | 0.62 | (0.43–0.89) | 0.01 | 0.74 | (0.42–1.30) | 0.30 |
| No information letter | 1.20 | (0.90–1.59) | 0.22 | 1.76 | (1.19–2.66) | 0.004 | 1.49 | (1.12–1.98) | 0.006 | 1.69 | (0.99–2.88) | 0.05 |
| 38 mg of iron | 0.18 | (0.12–0.27) | <0.0001 | 0.17 | (0.08–0.34) | <0.0001 | 0.27 | (0.18–0.41) | <0.0001 | 0.49 | (0.21–1.12) | 0.09 |
| 19 mg of iron | 0.18 | (0.12–0.29) | <0.0001 | 0.15 | (0.07–0.33) | <0.0001 | 0.22 | (0.15–0.35) | <0.0001 | 0.51 | (0.26–0.97) | 0.04 |
| Placebo | 1.32 | (0.96–1.82) | 0.09 | 1.48 | (1.01–2.16) | 0.04 | 1.39 | (0.98–1.98) | 0.07 | 2.32 | (1.30–4.20) | 0.005 |
Longitudinal logistic regression adjusting for race, sex, age, center, weight, smoking status, pregnancy history, menstrual status, number of donations in previous 2 years, and time since last donation.
Hb status at the end of the study
Among the 393 subjects completing the study, those randomized to the iron status letter, 19 mg of iron, or 38 mg of iron groups had equivalent mean Hb levels of 14.1 to 14.2 g/dL. These were 0.3 to 0.4 g/dL higher than the placebo or no information letter groups; however, the difference across groups was not significant (p =0.06; Table 3).
Changes in Hb status between enrollment and final visit
Examination of changes in Hb by group assignment between enrollment and final visit among the 393 subjects completing the study revealed improvement in venous Hb by 0.3 g/dL for those in the 38 mg of iron group (p =0.04) and by 0.4 g/dL for those in the 19-mg pill group (p =0.01). Subjects in the iron status letter, the no information letter, and placebo groups did not have significant changes in Hb (Table 4).
Hb status in longitudinal analyses of all visits by all enrolled subjects
Similar to the longitudinal assessment of iron status, repeated-measures analysis of Hb revealed that Hb increased by 0.3 g/dL in the 19 mg of iron and 38 mg of iron groups and decreased by 0.3 g/dL in the placebo and no information letter groups, all significant at p values of not more than 0.0002 (Table 5). Hb in the iron status letter group was unchanged. The odds for venous Hb of less than 12.5 g/dL increased by 69% in the no information letter group, more than doubled in the placebo group (p =0.05 and p =0.005, respectively), remained unchanged in the iron status letter group, and decreased by approximately 50% in the 19 and 38 mg of iron groups (Table 6).
Donor response to the iron status letter
Subject response to the information and guidance provided by letter was assessed at the end of the study. Most subjects in the iron status letter group took actions to protect their iron status. Of the 96 donors completing the study in this group, 16 never had ferritin levels of less than 26 ng/mL. Although not asked to do so, three of these 16 (19%) donors reported delaying donation. Of the 80 donors who received one or more iron status letters for having ferritin levels of less than 26 ng/mL, 27 (34%) began taking iron tablets at some time during the study, nine (11%) delayed subsequent next donation(s), 13 (16%) began taking iron tablets and delayed their subsequent donation(s), 21 (26%) did neither, and 10 (13%) did not provide information to assess response.
DISCUSSION
The efficacy of providing regular blood donors with iron status information (ferritin) or with 19- or 38-mg iron tablets for mitigation of blood donation–induced iron deficiency was examined in a multi-institutional, blinded, and placebo-controlled 2-year study of regular blood donors. Among the 393 subjects completing the study, the final iron status and Hb concentration were statistically equivalent between subjects randomized to the 19 mg of iron group, the 38 mg of iron group, and the iron status information group. This finding held true for all laboratory measures and diagnostic cutoffs examined with most measures indicating a reduction in the prevalence of iron deficiency of 50% or more. Longitudinal analyses of all visits by all enrolled subjects found that providing 19- or 38-mg iron pills were equivalent and effective means to mitigate iron deficiency and increase Hb, that providing iron status information mitigated iron deficiency, but not to the extent observed in subjects receiving iron pills, and that providing placebo or no information mostly resulted in worsening iron status and lower Hb.
