Abstract
Axonal loss contributes to induction of diabetic peripheral neuropathy. Sildenafil, a phosphodiesterase type 5 inhibitor, ameliorates neurological dysfunction in diabetic peripheral neuropathy. However, the direct effect of high glucose and sildenafil on axonal growth has not been extensively investigated. Using rat primary dorsal root ganglia (DRG) neurons cultured in a microfluidic chamber, we investigated the effect of axonal application of high glucose and sildenafil on distal axonal growth. We found that axonal, but not cell body, application of high glucose locally inhibited distal axonal growth. However, axonal application of sildenafil overcame high glucose-reduced axonal growth. Quantitative real-time RT-PCR (qRT-PCR) and Western blot analysis of distal axonal samples revealed that high glucose reduced axonal miR-146a levels and substantially increased miR-146a target genes, IRAK1 and TRAF6 in the axon. In contrast, sildenafil significantly reversed high glucose-reduced miR-146a levels and high glucose-increased IRAK1 and TRAF6. Gain- and loss-of function of miR-146a in DRG neurons revealed that miR-146a mediated the local effect of high glucose on the distal axonal growth. These in vitro data provide new insights into molecular mechanisms of diabetic peripheral neuropathy.
Keywords: peripheral neuropathy, diabetes, axonal growth, sildenafil, miR-146a
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes and affects more than 50% of patients with a >25 year history of diabetes (Greene et al., 1999). Pathological changes of DPN include microangiopathy, segmental demyelination, and axonal loss (Yagihashi, 1995). Excessive glucose can be removed by insulin from blood and stored in muscle, fat and liver tissues. However, this mechanism appears to be ineffective in the nervous system. Neuronal glucose uptake depends on the extracellular glucose levels. Hyperglycemia results in a fourfold increase in glucose levels in neurons, consequently leading to nerve damage (Tomlinson and Gardiner, 2008).
The most common subtype of DPN is diabetic sensorimotor polyneuropathy (Peltier et al., 2014). Degeneration usually begins from the most distal parts of the axon of dorsal root ganglia (DRG) neurons, and atrophy of the axon develops slowly towards the neuronal body (Morfini et al., 2009). Dying-back of distal axonal terminals likely contributes to these manifestations (Raff et al., 2002). Molecular mechanisms underlying DPN have not been fully explicated, and there is a paucity of reports studying the direct effect of high glucose (HG) on axonal growth.
MicroRNAs (miRNAs) play a crucial role in neuronal development (Fineberg et al., 2009, Im and Kenny, 2012, McNeill and Van Vactor, 2012). Moreover, miRNAs regulate biological function of DRG neurons and are involved in development of DPN (Natera-Naranjo et al., 2010, Kantharidis et al., 2011). MiR-146a has been extensively studied in the fields of innate immunity, inflammation and cancer development (Labbaye and Testa, 2012). We recently demonstrated that hyperglycemia downregulated miR-146a in DRG neurons and upregulation of miR-146a expression suppressed neuronal apoptosis induced by hyperglycemia (Wang et al., 2014), indicating that miR-146a positively affects neuronal survival. However, to our knowledge, whether miR-146a directly regulates axonal growth under hyperglycemia conditions has not been investigated. Emerging data indicate that miRNAs are enriched in axons and that axonal miRNAs locally regulate metabolism and axonal growth (Kaplan et al., 2013). Thus, it is important to investigate whether hyperglycemia locally affects miRNA levels in distal axons of DRG neurons, which may provide potential molecular mechanisms for the development of DPN.
Sildenafil, a potent phosphodiesterase type 5 (PDE5) inhibitor, is clinically used for treatment of pulmonary arterial hypertension and erectile dysfunction (Goldstein et al., 1998, Galie et al., 2005). We have demonstrated that treatment of DPN in the mouse with sildenafil ameliorates neuropathy and that elevation of miR-146a in DRG neurons by sildenafil contributes to the therapeutic effect of sildenafil for DNP (Wang et al., 2014). Recently, we also showed that sildenafil locally regulates miRNA levels in distal axons of embryonic cortical neurons (Zhang et al., 2015a).
In the present study, using a microfluidic chamber which separates distal axons from their parent neuronal somas, we investigated the local effect of HG on distal axonal growth of DRG neurons. Our data demonstrated that axonal application of HG led to reduction of axonal miR-146a levels and suppression of axonal growth, which were overcome by axonal application of sildenafil.
EXPERIMENTAL PROCEDURES
All experimental procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Cell culture of primary DRG neurons and treatment
DRG neurons were collected from embryonic day 18 Wistar rats (either sex; Charles River Laboratories). Cultures were prepared according to a published protocol with minor modifications (Zhang et al., 2013). Briefly, embryos were removed, and the DRG was dissected and then transferred into neurobasal medium (Invitrogen) containing 0.05% trypsin digestion for 30min, DRG neurons were mechanically triturated with a Pasteur pipette for 15 times and then the cells were passed through a 70μm cell strainer and counted to obtain a concentration of 3×107cells/ml.
A microfluidic chamber (Standard Neuron Device, catalog #SND450, Xona Microfluidics) was used (Taylor et al., 2005) to separate axons from neuronal cell bodies. Sterilized chambers were affixed to poly-D-lysine-coated (Sigma-Aldrich) dishes (35 mm, Corning). The DRG neurons were plated at a density of 6×105cells/chamber in DMEM with 5% FBS. The cells were incubated for 4 h and then cultured in the medium of neurobasal (Invitrogen), 2% B-27 (Invitrogen), 2mM GlutaMax, and 1% antibiotic-antimycotic (Zhang et al., 2013). 5-fluorodeoxyuridine was added to the neurobasal medium to purify the neurons. On day in vitro (DIV) 3, the medium was replaced with non-5-fluorodeoxyuridine neurobasal medium. Subsequently, the growth media was changed every other day. The small microgrooves embedded in the chamber permit only distal axons to sprout from their parental cell bodies located in the cell body compartment into the distal axonal compartment on the other side of the microgrooves (Taylor et al., 2005) (Fig. 1A).
