Abstract
Problem
Risk factors for preterm birth include placental abruption, giving rise to excessive decidual thrombin, and intrauterine bacterial infection. Human endometrial endothelial cells (HEECs) express Toll-like receptors (TLRs), and infection-derived agonists trigger HEECs to generate specific inflammatory responses. Since thrombin, in addition to inducing coagulation, can contribute to inflammation, its effect on HEEC inflammatory responses to the TLR4 agonist, bacterial lipopolysaccharide (LPS), was investigated.
Method of Study
HEECs were pretreated with or without thrombin or specific protease-activated receptor (PAR) agonists, followed by treatment with or without LPS. Supernatants were measured for cytokines and chemokines by ELISA and multiplex analysis.
Results
Thrombin significantly and synergistically augmented LPS-induced HEEC secretion of interleukin (IL)-6, IL-8, granulocyte colony stimulating factor (G-CSF), and growth regulated oncogene-alpha (GRO-α); and significantly augmented monocyte chemotactic protein (MCP)-1, tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF) secretion additively. Similar to thrombin, a PAR1 agonist synergistically augmented the LPS-induced HEEC secretion of inflammatory IL-6, IL-8, G-CSF, and GRO-α.
Conclusion
Thrombin, via PAR1 activation, synergistically augments LPS-induced HEEC production of chemokines involved in immune cell recruitment and survival, suggesting a mechanism by which intrauterine abruption and bacterial infection may together be associated with an aggravated uterine inflammatory response.
Keywords: Endothelial, Infection, Inflammation, Pregnancy, Thrombin
Introduction
Preterm birth affects 9.6% of US births 1 and is the leading cause of morbidity and mortality in newborns 2. Preterm infants have increased rates of respiratory illnesses, feeding difficulties, and neurodevelopmental issues. Every year, over $26 billion are spent in the US on healthcare costs related to preterm birth 3 with no significant decrease in mortality from 2000 to 2010 4. Our understanding of preterm birth is still evolving and our ability to prevent preterm birth remains limited. Therefore, a better understanding of the molecular basis of preterm birth is needed to predict and prevent this disorder.
Elucidation of the mechanism(s) underlying preterm birth is complicated by its heterogeneous antecedents including bacterial infection, placental abruption, cervical incompetence, and disparate risk factors including prior preterm birth, smoking, prior cervical surgery, and low pre-pregnancy weight 5. However, a likely common pathway leading to preterm birth is inflammation at the maternal-fetal interface 6–8. Since bacterial infection and abruption are both important risk factors for preterm birth 9, this study sought to determine the relationship between decidual infection and hemorrhage at the molecular level and how this might impact pregnancy outcome.
The convergence of placental abruption and bacterial infection is commonly seen in the setting of preterm premature rupture of membranes (PPROM) 10. In this clinical scenario, chronic placental abruption and indolent inflammation can lead to preterm labor. In many cases of PPROM, women do not display overt clinical signs of infection; confirmation of infection and inflammatory changes is often noted upon pathologic analysis of the placenta and fetal membranes. Similarly, the patient may not acutely hemorrhage, but may have small but persistent amounts of vaginal bleeding, suggesting a chronic placental abruption 9.
During placental abruption, the decidua is exposed to blood borne clotting factors to generate high levels of thrombin 11, which can act directly on the endothelium 12. Thrombin is the main effector protease of the coagulation cascade that converts fibrinogen into fibrin, facilitating clot formation. However, thrombin can mediate a number of inflammatory effects by binding to the protease-activated receptors (PARs), PAR1, PAR3 and PAR4, which stimulate endothelial cells and other cell types to express cytokines, chemokines and other inflammatory mediators 13, 14. Similarly, inflammation can influence the coagulation process 14.
Intrauterine bacterial infections can present as an ascending infection, a systemic infection originally stemming from a distant site and now circulating in the maternal bloodstream, or a descending infection from another intra-abdominal organ 8. For systemic infections, the endothelium is the first tissue exposed to invasive pathogens in the bloodstream. The endothelial response to infection or tissue injury is to become activated to recruit immune cells and express adhesion molecules that allow their extravasation into tissues for host defense 15. Thus, this study questioned the impact a systemic bacterial infection would have on the uterine endothelium in the context of excess thrombin production arising from abruption.
We previously demonstrated that human endometrial endothelial cells (HEECs) express Toll-like receptors (TLRs) and following their activation by infection-derived agonists mediate specific innate immune inflammatory responses 16, 17. When HEECs, expressing TLR4 18, are activated by bacterial LPS, they secrete elevated levels of inflammatory cytokines and chemokines 17.