Previous studies have shown that an iron-rich diet does not prevent iron deficiency in blood donors.3,13,14 Therefore, subjects in the iron status letter group were advised to take iron supplements or delay donation when their ferritin level was less than 26 ng/mL. Subjects took actions in response to these recommendations and had iron status and Hb indistinguishable from those in the 19 or 38 mg of iron groups at the end of the study. However, in the paired analyses comparing changes in iron and Hb status from enrollment to final visit, as well as in the longitudinal analyses of all visits by all subjects enrolled in the study, subjects randomly assigned to the iron status letter group did not improve their iron status as much as subjects taking iron pills. These differences between the analyses can be attributed to the diverse responses of subjects in the iron status information group when provided the opportunity to choose whether, when, and how to intervene in response to being told they have iron deficiency. Some subjects took iron pills, some delayed donation, some did both, and some did neither. In addition, some subjects in this group did not have ferritin levels of less than 26 ng/mL and, therefore, were never advised to take actions to mitigate iron deficiency. Thus, the iron status information group represents a mixture of subjects who took different actions for different periods of time during the study. The positive effects on iron status in subjects randomized to the iron status information group demonstrate that donors are interested and capable of modifying their behavior to prevent iron deficiency when provided accurate information about their iron status. These findings are similar to those of O’Meara and coworkers,20 who also suggested that donors will voluntarily take actions to prevent iron deficiency when provided with their ferritin value.
There are now ample data available to guide blood center management in designing programs to mitigate donor iron deficiency. Important findings from this study are that 19 mg of iron daily is as effective as 38 mg and that adverse events were no more common in the placebo group than in iron pill groups. These data suggest that perhaps the simplest program involves educating successful whole blood donors about the value in taking a once daily multiple vitamin with 18 to 19 mg of iron for 60 to 90 days after each whole blood donation. A more comprehensive program for frequent donors might involve measuring ferritin after each donation, providing the result to the donor, and providing pills containing 19 mg of iron for those with low ferritin. Whether ferritin of not more than 26 ng/mL is the optimal cutoff value is uncertain as another recent study found that iron supplementation benefits donors with ferritin of up to 50 ng/mL.2 Irrespective of providing iron supplements, providing donors with ferritin status along with appropriate messaging regarding their donation appears to be an important operational component of a comprehensive donor iron management program, since it provides donors with an indicator of their iron status and their individual need for iron supplements.
A potential limitation to these results is the large number of subjects not completing the 2-year follow-up. Overall, only 57% (393 of 692) of randomized subjects completed a final visit, with statistical differences across treatment arms. Subjects in pill groups deenrolled over five times more frequently than those in the letter groups. This was recognized in analyses examining the first 60 days after enrollment18 and continued throughout the study. Approximately 20% of the pill group deenrollments were from adverse events, which occurred statistically equally in subjects receiving iron or placebo, indicating that 19 or 38 mg of iron daily is well tolerated by blood donors, as previously reported.16 Cohort studies, including randomized trials, are potentially subject to selection bias if loss to follow-up is associated with both the treatment and the outcome(s). In the STRIDE study, while there were large differences in completion across the five arms, comparisons within the letter and pill groups separately are most appropriate. A mean of 73% (201/276) of donors receiving the iron status or the control letter completed the study, with no difference between groups. Across the three pill groups, only 46% (192/416) completed the study. Available evidence, however, is against the likelihood of significant bias due to loss to follow-up. Many of the subjects requesting withdrawal simply appeared to not want to take pills under the study conditions. Subjects receiving pills were not informed of their iron status and did not know what type of pill they were taking. Consequently, some subjects deenrolled because they thought they were taking a placebo pill and wanted to take iron. Thus, it is possible that deenrollments from the pill groups overestimate the percentage of donors who will not take pills in an operational setting. However, the donors for STRIDE were frequent blood donors and may have been more compliant with iron recommendations than other donors. In either case, better compliance is anticipated when the donor knows the iron pill content and their ferritin value.
Despite remote3,4 and more recent studies15–17 demonstrating that blood donation causes iron deficiency, and that this outcome can be mitigated by use of oral iron supplements, few blood-collecting organizations in the United States have implemented iron replacement programs. One concern relates to the possibility of providing iron to donors with undiagnosed hemochromatosis.21 However, this can be avoided by an initial ferritin test or by educating donors with a family history of hemochromatosis to speak with their personal physician before taking iron supplements. There are also concerns that iron supplementation may delay the recognition and diagnosis of occult gastrointestinal bleeding.21 However, iron supplementation typically only replaces iron lost from blood donation17 and, therefore, should not delay these diagnoses beyond that if donation had not occurred. In addition, blood centers are part of the health care system and should encourage all donors over 50 years old to have a colonoscopy as recommended by the US Preventive Services Task Force.22 In sum, while the historical justifications for not acting are based on legitimate concerns, these issues are not insuperable and can be addressed with appropriate coordination and donor education.