FIGURE 1. The effect of high glucose and sildenafil on axonal growth of DRG neurons.
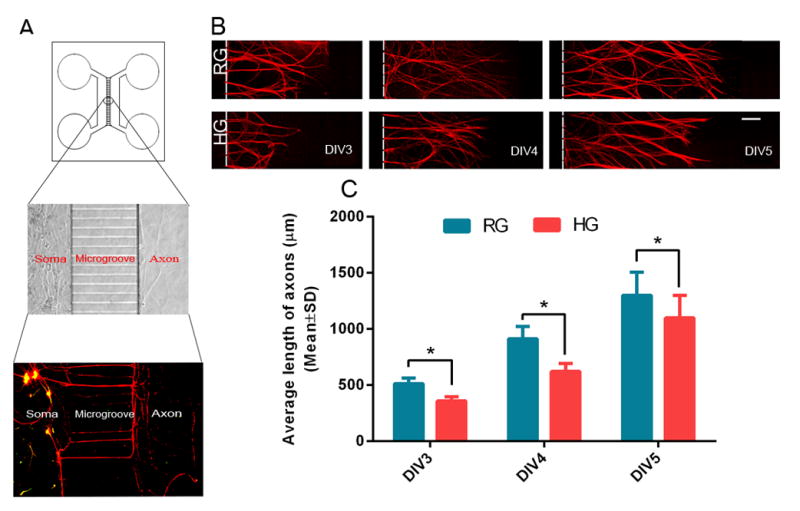
Panel A is a schematic of the microfluidic device and a representative fluorescent image showing the cell bodies and dendrites of DRG neurons cultured within the cell body compartment (soma) and distal axons within the axonal compartment that was separated from the cell body compartment by microgrooves. Panel B shows representative microscopic images of distal pNFH+ axons of DRG neurons DIV3 to DIV5 under regular (RG) and high glucose (HG) conditions. Regular and high glucose was applied to the cell body and axonal compartments. Panel C shows quantitative data of axonal growth under RG and HG conditions. n = 6 chambers/3 individual experiments/ group. * P< 0.05. Scale bars = 100μm.
To investigate the effect of HG on whole DRG neurons, we cultured DRG neurons under HG conditions. The HG medium was added to the cell body and distal axonal chambers on DIV 1. Additional sildenafil was applied to examine its effect on DRG neurons under HG conditions. We then examined the effect of HG and sildenafil on local axonal outgrowth. The HG and sildenafil were added to the cell body chamber or distal axonal chamber on DIV4 when all of the microgrooves were fully filled with axons, which blocks interchange of medium used in the present study between the cell body and distal axonal chambers (Taylor et al., 2009, Zhang et al., 2013).
In the present study, the regular glucose (RG) was defined as 25 mM that was present in neurobasal medium. This glucose concentration is optimal for the growth of primary DRG neurons, which does not affect osmotic pressure, and is commonly used for the in vitro hyperglycemia experiments (Russell et al., 1999, Cnop et al., 2005, Wang et al., 2014). HG was defined as 45 mM (Cnop et al., 2005, Wang et al., 2014).
Sildenafil treatment
To examine the effect of sildenafil on axonal growth, DRG neurons were cultured with sildenafil at 300 nM according to our published protocol (Wang et al., 2011). The cells were treated from the first day when the DMEM was replaced with neurobasal medium.
Transfection of DRG neurons by miR-146a mimics, and inhibitors and siRNAs against IRAK1 and TRAF6
To examine the effect of miR-146a on axonal growth, DRG neurons were transfected by miR-146a mimics, miR-146a hairpin inhibitor, and their corresponding controls (Dharmacon, Pittsburgh, PA, USA) by means of Nucleofector™ kit (Lonza, Walkersville, MD, USA). Briefly, miRNA mimics, inhibitors or negative control at 200 pmol/well were mixed with 100μl of Nucleofector solution. DRG neurons were added to transfection solution and then were transferred into a cuvette. The program O-03 was used for electroporation (Zhang et al., 2013). Using the same electroporation protocol, DRG neurons were also transfected by siRNAs against IRAK1 and TRAF6, or control siRNA-A (0.1 μM, Santa Cruz).
Isolation of total RNA
On DIV6, total RNA in the axonal or somal compartment was collected separately using the miRNeasy Mini kit (Qiagen) according to our published protocol (Zhang et al., 2015b). First, culture medium in the axonal compartment was removed and washed with PBS, and 20 μl/chamber of Qiazol reagent was applied to the axonal compartment for less than 1 min, and then was collected. Medium in the somal compartment was retained during axonal sample collection. After that, the somal samples were collected with fresh Qiazol reagent (20μl/chamber).
Quantitative Real-Time PCR (qRT-PCR) analysis
Generally, we acquired 1.5–2.0 μg of total RNA from the axonal or cell body samples. According to the manufacturer’s instructions, the reverse transcription was then performed using random hexamers and M-MLV reverse-transcriptase (Invitrogen). The qRT-PCR was performed according to published methods (Zhang et al., 2013, Wang et al., 2014). The SYBR green was used to measure mRNA levels by means of a ViiA7 Instrument (Applied Biosystems). Each sample was tested in triplicate, and at least three samples obtained from independent experiments were examined. The cDNA template of 1.5μl for each reaction was used. The program was used as follows: 2 min at 50°C, 10 min at 95 °C, and then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The mRNA of glyceraldehyde-3-phosphatedehydrogenase (GAPDH) was used as a reference. The primers were listed below: GAPDH (FWD, AGAACATCATCCCTGCATCC; REV, CACATTGGGGGTAGGAACAC), IRAK1 (FWD, GAGACCCTTGCTGGTCAGAG; REV, GCTACACCCACCCACAGAGT), TRAF6 (FWD, GCCCAGGCTGTTCATAAGT; REV, CGGATCTGATGGTCCTGTCT). Relative levels of mRNAs were calculated by means of the formula 2−ΔΔCT after normalizing Δ ΔCT values to a reference level of GAPDH mRNA (Natera-Naranjo et al., 2010).