Given that thrombin is a critical component of the inflammatory and coagulation cascades, and that HEECs express a specific inflammatory profile in response to bacterial LPS 17, we hypothesized that thrombin, as itself a mediator of inflammation, may act to strengthen this pro-inflammatory response of HEECs to bacterial stimulation by modulating the TLR4 pathway through PAR activation.
Materials and Methods
Human Endometrial Endothelial Cell (HEEC) Culture and Treatments
The HEECs used in this study were isolated, immortalized, and characterized as previously described 18–20. Previous studies have demonstrated that these cells express the same factors and cytokines as the intact physiological endothelium 19, 20. Cell culture was performed as previously described 16, 20. For treatment experiments, HEECs were plated in 60mm tissue culture plates coated with 2% gelatin and grown until 70% confluent. Media was then replaced with serum-free OptiMEM (Gibco-Invitrogen; Grand Island, NY) with or without physiological concentrations of thrombin (Sigma-Aldrich, St. Louis, MO; 0.25 U/ml) and incubated for 1 hr. Without changing the media, cells were then treated with or without LPS (isolated from Escherichia coli 0111:B4; Sigma-Aldrich) at 1μg/ml, and the cells incubated for an additional 24 or 48 hrs. In subsequent treatment experiments, in place of thrombin, HEECs were treated with either a specific PAR1 agonist (TFLLR-NH2); a specific PAR2 agonist (SLIGKV-NH2); or a specific PAR4 agonist (AYPGKF-NH2) at 1mM (Sigma-Aldrich). After each time point, cell-free culture supernatants were collected and stored at −80°C. Cells were lysed for either RNA or protein isolation.
Cytokine and Chemokine Analysis
Supernatants were measured for IL-8 by ELISA (Assay Designs/Enzo Life Sciences; Farmingdale, NY). Supernatants were also measured for levels of interleukin (IL) 1β, IL-6, IL-10, IL-12, IL-17, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFNγ), Monocyte chemotactic protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1a (MIP-1α/CCL3), MIP-1β (CCL4), regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), tumor necrosis factor alpha (TNFα), vascular endothelial growth factor (VEGF), growth regulated oncogene-alpha (GRO-α, and interferon gamma-induced protein 10RO-(IP-10/CXCL10) by multiplex analysis (BioRad; Hercules, CA) as previously described 21.
Quantitative Real Time RT-PCR
Quantitative RT-PCR was performed as previously described 22, 23. Briefly, total RNA was extracted from cells, isolated, and reverse transcribed, after which quantitative real-time PCR was performed for TLR4 (Forward: 5′-CAGAGTTTCCTGCAATGGATCA-3′; Reverse: 5′-GCTTATCTGAAGGTGTTGCACAT-3′); PAR1 (Forward: 5′-AGTAGGCTATTCCTGAGAGCTGCAT-3′; Reverse: 5′-ATGGCCCTGGCATGTGTCT-3′); PAR2 (Forward: 5′-GGCAGGTGAGAGGCTGACTT-3′; Reverse: 5′-CGCCACCGATTCGAAACT-3′); PAR3 (Forward: 5′-TTGTGATTTTTACCATTTGCTTTG-3′; Reverse: 5′-TTTGTAAGGTAAGCAGTGGAGTGA-3′); PAR4 (Forward: 5′-GGGTCCCTTCCCCCACTT-3′; Reverse: 5′-GACAGTTGTAACAACCCTATTTCCAAA-3′); and GAPDH (Forward: 5′-CAGCCTCCCGCTTCGCTCTC-3′; Reverse: 5′-CCAGGCGCCCAATACGACCA-3′). Cycle threshold (CT) values were normalized to GAPDH and then presented as either relative abundance or fold change relative to the no treatment (NT) control.
Western Blot Analysis
HEEC protein was isolated and analyzed by Western blot as previously described 24. Membranes were probed for SOCS1 and SOCS3 using antibodies from Cell Signaling (Danvers, MA; Cat #3950 (1:500) and #2923 (1:1000)). Hsp90 (H-114; Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:1000 dilution as internal control to validate the amount of protein loaded onto the gels. Images were recorded and semi-quantitative densitometry performed using the Gel Logic 100 system and Carestream software (Carestream Molecular Imaging, Woodbridge, CT).
Statistical Analysis
Experiments were performed at least three times and data presented as mean ± SEM. Statistical significance (p<0.05) was determined by analysis of variance (ANOVA) followed by Bonferroni correction for multiple comparisons using Prism software (Graphpad Software Inc, La Jolla, CA).