Key findings from the present study are: 1) once daily 19-mg iron pills are as effective as once daily 38-mg iron pills given for 60 days to mitigate iron deficiency in blood donors, 2) providing accurate information about iron status through measurement of ferritin and allowing the donor to choose whether to take iron supplements or delay donation is an effective method for mitigating iron deficiency in blood donors, 3) interventions to mitigate iron deficiency also improve donor Hb status in the context of frequent donation, and 4) frequent donors who do not take actions to prevent iron deficiency become progressively more iron deficient with continued donation. Therefore, directly providing iron pills to donors or providing iron status information in the form of a ferritin test result are operationally effective means that will mitigate iron deficiency in blood donors.
Supplementary Material
Percent of subjects with iron deficiency defined by different laboratory cut-offs at enrollment and final visits.
Acknowledgments
This work was supported by NHLBI grant 1R01HL105809.
ABBREVIATIONS
- ID(s)
identification number(s)
- sTfR
soluble transferrin receptor
Footnotes
ClinicalTrials.gov Identifier: NCT02245321.
CONFLICT OF INTEREST
AEM has received honoraria from Novo Nordisk and Portola and receives research grant funding from Novo Nordisk. The other authors have disclosed no conflicts of interest.
References
- 1.Mast AE, Lee TH, Schlumpf KS, et al. The impact of HFE mutations on haemoglobin and iron status in individuals experiencing repeated iron loss through blood donation. Br J Haematol. 2012;156:388–401. doi: 10.1111/j.1365-2141.2011.08952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–83. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245:2038–43. [PubMed] [Google Scholar]
- 4.Finch CA, Cook JD, Labbe RF, et al. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50:441–7. [PubMed] [Google Scholar]
- 5.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–22. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer BR, Kleinman S, Wright DJ, et al. Restless legs syndrome, pica, and iron status in blood donors. Transfusion. 2013;53:1645–52. doi: 10.1111/trf.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant BJ, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637–44. doi: 10.1111/trf.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutler E, Larsh SE, Gurney CW. Iron therapy in chronically fatigued, nonanemic women: a double-blind study. Ann Intern Med. 1960;52:378–94. doi: 10.7326/0003-4819-52-2-378. [DOI] [PubMed] [Google Scholar]
- 9.Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118:3222–7. doi: 10.1182/blood-2011-04-346304. [DOI] [PubMed] [Google Scholar]
- 10.Brownlie T, Utermohlen V, Hinton PS, et al. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–43. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 11.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–87. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- 12.Jahanshad N, Kohannim O, Hibar DP, et al. Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene. Proc Natl Acad Sci U S A. 2012;109:E851–9. doi: 10.1073/pnas.1105543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garry PJ, Koehler KM, Simon TL. Iron stores and iron absorption: effects of repeated blood donations. Am J Clin Nutr. 1995;62:611–20. doi: 10.1093/ajcn/62.3.611. [DOI] [PubMed] [Google Scholar]
- 14.Mast AE, Foster TM, Pinder HL, et al. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48:2197–204. doi: 10.1111/j.1537-2995.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 15.Simon TL, Hunt WC, Garry PJ. Iron supplementation for menstruating female blood donors. Transfusion. 1984;24:469–72. doi: 10.1046/j.1537-2995.1984.24685066802.x. [DOI] [PubMed] [Google Scholar]
- 16.Radtke H, Tegtmeier J, Röcker L, et al. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double-blind, placebo-controlled study. Transfusion. 2004;44:1427–32. doi: 10.1111/j.1537-2995.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 17.Bryant BJ, Yau YY, Arceo SM, et al. Iron replacement therapy in the routine management of blood donors. Transfusion. 2012;52:1566–75. doi: 10.1111/j.1537-2995.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialkowski W, Bryant BJ, Schlumpf KS, et al. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrollment and early retention. Vox Sang. 2015;108:178–85. doi: 10.1111/vox.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II donor iron status evaluation (RISE) study. Transfusion. 2012;52:702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Meara A, Infanti L, Stebler C, et al. The value of routine ferritin measurement in blood donors. Transfusion. 2011;51:2183–8. doi: 10.1111/j.1537-2995.2011.03148.x. [DOI] [PubMed] [Google Scholar]
- 21.Bianco C, Brittenham G, Gilcher RO, et al. Maintaining iron balance in women blood donors of childbearing age: summary of a workshop. Transfusion. 2002;42:798–805. doi: 10.1046/j.1537-2995.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent of subjects with iron deficiency defined by different laboratory cut-offs at enrollment and final visits.