QRT-PCR analysis of precursor and mature miR-146a
For precursor and mature miRNAs, qRT-PCR were performed on ABI 7000 and ABI ViiA 7 PCR instrument (Applied Biosystems) (Wang et al., 2014). Briefly, for the reverse transcription, fifteen μl of reverse transcription reactions were used, including 1–10 ng total RNA, 5U MultiScribe Reverse Transcriptase, 0.5 mM each of dNTPs, 1x reverse transcription buffer, 4U RNase inhibitor, and nuclease-free water. The running program was as follows: 16 °C for 30 min, 42 °C for 30 min, 85 °C for 5 min. For qRT-PCR, twenty μl qRT-PCR reactions were used, including 1x TaqMan Universal PCR Master Mix No AmpErase UNG, 1x TaqMan miRNA assay, 1.33μl of undiluted cDNA and nuclease-free water. The running program was: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Each sample was tested in triplicate, and at least three samples obtained from independent experiments were examined. The following hydrolysis miRNA primers were used: miR-18a (mature sequence: UAAGGUGCAUCUAGUGCAGAUAG); miR-19b (mature sequence: UGUGCAAAUCCAUGCAAAACUGA); miR-29b (mature sequence: UAGCACCAUUUGAAAUCAGUGUU); miR-139 (mature sequence: UCUACAGUGCACGUGUCU); miR-146a (mature sequence: UGAGAACUGAAUUCCAUGGGUU). Relative levels of miRNAs were calculated by means of the formula 2−ΔΔCT after normalizing Δ ΔCT values to a reference miRNA U6. Precursor-miR-146a (pre-miR-146a) measurement was performed with SYBR Green according to the manufacturer’s instruction. Briefly, miScript II RT Kit (Qiagen, Cat. # 218161) was used for reverse transcription of total RNA, miScript SYBR Green PCR Kit (Qiagen) and pre-miR-146a primer (Qiagen, Cat. # MP00000938) were used for detecting pre-miR-146a. The miScript PCR Control for RNU6-2 (Qiagen, Cat. #MS00000001) was used for normalization. CT values and melt curve were checked. The method of 2−ΔΔCT was used to calculate the relative levels.
Western blot analysis
On DIV6, total proteins in the cell body and axonal compartments were extracted according to published methods, in which we have demonstrated that proteins extracted from the axonal compartment were not contaminated by materials from the soma compartment (Zhang et al., 2013, Zhang et al., 2015a). Briefly, starting from the axonal compartment, culture medium within the axonal compartment was removed and washed with PBS, and then 10 μl of lysis buffer was applied and collected. Medium in the somal compartment was kept intact during axonal lysis to prevent lysis buffer in the axonal compartment flowing back to the cell body compartment, which minimizes cell body compartment contamination. After that, the sample within the somal compartment was collected. During the entire period, the chamber was placed horizontally on ice. Samples from 4 individual compartments were pooled for one Western blot. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology). Western blot was performed according to previously described methods (Zhang et al., 2013, Wang et al., 2014, Zhang et al., 2015a). Briefly, equal amounts of proteins were loaded. Primary antibodies used were rabbit anti-interleukin-1 receptor-associated kinase 1(IRAK1), rabbit anti-TNF receptor-associated factor 6 (TRAF6) (1:1000, Cell Signaling Technology), rabbit anti-Argonaute 2 (Ago2), and rabbit against Dicer (1:1000, Abcam). The optical density of protein bands was measured and calculated by means of Fluorchem E instrument (ProteinSimple). .
Immunofluorescent staining and axonal measurement
Immunofluorescent staining and axonal measurement were performed as previously described (Zhang et al., 2013, Wang et al., 2014). Briefly, a monoclonal antibody against phosphorylated neurofilament heavy protein (pNFH) (1:500; SMI31, Covance) was used. The length of the 15 longest axons in each chamber was measured using a microscopic computer imaging device (MCID) system. The axonal length was recorded for 3 days from DIV3 to DIV5, or from DIV4 to DIV6. To detect apoptotic cells, cultured DRG neurons were stained with the Apoptag fluorescein In Situ Detection Kit (Millipore) and were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) (1:10000) for detecting cell nuclei.
Statistical analysis
One-way ANOVA with post hoc Bonferroni test was used for multiple group analysis. Student’s test was used for two group comparisons. Values are presented as mean ± standard deviation (SD). A value of P< 0.05 was considered as significant.
RESULTS
High glucose in the distal axon locally suppresses axonal growth
To examine the effect of hyperglycemia on axons, the primary DRG neurons were cultured in a microfluidic chamber device that permits distal axons to grow into the axonal compartment (Taylor et al., 2005) (Fig. 1A). Using culture dishes, we previously demonstrated that hyperglycemia inhibits DRG axonal growth (Wang et al., 2015). Thus, we first examined whether we could reproduce our previous findings when DRG neurons are cultured in the microfluidic device. Glucose at 25 mM (RG) or 45 mM (HG) was added into both cell body and axonal compartments. Distal axonal length in the axonal compartment was measured daily for 3 days starting on DIV3. DRG neurons cultured under the HG condition exhibited significant (p<0.05) reduction of distal axonal length compared to DRG neurons cultured under the RG condition (Fig. 1B, C), which is consistent with our previous findings (Wang et al., 2015).
We then examined the local effect of HG on axonal growth by axonal application of HG. Addition of HG into the axonal compartment significantly (p<0.05) reduced distal axonal growth compared to axonal application of RG (Fig. 2A, B). In contrast, application of HG into the cell body compartment did not significantly reduce axonal growth (Fig. 2A, B).