Results
Thrombin augments LPS-induced HEEC inflammation
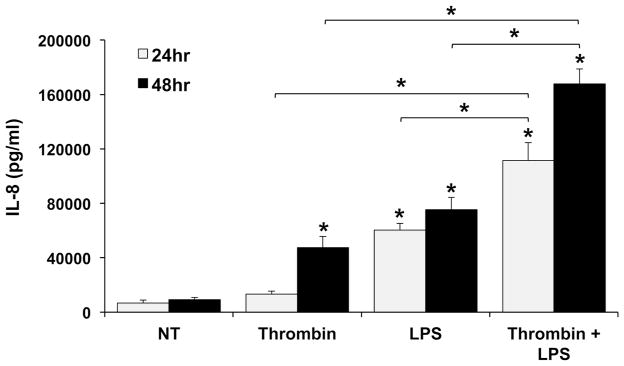
We previously demonstrated that treatment of HEECs with LPS induces the secretion of inflammatory IL-8, IL-6 and G-CSF 17. In this study, we sought to expand our knowledge of HEEC-derived factors regulated by LPS and to investigate if this response was modified when cells were pre-treated with thrombin. We first evaluated IL-8 secretion after 24 and 48hrs. As shown in Figure 1, after 24 hours, IL-8 secretion was not significantly elevated in HEECs treated with thrombin alone compared to the no treatment (NT) control. However, at the same time point, IL-8 levels were significantly increased in HEECs treated with LPS alone compared to the NT control. Furthermore, HEECs treated with both thrombin and LPS exhibited a synergistic 1.89 ± 0.21 fold increase in IL-8 secretion compared to LPS alone. After 48 hours, IL-8 secretion was significantly elevated in HEECs treated with either thrombin alone or LPS alone compared to the NT control (Figure 1). Moreover, HEECs treated with both thrombin and LPS exhibited a synergistic 3.09 ± 0.71 fold increase in IL-8 secretion compared to LPS alone, and a synergistic 4.44 ± 0.50 fold increase in IL-8 secretion compared to thrombin alone (Figure 1). Given the pronounced IL-8 secretion by HEECs treated for 48hr with thrombin or LPS, either alone or in combination, all subsequent cytokine/chemokine data were collected at this time point.
Figure 1. Thrombin augments basal and LPS-induced IL-8 secretion by HEECs in a time-dependent manner.
HEECs were pretreated with no treatment (NT) or thrombin (0.25 U/ml) for 1hr followed by NT or treatment with LPS (1μg/ml) for 24 hours (n=4) and 48 hours (n=5). Supernatants were collected and IL-8 measured by ELISA. *p<0.05 vs. the NT control unless indicated otherwise.
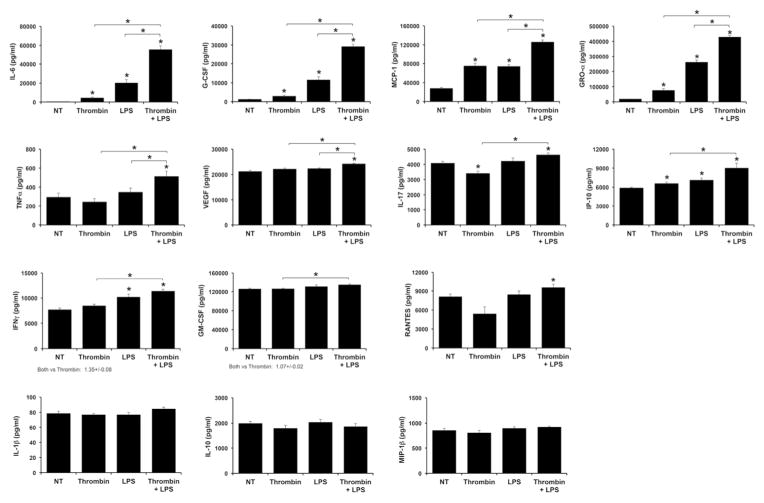
Next, we sought to delineate HEEC secretion of other cytokines, chemokines and growth factors in response to the thrombin and LPS treatments. As shown in Figure 2, thrombin alone significantly increased HEEC secretion of inflammatory IL-6, G-CSF, MCP-1, GRO-α and IP-10, and significantly reduced secretion of IL-17, when compared to the NT control. Thrombin alone had no significant effect on the remaining factors: TNFα, VEGF, IP-10, IFNγ, GM-CSF, RANTES, IL-1β, IL-10 and MIP-1β (Figure 2). LPS alone significantly increased HEEC secretion of inflammatory IL-6, G-CSF, MCP-1, GRO-α, IP-10, and IFNγ when compared to the NT control, without altering the secretion of TNFα, VEGF, IL-17, GM-CSF, RANTES, IL-1β, IL-10 or MIP-1β (Figure 2). When compared to LPS alone, thrombin significantly and synergistically augmented the LPS-induced HEEC secretion of inflammatory