FIGURE 2. The effect of axonal application of high glucose and sildenafil on axonal growth and neuronal apoptosis.
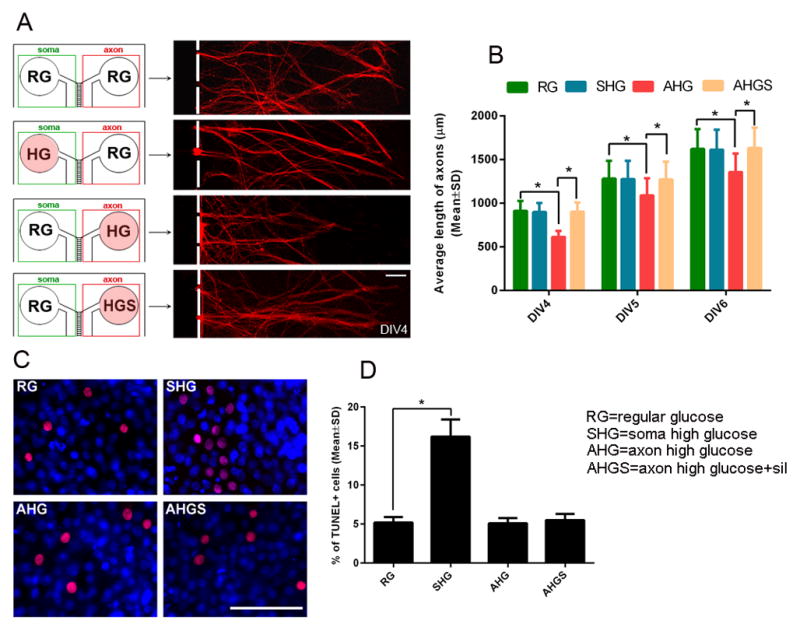
Panel A shows representative microscopic images of distal pNFH+ axons DIV6 under conditions of regular glucose (RG), high glucose (HG) in the cell body compartment (soma) or in the axonal compartment (axon) and high glucose along with sildenafil (HGS) in the axonal compartment (axon). A red circle in panel A indicates HG or HGS was applied. Panel B shows quantitative data of axonal length during 3 days under conditions of regular glucose (RG), high glucose in the soma compartment (SHG) or in the axonal compartment (AHG) and high glucose along with sildenafil in the axonal compartment (AHGS). Panel C shows representative microscopic images of TUNEL+ cells (red) under conditions of regular glucose in both soma and axonal compartment (RG), high glucose in the soma compartment (SHG), or high glucose (AHG) and high glucose with sildenafil in the axonal compartment (C, AHGS). Panel D shows quantitative data of TUNEL+ cells under these conditions. n = 6 chambers/3 individual experiments/group. * P< 0.05. Scale bar = 100μm.
To examine whether axonal application of HG induces neuronal death, leading to reduction of axonal growth, the number of TUNEL positive cells were measured. Interestingly, axonal application of HG did not significantly increase the number of TUNEL positive cells compared to axonal application of RG (Fig. 2C, D). However, addition of HG into the cell body compartment significantly (P<0.05) increased the number of TUNEL positive cells (Fig. 2C, D). These data suggest that axonal application of HG does not induce neuronal death, but locally regulates distal axonal growth.
Reduction of axonal miR-146a levels by local HG mediates HG-inhibited axonal growth
We previously demonstrated that hyperglycemia suppresses miR-146a expression in DRG neurons (Wang et al., 2014). In the present study, we found that when HG was added into both the cell body and axonal chambers of cultured DRG neurons, it suppressed expression of miR-146a in DRG neurons (0.6 ± 0.05, n=6, p<0.05) compared to RG (1 ± 0.07, n=6), which confirms our previous findings (Wang et al., 2014). Axons contain abundant miRNAs (Jung et al., 2012). To examine the local effect of HG on miR-146a levels, HG was applied into the cell body or axonal compartments, respectively. RNA samples were individually collected from the cell body or axonal compartments. QRT-PCR analysis revealed that axonal application of HG significantly reduced axonal miR-146a levels compared to axonal application of RG, whereas axonal application of HG did not alter miR-146a levels in the samples obtained from the cell body compartment (Fig. 3A). Moreover, axonal application of HG did not significantly change axonal levels of other miRNAs known to regulate axonal growth: miR-18a (1.08 ± 0.10 vs 1 ± 0.07 in RG, n=6), miR-19b (0.92 ± 0.06 vs 1 ± 0.09 in RG, n=6), miR-29b (0.95 ± 0.11 vs 1 ± 0.08 in RG, n=6), and miR-139(0.98 ± 0.12 vs 1 ± 0.09 in RG, n=6). Reduction of axonal miR-146a levels by axonal application of HG was associated significant elevations of axonal IRAK1 and TRAF6 mRNAs (Fig. 3C, D) and proteins (Fig. 3E, F) that are validated genes targeted by miR-146a. Axonal application of HG did not change IRAK1 and TRAF6 expression in cell body samples (Fig. 3C to F). In contrast, application of HG into the cell body compartment significantly reduced miR-146a levels in the somal samples, but did not significantly alter miR-146a levels in axonal samples (Fig. 3B). The role of miR-146a in mediating axonal growth has not been investigated. We thus examined whether gain- and loss-of function of miR-146a affects axonal growth by transfecting DRG neurons with miR-146a mimics and inhibitors, respectively. Due to technical challenges to locally transfect axons, gain- and loss-of function experiments were performed in transfected DRG neurons. We seeded transfected DRG neurons into the microfluidic device and HG was applied into both the cell body and axonal chambers. RNA and protein samples were collected on DIV6. QRT-PCR analysis showed that the transfection of DRG neurons with miR-146a mimics significantly (p<0.05) increased the miR-146a levels (Fig. 4A) and reduced the mRNA levels of IRAK1 (Fig.4B) and TRAF6 (Fig. 4C) compared to the miR-146a mimic control. In contrast, the transfection of the neurons with miR-146a inhibitor reduced the miR-146a levels (Fig. 4A) and increased the mRNA levels of IRAK1 (Fig. 4B) and TRAF6 (Fig. 4C) compared to the miR-146a inhibitor control. Western blot analysis showed transfection of DRG neurons with miR-146a mimics and inhibitors reduced and increased, respectively, protein levels of IRAK1 (Fig. 4D, E) and TRAF6 (Fig. 4D, F).
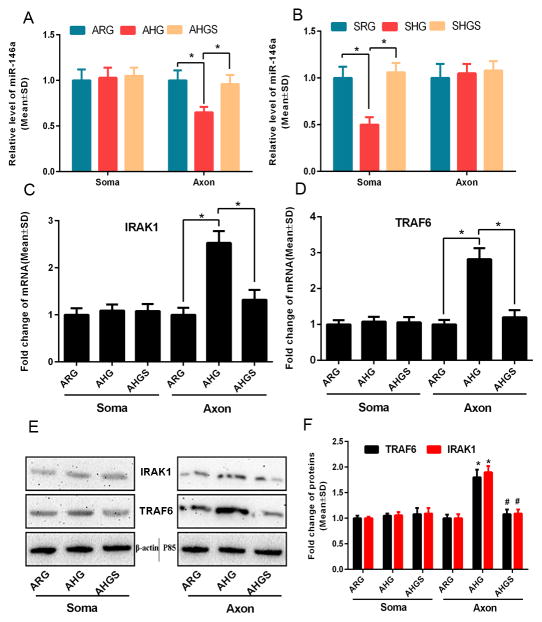
FIGURE 3. The effect of high glucose and sildenafil on levels of miR-146a, IRAK1 and TRAF6 in DRG neurons.
Panels A to D are qRT-PCR data and panels E and F are Western blot data. Panels A and B show soma and axonal miR-146a levels when high glucose or high glucose along with sildenafil were added into the axonal (A) or soma (B) compartment, respectively, compared to regular glucose condition. Panels C and D show soma and axonal mRNA levels of IRAK1 (C) and TRAF6 (D) when high glucose (AHG) or high glucose along with sildenafil (AHGS) were applied into the axonal compartment. Panels E and F show representative Western blots of soma and axonal protein levels of IRAK1 and TRAF6 (E) and their quantitative data (F) when high glucose (AHG) or high glucose along with sildenafil (AHGS) were added into the axonal compartment. n=6 chambers/3 individual experiments/group for panels A to D and n=4 chambers/3 individual experiments/group for Western blot data (F). * P< 0.05 versus ARG. # P< 0.05 versus AHG. ARG=axonal regular glucose. AHG=axonal regular glucose. AHGS=axonal high glucose + sildenafil. SRG=soma regular glucose. SHG=soma high glucose. SHGS=soma high glucose + sildenafil.
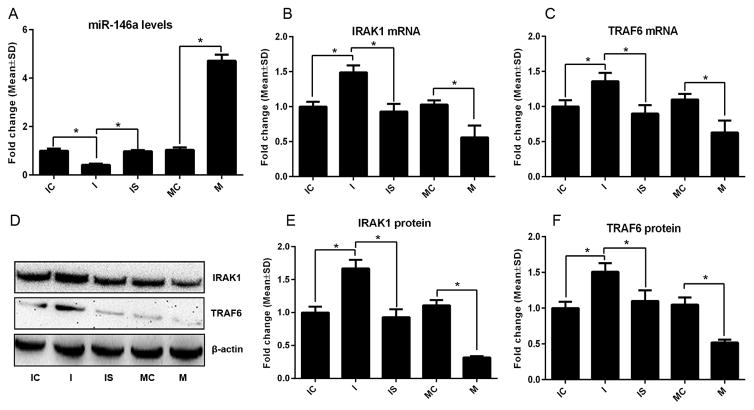
FIGURE 4. The effect of gain- and loss-of function of miR-146a on expression of IRAK1 and TRAF6 in DRG neurons under high glucose and high glucose along with sildenafil conditions.
Panels A to C and D to F are qRT-PCR and Western blot data, respectively. Panels A to C show levels of miR-146a (A), IRAK1 mRNA (B), and TRAF6 mRNA (C) in DRG neurons transfected with the miR-146a inhibitor (I), inhibitor control (IC), miR-146a mimics (M) and mimic control (MC) as well as when sildenafil was applied to DRG neurons transfected with the miR-146a inhibitor (IS). Panels D to F show representative Western blots (D) and quantitative data of protein levels of IRAK1 (D, E) and TRAF6 (D, F) under above listed conditions. n=6 chambers/3 individual experiments/group for qRT-PCR data and n=4 chambers/3 individual experiments/group for Western blot data. * P< 0.05.
We then examined whether alteration of miR-146a levels affects axonal growth under HG conditions by measuring axonal length for 3 days on DIV3, DIV4 and DIV5. DRG neurons transfected by the miR-146a mimics significantly increased the axonal length compared to the neurons transfected by the miR-146a mimic negative control (Fig. 5A, B). When transfected by the miR-146a inhibitor, axonal length was significantly reduced compared to miR-146a inhibitor negative control (Fig. 5A, B). Moreover, we attenuated IRAK1 and TRAF6 expression in DRG neurons by means of the siRNA approach (Fig. 6A to C) and then cultured these DRG neurons under HG condition (both soma and axonal compartments). We found that downregulation of either IRAK1 or TRAF6 overcame the inhibitory effect of HG on axonal growth (Fig. 6D). These data indicate that miR-146a, IRAK1 and TRAF6 regulate axonal growth under HG condition.
FIGURE 5. The effects of gain- and loss-of-function of miR-146a on axonal growth under high glucose and high glucose along with sildenafil conditions.