IL-6 (3.14 ± 0.36 fold); G-CSF (12.28 ± 2.19 fold); and GRO-α (1.69 ± 0.14 fold) (Figure 2). When compared to LPS alone, the combination of thrombin and LPS also significantly augmented HEEC secretion of inflammatory MCP-1 (1.75 ± 0.12 fold); TNF-α (1.51 ± 0.09 fold); and VEGF (1.08 ± 0.02 fold), although these were additive responses (Figure 2). Compared to LPS alone combination thrombin and LPS had no significant effect on HEEC secretion of any of the other factors tested. However, compared to thrombin alone, thrombin and LPS significantly augmented HEEC secretion of IL-17 (1.38 ± 0.07 fold); IP-10 (1.36 ± 0.07 fold); IFNγ (1.35 ± 0.08 fold); and GM-CSF (1.07 ± 0.02 fold) (Figure 2). IL-12 and MIP-1α were undetectable for all treatments (data not shown).
Figure 2. Thrombin augments basal and LPS-induced HEEC cytokine and chemokine secretion by HEECs.
HEECs were pretreated with no treatment (NT) or thrombin (0.25 U/ml) for 1hr followed by NT or treatment with LPS (1μg/ml) for 48 hours (n=4). Supernatants were collected and measured by multiplex analysis. Barcharts IL-6, G-CSF, MCP-1, GRO-α, TNFα, VEGF, IL-17, IP-10, IFNγ, GM-CSF, RANTES, IL-1β, IL-10 and MIP-1β secretion. *p<0.05 vs. the NT control unless indicated otherwise.
Thrombin synergistically augments LPS-induced HEEC inflammation through PAR-1 activation
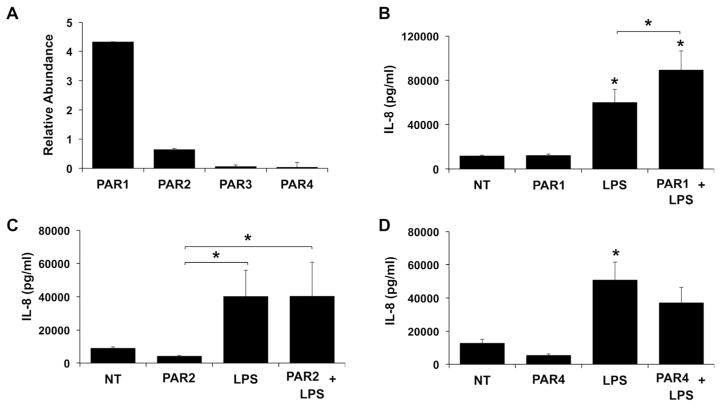
Having demonstrated that HEECs treated with thrombin or LPS generate specific inflammatory cytokine/chemokine profiles, and that thrombin augments the HEEC inflammatory response to LPS both synergistically and additively, we sought to clarify the mechanisms involved. Given that thrombin activates PAR1, PAR3, and PAR4 13, we investigated whether the effects of thrombin on basal and LPS-induced HEEC inflammation was mediated in a PAR-dependent mechanism. As shown in Figure 3A, all four PARs are expressed by HEECs under basal conditions, with PAR1 being the most highly expressed (Figure 3A). Having confirmed PAR1-4 expression by HEECs, their functional role in modulating HEEC responses to LPS was tested using specific agonists. Since a specific PAR3 agonist was not available for these studies, we focused on PAR1 and PAR4. Since thrombin does not activate PAR2 13, a specific agonist for this receptor was also tested to serve as a negative control. As shown in Figure 3(B–D), none of the PAR agonists alone altered HEEC IL-8 secretion. However, the PAR1 agonist significantly and synergistically augmented the LPS-induced IL-8 response by 1.49 ± 0.01 fold when compared to LPS alone (Figure 3B). In contrast, neither the PAR2 (Figure 3C) nor the PAR4 (Figure D) agonist significantly altered the HEEC IL-8 response to LPS.
Figure 3. Expression and function of PAR receptors in HEECs.