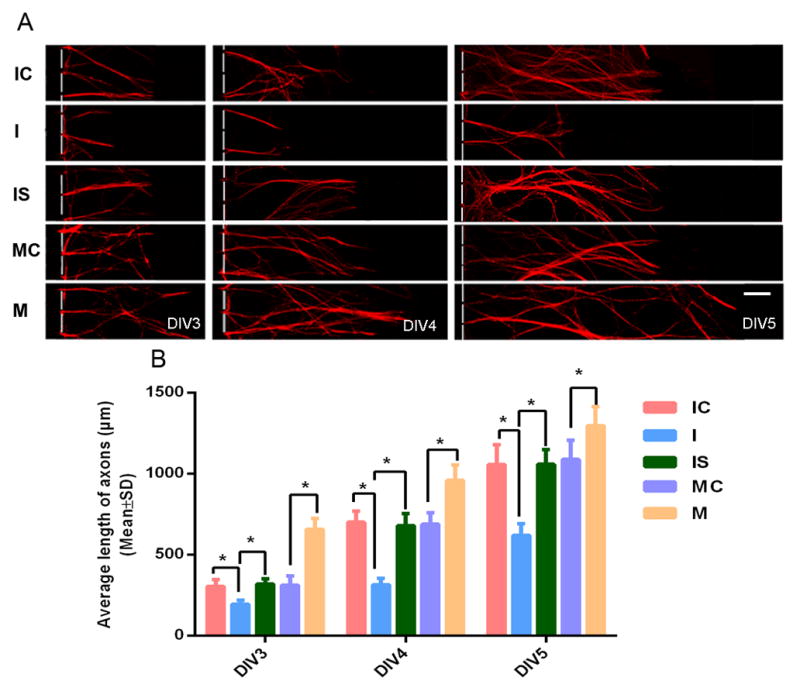
Panel A shows representative microscopic images of distal pNFH+ axons of DRG neurons transfected with the miR-146a inhibitor (I), inhibitor control (IC), miR-146a inhibitor + sildenafil (IS), miR-146a mimics (M) and mimic control (MC) during DIV3 to DIV5. Panel B shows quantitative data of distal axon length in DRG neurons under above listed conditions. n = 6 chambers/3 individual experiments /group.* P< 0.05. Scale bars = 100μm.
FIGURE 6. The effects of siRNAs of IRAK1 and TRAF6 on axonal growth under high glucose conditions.
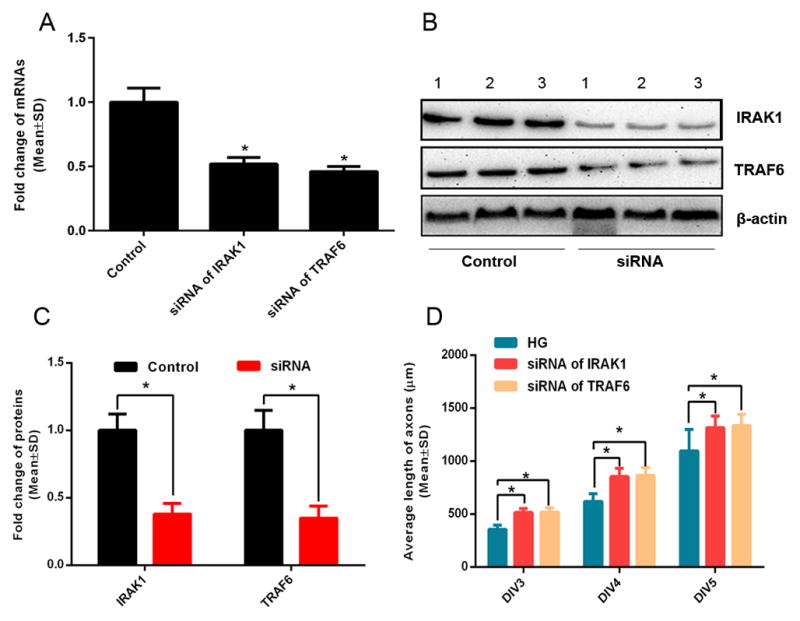
Panels A to C show qRT-PCR (A) and Western blot (B, C) data of IRAK1 and TRAF6 mRNA (A) and protein (B, C) levels in DRG neurons transfected siRNAs against IRAK1 or TRAF6 under high glucose conditions. Numbers in representative images (B) represent individual axonal samples. Panel D shows quantitative data of axonal length of DRG neurons transfected by siRNAs against IRAK1 or TRAF6 during 3 days in culture under high glucose conditions. Numbers in panel B represents individual experiment samples. n=6 chambers/3 individual experiments /group.* P< 0.05.
Axonal application of sildenafil overcomes the HG-inhibited axonal growth
Sildenafil exerts the therapeutic effect on diabetic peripheral neuropathy (Wang et al., 2014). To examine whether sildenafil can locally overcome the inhibitory effect of HG on axonal growth, sildenafil was added into the axonal compartment in the presence of HG. Axonal application of sildenafil overcame the inhibitory effect on axonal growth induced by axonal application of HG (Fig. 2A, B). In addition, axonal application of sildenafil reversed HG-reduced miR-146a levels (Fig. 3A) and their target gene, IRAK1 (Fig. 3C, E, F) and TRAF6 (Fig. 3D, E, F) mRNAs (Fig. 3C, D) and proteins (Fig. 3E, F) in the distal axons.
To examine whether sildenafil can rescue the inhibitory effect of miR-146a downregulation on axonal growth, DRG neurons transfected by siRNA against miR-146a were treated with sildenafil. QRT-PCR and Western blot analysis showed that sildenafil treatment restored levels of miR-146a in DRG neurons transfected by siRNA-miR-146a to levels in the neurons transfected by scramble siRNAs (Fig. 4A) and eliminated the effect of reduced-miR-146a on IRAK1 (Fig. 4B, D, E) and TRAF6 (Fig. 4C, D, F). In addition, sildenafil abolished the inhibitory effect of siRNA-miR-146a on axonal growth (Fig. 5A, B).
HG and sildenafil affect Ago2 levels in the distal axon
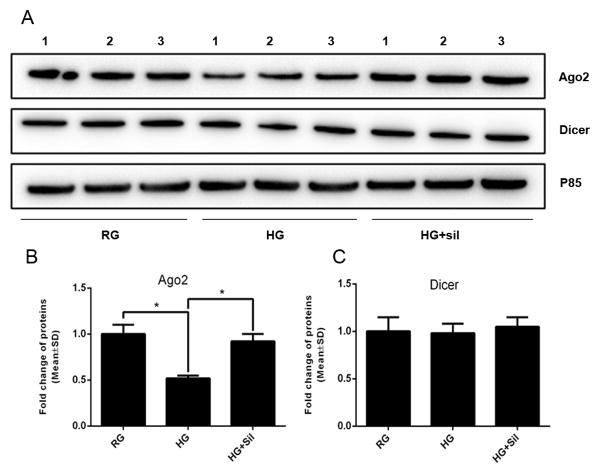
To examine whether miRNA biogenesis is involved in reduction and augmentation of miR-146a in distal axons by HG and sildenafil, respectively, the pre-miR-146a in the distal axons was measured. QRT-PCR analysis showed that HG and sildenafil did not alter levels of pre-miR146a (HG=1.08 ± 0.12, sildenafil=1.05 ± 0.11, vs control=1.00 ± 0.08, n=6/group, p>0.05). Axons locally contain miRNA biogenesis proteins, Dicer and Ago2 (Hengst et al., 2006, Aschrafi et al., 2008, Zhang et al., 2013). Western blot analysis of axonal samples confirmed the presence of these two proteins in the RG group (Fig. 7A to C). HG markedly reduced Ago2 levels, which was reversed by sildenafil (Fig. 7A, B); however, HG and sildenafil did not affect Dicer levels (Fig. 7A, C). Ago2 has been shown to regulate miR-146a stability (Srivastava et al., 2015). These data, thus, suggest that changes of axonal Ago2 levels induced by HG and sildenafil may affect the observed alteration of mature miR-146a in the distal axons.
FIGURE 7. The effects of axonal application of high glucose on Ago2 and Dicer.
Panels A to C show representative Western blots (A) and quantitative data of axonal Ago2 (B) and Dicer (C) proteins under regular glucose (RG), high glucose (HG), and high glucose along with sildenafil (HG+sil). Numbers in representative images (A) represent individual axonal samples. n=6 chambers/3 individual experiments /group.* P< 0.05. Axonal loss contributes to introduction of diabetic peripheral neuropathy. MiR-146a mediated the local effect of high glucose on the distal axonal growth. Distal axons are sensitive to local high glucose stress. Our findings provide a target for treatment of diabetic peripheral neuropathy.
DISCUSSION
In the present study, we demonstrated that HG locally inhibits distal axonal growth of DRG neurons, which can be overcome by axonal application of sildenafil. Moreover, we found that miR-146a, an important modulator in autoimmunity and innate immune response, mediates axonal growth under HG and sildenafil conditions. Thus, this in vitro study provides new insights into molecular mechanisms of DPN.
Axons are vulnerable to several insults including HG (Raff et al., 2002, Wang et al., 2012, Biessels et al., 2014, Wang et al., 2015). However, the direct effect of HG on axons of DRG neurons has not been extensively investigated. Using a microfluidic chamber which separates axons from their parental neuronal somata, we found that HG locally blocked distal axon growth of DRG neurons and that an elevation of distal axonal glucose levels did not induce cell apoptosis, suggesting that the process of HG-inhibited axonal growth is independent of cell death of their parental cell bodies. Our data further confirm that axons of DRG neurons are vulnerable to HG stress, which may contribute to axonal “die-back” observed in DPN (Raff et al., 2002). Our recently published data showed that elevation of axonal cGMP levels by sildenafil enhances axonal growth of embryonic cortical neurons (Zhang et al., 2015a). Sildenafil ameliorates peripheral neuropathy in a mouse model of type II diabetes (Wang et al., 2015). The present study shows that sildenafil locally promotes axonal growth of DRG neurons under HG conditions. Thus, in addition to cortical neurons, sildenafil can locally regulate distal axonal elongation of DRG neurons.
MiR-146a regulates the innate immune system through down-regulation of IRAK1 and TRAF6 genes (Taganov et al., 2006). MiR-146a plays a role in pathophysiological complications of diabetes. Reduction of miR-146a exacerbates diabetic wound-healing by increasing expression of its proinflammatory target genes (Xu et al., 2012). MiR-146a also contributes to the transcriptional regulation of the extracellular matrix protein fibronectin which is involved in development of diabetic retinopathy (Feng et al., 2011). We previously demonstrated that HG suppresses the expression of miR-146a and increases DRG neuron apoptosis (Wang et al., 2014). However, it is not known whether miR-146a regulates axonal growth. Axons contain abundant miRNAs, and axonal miRNAs locally regulate energy metabolism in the axon (Kaplan et al., 2013). The present study demonstrated that HG locally reduced miR-146a levels in the distal axons of DRG neurons. The effect of HG on axonal miR-146a appears specific because axonal HG did not significantly alter several other miRNAs that have been shown to be present in the axon (Zhang et al., 2013, Li et al., 2014). Our gain- and loss-of function data indicate that miR-146a and its target genes, IRAK1 and TRAF6, regulate axonal growth, and that elevated-miR-146a and reduced-IRAK1 and TRAF6 suppress HG-inhibited axonal growth. In addition to translational repression, miRNAs destabilize target mRNAs (Iwakawa and Tomari, 2015). Studies by others have shown that miR-146a regulates posttranscriptional gene expression of IRAK1 and TRAF6 either on mRNAs (Nahid et al., 2009, Xu et al., 2012, Jiang et al., 2014) or on proteins (Taganov et al., 2006, Ho et al., 2014). Our data suggest that miR-146a in the DRG neurons affects IRAK1 and TRAF6 genes at mRNA and protein levels. Our novel findings indicate that that miR-146a and its target genes, IRAK1 and TRAF6, may play multiple roles in the process of DPN.