(A) RNA was isolated from untreated HEEC cultures (n=3). qRT-PCR was performed for PAR1-4. PAR mRNA levels are expressed as relative abundance after normalization to GAPDH. (B–D) HEECs were pretreated for 1hr with no treatment (NT) or either: (B) a PAR-1 agonist (1mM); (C) a PAR-2 agonist (1mM); or (D) a PAR-4 agonist (1mM). All cells were then treated with either NT or LPS (1μg/ml) for 48 hours (n=3). Supernatants were collected and IL-8 measured by ELISA. *p<0.05 vs. the NT control unless indicated otherwise.
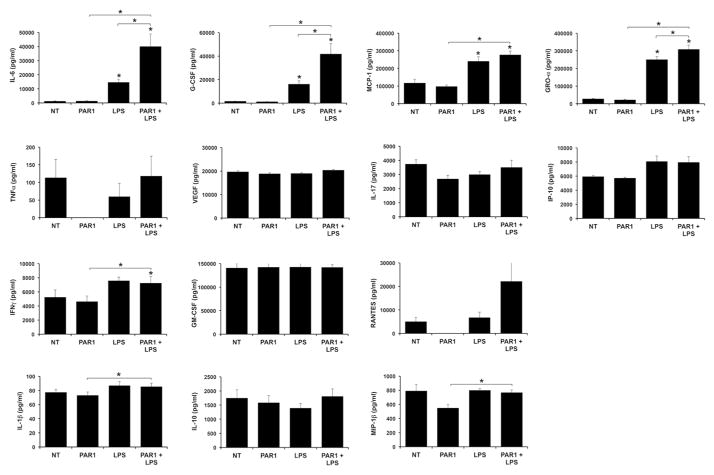
Since the PAR1 agonist augmented LPS-induced HEEC IL-8, similarly to thrombin, the ability of this agonist to modulate other HEEC cytokine/chemokine responses to LPS was examined. In contrast to thrombin, the PAR1 agonist alone had no effect on the basal secretion of any of the factors tested; consequently no additive effects of the PAR1 agonist and LPS was observed as we had with thrombin and LPS on factors such as MCP-1, TNFα, and VEGF (Figure 4). However, similarly to thrombin, pretreatment of HEECs with the PAR1 agonist significantly and synergistically augmented LPS-induced secretion of IL-6 (2.54 ± 0.27 fold); G-CSF (2.48 ± 0.21 fold); and GRO-α (1.23 ± 0.03 fold) when compared to LPS alone (Figure 4).
Figure 4. PAR1 agonist augments LPS-induced HEEC cytokine and chemokine secretion by HEECs.
HEECs were pretreated with no treatment (NT) or a PAR-1 agonist (1mM) for 1hr followed by NT or treatment with LPS (1μg/ml) for 48 hours (n=3). Supernatants were collected and measured by multiplex analysis. Barcharts IL-6, G-CSF, MCP-1, GRO-α, TNFα, VEGF, IL-17, IP-10, IFNγ, GM-CSF, RANTES, IL-1β, IL-10 and MIP-1β secretion. IL-12 and MIP-1α were below the assay’s detection limit. *p<0.05 vs. the NT control unless indicated otherwise.
Thrombin modulates HEEC suppressors of cytokine signaling expression
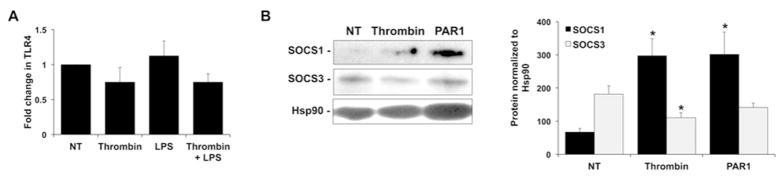
Having found that thrombin, through PAR1 activation, acts to synergistically augment the secretion of specific HEEC cytokines and chemokines in response to LPS, we further investigated the mechanisms involved. Since LPS responses are mediated by TLR4, we first sought to determine if thrombin increased HEEC TLR4 expression. As shown in Figure 5A, treatment of HEECs with either thrombin or LPS, alone or in combination had no effect on TLR4 mRNA levels. We next examined whether thrombin altered the expression of TLR inhibitors to allow TLR4 activation to cause a more aggravated inflammatory response. Since the suppressors of cytokine signaling 1 (SOCS1) and SOCS3 proteins inhibit TLR4-mediated induction of inflammatory factors 25, their expression in HEECs in response to thrombin and the PAR1 agonist was investigated. As shown in Figure 5B treatment of HEECs with thrombin or the PAR1 agonist significantly increased SOCS1 protein expression when compared to the NT control (Figure 5B). Treatment of HEECs with thrombin significantly decreased SOCS3 protein expression by 26.9 ± 8.3% compared to the NT control, however the PAR1 agonist had no effect on SOCS3 protein expression (Figure 5B).