The present study showed that HG and sildenafil did not alter levels of pre-miRNA 146a and Dicer protein levels in the distal axons of DRG neurons. In cytoplasm, Dicer, an RNase enzyme, cleaves pre-miRNAs to produce the 22-nt mature miRNA (Arroyo et al., 2011). Thus, our data suggest that maturation processes of miR-146a may not be involved in the observed alteration of mature miR-146a in the distal axons. Recent studies have shown that Ago2, one of proteins in RNA-induced silencing complex (RISC), binds to matured miRNAs including miR-146a, which leads to an increase of miRNA stability (Arroyo et al., 2011, Srivastava et al., 2015). Our data showed that HG and sildenafil reduced and increased, respectively, Ago2 protein levels in the distal axons, which was associated with levels of miR-146a. Thus, we speculate that Ago2-related miRNA stability may contribute to the observed alteration of miR-146a in the distal axons. Additional experiments are warranted to validate this possibility.
Axonal loss contributes to introduction of diabetic peripheral neuropathy.
MiR-146a mediated the local effect of high glucose on the distal axonal growth.
Distal axons are sensitive to local high glucose stress.
Our findings provide a target for treatment of diabetic peripheral neuropathy.
Acknowledgments
This work was supported by NINDS grants RO1 NS075084 (LW) and RO1 NS075156 (ZGZ), R01 NS 088656 (MC), and NIDDK RO1 DK097519 (LW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12581–12590. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Bril V, Calcutt NA, Cameron NE, Cotter MA, Dobrowsky R, Feldman EL, Fernyhough P, Jakobsen J, Malik RA, Mizisin AP, Oates PJ, Obrosova IG, Pop-Busui R, Russell JW, Sima AA, Stevens MJ, Schmidt RE, Tesfaye S, Veves A, Vinik AI, Wright DE, Yagihashi S, Yorek MA, Ziegler D, Zochodne DW. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab) Journal of the peripheral nervous system : JPNS. 2014;19:77–87. doi: 10.1111/jns5.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, Feldman RD, Chakrabarti S. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G Sildenafil Use in Pulmonary Arterial Hypertension Study G. Sildenafil citrate therapy for pulmonary arterial hypertension. The New England journal of medicine. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. The New England journal of medicine. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. The American journal of medicine. 1999;107:2S–8S. doi: 10.1016/s0002-9343(99)00007-8. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends in neurosciences. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nature reviews Neuroscience. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BB, Kar AN, Gioio AE, Aschrafi A. MicroRNAs in the axon and presynaptic nerve terminal. Frontiers in cellular neuroscience. 2013;7:126. doi: 10.3389/fncel.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbaye C, Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. Journal of hematology & oncology. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mao S, Wang H, Zen K, Zhang C, Li L. MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein & cell. 2014;5:160–169. doi: 10.1007/s13238-014-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill E, Van Vactor D. MicroRNAs shape the neuronal landscape. Neuron. 2012;75:363–379. doi: 10.1016/j.neuron.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natera-Naranjo O, Aschrafi A, Gioio AE, Kaplan BB. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. Rna. 2010;16:1516–1529. doi: 10.1261/rna.1833310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. Bmj. 2014;348:g1799. doi: 10.1136/bmj.g1799. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiology of disease. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Duan G, Kershaw NJ, Athanasopoulos V, Yeo JH, Ose T, Hu D, Brown SH, Jergic S, Patel HR, Pratama A, Richards S, Verma A, Jones EY, Heissmeyer V, Preiss T, Dixon NE, Chong MM, Babon JJ, Vinuesa CG. Roquin binds microRNA-146a and Argonaute2 to regulate microRNA homeostasis. Nature communications. 2015;6:6253. doi: 10.1038/ncomms7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in uninjured and regenerating cortical mammalian axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nature reviews Neuroscience. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. The Journal of cell biology. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Jia L, Lu X, Szalad A, Zhang Y, Zhang R, Zhang ZG. Therapeutic Benefit of Extended Thymosin beta4 Treatment Is Independent of Blood Glucose Level in Mice with Diabetic Peripheral Neuropathy. Journal of diabetes research. 2015;2015:173656. doi: 10.1155/2015/173656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Szalad A, Liu Z, Bolz M, Alvarez FM, Lu M, Zhang L, Cui Y, Zhang RL, Zhang ZG. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience. 2011;193:399–410. doi: 10.1016/j.neuroscience.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Szalad A, Zhang Y, Wang X, Zhang RL, Liu XS, Jia L, Zhang ZG. The role of miR-146a in dorsal root ganglia neurons of experimental diabetic peripheral neuropathy. Neuroscience. 2014;259:155–163. doi: 10.1016/j.neuroscience.2013.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, Liechty KW. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012;61:2906–2912. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagihashi S. Pathology and pathogenetic mechanisms of diabetic neuropathy. Diabetes/metabolism reviews. 1995;11:193–225. doi: 10.1002/dmr.5610110304. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Liu X, Kassis H, Wang X, Li C, An G, Gang Zhang Z. MicroRNAs in the axon locally mediate the effects of chondroitin sulfate proteoglycans and cGMP on axonal growth. Developmental neurobiology. 2015a doi: 10.1002/dneu.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Liu XS, Kassis H, Wang X, Li C, An G, Zhang ZG. MicroRNAs in the axon locally mediate the effects of chondroitin sulfate proteoglycans and cGMP on axonal growth. Dev Neurobiol. 2015b doi: 10.1002/dneu.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]