Figure 5. Effect of thrombin on HEEC TLR4 and SOCS expression.
(A) HEECs were pretreated with no treatment (NT) or thrombin (0.25 U/ml) for 1hr followed by NT or treatment with LPS (1μg/ml) for 24 hours (n=3). Cellular RNA was isolated and qRT-PCR performed for TLR4. Barchart shows TLR4 mRNA expressed as fold change relative to the NT control. (B) HEECs were treated with either no treatment (NT), thrombin (0.25 U/ml) or a PAR-1 agonist (1mM) for 24 hours after which protein was isolated and Western blot performed for SOCS1, SOCS3 and Hsp90. Blots are from one representative experiment. Barchart shows SOCS1 and SOCS3 protein expression determined by densitometry and normalized to Hsp90 (*p<0.05 vs. NT; n=3–5).
Discussion
Bacterial infection and placental abruption are important risk factors for preterm birth 9. Therefore, the objective of this study was to provide further insight into the relationship between coagulation and inflammation in the uterine endothelium. Bacterial infections gain access to the maternal-fetal interface either by ascending into the uterus from the lower reproductive tract; by disseminating via the maternal circulation from a distant site; or rarely, by descending into the uterus from the peritoneal cavity 26. The endothelium is the first tissue exposed to invasive pathogens in the bloodstream. Its response to infection or tissue injury is to become activated to recruit immune cells and express adhesion molecules that allow their extravasation into tissues for host defense 15. Thus, this study questioned the impact a systemic bacterial infection would have on the uterine endothelium in the context of excess thrombin production arising from abruption.
We previously demonstrated that bacterial LPS isolated from Escherichia coli 055:B5 induced an inflammatory response in HEECs by upregulating their secretion of the cytokine IL-6; the chemokine IL-8; and the growth factor G-CSF, via TLR4 activation 17. In this current study we examined the HEEC inflammatory response to bacterial LPS isolated from Escherichia coli 0111:B4 at the same dose 17; thrombin; or both in combination. The bacterial LPS used in this study not only upregulated the secretion of IL-6, IL-8 and G-CSF as previously reported 17, but also increased secretion of MCP-1, GRO-α, IP-10 and IFNγ, although the IP-10 and IFNγ responses were very mild. The production of these additional inflammatory mediators is likely due to a difference in the strain of bacterial LPS used 27. Thrombin alone induced a similar, yet distinct, inflammatory profile in the HEECs by upregulating their secretion of IL-6, IL-8, G-CSF, MCP-1, GRO-α and IP-10 while reducing basal levels of IL-17. Furthermore, most of these responses were less robust than those induced by LPS. This suggests that LPS and thrombin induce HEEC inflammation through distinct mechanisms. Our findings are, however, in keeping with studies in endothelial cells from other sites, such as the brain and umbilical cord, showing that thrombin induces a similar chemokine profile to the HEECs 28–30.
When HEECs were pretreated with thrombin and then exposed to LPS, two primary responses were observed: a synergistic upregulation of IL-6, IL-8, G-CSF and GRO-α secretion; and an additive upregulation of MCP-1, TNFα and VEGF secretion. Interestingly, there were no changes in the levels of IL-1β secretion in HEECs treated with LPS or thrombin, either alone or in combination. This was surprising since IL-1β is an important mediator of preterm birth 31–33, and thrombin induces IL-1β in microglia and astrocytes 34, 35. However, to our knowledge, no other studies have reported thrombin-induced IL-1β production by the endothelium 30. Instead, our results suggest that under conditions of infection and/or abruption, HEECs produce factors such as IL-8, MCP-1, GRO-α and G-CSF that are important for the recruitment and survival of immune cells such as monocytes 36. Once they undergo diapedesis and entered the decidual tissue, it is these peripheral-derived macrophages and other immune cells 37, 38 that may serve as a localized source of IL-1β 39 in the setting of intrauterine infection and/or intrauterine hemorrhage.
PAR1, PAR3 and PAR4 are all stimulated by thrombin 13. HEECs express all four receptors, with PAR1 being predominant, which in keeping with their expression in other endothelial cells 40. The synergistic effect of thrombin on HEEC inflammatory responses to LPS were mediated by PAR1, since a specific receptor agonist induced the same synergistic augmentation of these factors by HEECs when exposed to LPS. However, the additive effect of thrombin on HEEC responses to LPS was independent of PAR1 since the receptor agonist alone had no effect on HEEC basal cytokine/chemokine production, unlike thrombin alone. Thus, another pathway may also be involved that mediates the effects of thrombin alone on HEEC cells and is responsible for the additive inflammatory response seen in combination with LPS. Thus, it remains possible that PAR3, together with PAR1, mediates the additional modulation of the LPS-induced inflammation. Alternatively, studies in amniotic mesenchymal cells showed that thrombin induced cell activation directly via PAR1 and potentially indirectly via TLR4 41.
How thrombin exerts its synergistic effects on HEEC responses to LPS downstream of PAR1 activation was also examined. PAR1 stimulation can activate the NFκB pathway 42, and we speculated that this might lead to augmented TLR4 function by modulating receptor expression. However, our findings suggest that TLR4 expression was not altered in HEECs by the presence of thrombin or LPS, either alone or in combination, and thus was not the cause for the augmented inflammatory response. Instead we found that the negative regulator of TLR4 signaling, SOCS1 and SOCS3 25 were modulated. At the protein level, while thrombin downregulated HEEC SOCS3 expression, this was not altered in response to PAR1 activation by its agonist. Thus, while suppression of SOCS3 may play a role in the HEEC LPS-mediated inflammation, it may not be the mechanism by which PAR1 augments this response. Of interest other studies investigating the role of thrombin and SOCS3, found that thrombin and PAR1 activation directly stimulated SOCS3 expression in microglia and suppressed the LPS response in microglia 43, 44.
We acknowledge that the maternal-fetal interface involves the placental trophoblast, the decidual stroma, and the uterine endothelium, and each of these components may individually participate in the pathogenesis of preterm birth. While we recognize the multiple pathways may contribute to the inflammatory cascade in response to thrombin and bacterial LPS, the focus our investigation was at the uterine microvasculature. Another limitation of our study is that our experimental model examined HEECs exposed to thrombin prior to LPS. While our results clearly demonstrate that thrombin actively alters the LPS inflammatory response, whether there is a similar effect seen when thrombin and LPS are combined simultaneously, or when HEECs are exposed to LPS before thrombin would warrant further study.
In summary, this study demonstrates that thrombin and LPS induce distinct inflammatory profiles in HEECs. Moreover, thrombin, via PAR1 activation, synergistically augments LPS-induced production of chemokines involved in immune cell recruitment and survival. These findings suggest a mechanism by which preterm birth in the context of intrauterine abruption and bacterial infection may be associated with an aggravated uterine inflammatory response.
Acknowledgments
This study was supported by grant PO1HD054713 from the NICHD, NIH.
References
- 1.March of Dimes. National Center for Health Statistics, 2014 final natality data. 2015. 2015 Premature Birth Report Cards. [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64:1–65. [PubMed] [Google Scholar]
- 3.Behrman RE, Butler AS, editors. Committee on Understanding Premature Birth and Assuring Healthy Outcomes BoHSP. Societal Costs of Preterm Birth. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): 2007. pp. 398–429. [Google Scholar]
- 4.Malloy MH. Changes in infant mortality among extremely preterm infants: US vital statistics data 1990 vs 2000 vs 2010. Journal of perinatology: official journal of the California Perinatal Association. 2015 doi: 10.1038/jp.2015.91. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Practice Bulletins-Obstetrics TACoO, Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstetrics and gynecology. 2012;120:964–973. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 6.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–647. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhimschi CS, Schatz F, Krikun G, Buhimschi IA, Lockwood CJ. Novel insights into molecular mechanisms of abruption-induced preterm birth. Expert Rev Mol Med. 2010;12:e35. doi: 10.1017/S1462399410001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nath CA, Ananth CV, Smulian JC, Shen-Schwarz S, Kaminsky L New Jersey-Placental Abruption Study I. Histologic evidence of inflammation and risk of placental abruption. Am J Obstet Gynecol. 2007;197:319 e311–316. doi: 10.1016/j.ajog.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol. 2005;167:1443–1449. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krikun G, Huang ST, Schatz F, Salafia C, Stocco C, Lockwood CJ. Thrombin activation of endometrial endothelial cells: a possible role in intrauterine growth restriction. Thromb Haemost. 2007;97:245–253. [PubMed] [Google Scholar]
- 13.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 14.van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol. 2012;10:632–638. doi: 10.2174/157016112801784549. [DOI] [PubMed] [Google Scholar]
- 15.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krikun G, Potter JA, Abrahams VM. Human Endometrial Endothelial Cells Generate Distinct Inflammatory and Antiviral Responses to the TLR3 agonist, Poly(I:C) and the TLR8 agonist, viral ssRNA. Am J Reprod Immunol. 2013;70:190–198. doi: 10.1111/aji.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krikun G, Trezza J, Shaw J, Rahman M, Guller S, Abrahams VM, Lockwood CJ. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am J Reprod Immunol. 2012;68:233–237. doi: 10.1111/j.1600-0897.2012.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krikun G, Lockwood CJ, Abrahams VM, Mor G, Paidas M, Guller S. Expression of Toll-like receptors in the human decidua. Histol Histopathol. 2007;22:847–854. doi: 10.14670/HH-22.847. [DOI] [PubMed] [Google Scholar]
- 19.Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ. Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells. Histol Histopathol. 2005;20:719–724. doi: 10.14670/HH-20.719. [DOI] [PubMed] [Google Scholar]
- 20.Schatz F, Soderland C, Hendricks-Munoz KD, Gerrets RP, Lockwood CJ. Human endometrial endothelial cells: isolation, characterization, and inflammatory-mediated expression of tissue factor and type 1 plasminogen activator inhibitor. Biol Reprod. 2000;62:691–697. doi: 10.1095/biolreprod62.3.691. [DOI] [PubMed] [Google Scholar]
- 21.Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;90:39. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod. 2015;92:17. doi: 10.1095/biolreprod.114.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. 2011;65:542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. The Journal of biological chemistry. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 26.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–637. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Jain S, Carl DJ, Paolella L, Darveau RP, Gravett MG, Adams Waldorf KM. Differential host response to LPS variants in amniochorion and the TLR4/MD-2 system in Macaca nemestrina. Placenta. 2010;31:811–817. doi: 10.1016/j.placenta.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alabanza LM, Bynoe MS. Thrombin induces an inflammatory phenotype in a human brain endothelial cell line. J Neuroimmunol. 2012;245:48–55. doi: 10.1016/j.jneuroim.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Kadner SS, Guller S. Differential effects of lipopolysaccharide and thrombin on interleukin-8 expression in syncytiotrophoblasts and endothelial cells: implications for fetal survival. Ann N Y Acad Sci. 2004;1034:236–244. doi: 10.1196/annals.1335.025. [DOI] [PubMed] [Google Scholar]
- 30.Kaplanski G, Fabrigoule M, Boulay V, Dinarello CA, Bongrand P, Kaplanski S, Farnarier C. Thrombin induces endothelial type II activation in vitro: IL-1 and TNF-alpha-independent IL-8 secretion and E-selectin expression. J Immunol. 1997;158:5435–5441. [PubMed] [Google Scholar]
- 31.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17:619–628. doi: 10.1177/1933719110373148. [DOI] [PubMed] [Google Scholar]
- 33.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. Bjog. 2011;118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci. 2003;23:5877–5886. doi: 10.1523/JNEUROSCI.23-13-05877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J Neuropathol Exp Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- 36.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wicherek L, Galazka K, Lazar A. RCAS1 decidual immunoreactivity during placental abruption: immune cell presence and activity. Am J Reprod Immunol. 2007;58:46–55. doi: 10.1111/j.1600-0897.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 39.Aldo PB, Racicot K, Craviero V, Guller S, Romero R, Mor G. Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am J Reprod Immunol. 2014;72:270–284. doi: 10.1111/aji.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol. 2004;24:41–53. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- 41.Mogami H, Keller PW, Shi H, Word RA. Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth. J Biol Chem. 2014;289:13295–13307. doi: 10.1074/jbc.M114.550541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112:876–882. doi: 10.1160/TH14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang MS, Lee J, Ji KA, Min KJ, Lee MA, Jou I, Joe E. Thrombin induces suppressor of cytokine signaling 3 expression in brain microglia via protein kinase Cdelta activation. Biochemical and biophysical research communications. 2004;317:811–816. doi: 10.1016/j.bbrc.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 44.Fabrizi C, Pompili E, Panetta B, Nori SL, Fumagalli L. Protease-activated receptor-1 regulates cytokine production and induces the suppressor of cytokine signaling-3 in microglia. International journal of molecular medicine. 2009;24:367–371. doi: 10.3892/ijmm_00000241. [DOI] [PubMed] [Google Scholar]