Abstract
Francisella tularensis (Ft) is a Category A biothreat agent for which there currently is no FDA-approved vaccine. Thus, there is a substantial effort underway to develop an effective tularemia vaccine. While it is well established that gender can significantly impact susceptibility to primary infection, the impact of gender on vaccine efficacy is not well established. Thus, development of a successful vaccine against tularemia will require an understanding of the impact gender has on vaccine-induced protection against this organism. In this study, a role for gender in vaccine-induced protection following Ft challenge is identified for the first time. In the present study, mucosal vaccination with inactivated Ft (iFt) LVS elicited gender-based protection in C57BL/6Tac mice against respiratory challenge with Ft LVS. Specifically, vaccinated male mice were more susceptible to subsequent Ft LVS challenge. This increased susceptibility in male mice correlated with increased bacterial burden, increased tissue inflammation, and increased proinflammatory cytokine production late in post-challenge infection. In contrast, improved survival of iFt-vaccinated female mice correlated with reduced bacterial burden and enhanced levels of Ft-specific Abs in serum and broncho-alveolar lavage (BAL) fluid post-challenge. Furthermore, vaccination with a live attenuated vaccine consisting of an Ft LVS superoxide dismutase (SodB) mutant, which has proven efficacious against the highly virulent Ft SchuS4 strain, demonstrated similar gender bias in protection post-Ft SchuS4 challenge. Of particular significance is the fact that these are the first studies to demonstrate that gender differences impact disease outcome in the case of lethal respiratory tularemia following mucosal vaccination. In addition, these studies further emphasize the fact that gender differences must be a serious consideration in any future tularemia vaccine development studies.
Keywords: Tularemia, Gender differences, Host susceptibility, Intranasal vaccine, Inactivated vaccine, Humoral immunity, Proinflammatory cytokines
1. Introduction
It is well documented that gender can play an important role in determining the outcome of primary infection in that the gender of a host can significantly affect susceptibility to infection [1]. Epidemiological studies have shown that males and females handle infections differently [2,3]. Most notably, males can be at increased risk of susceptibility to major bacterial and viral infections versus females [4,5]. In the case of primary infection, consistent correlations between sex, immunity, and protection have been observed. For example, females have greater humoral and cell-mediated immune responses to antigenic stimulation by infectious agents as compared to males [6–8]. In contrast, males have higher levels of expression of pattern-recognition receptors for bacterial lipopolysaccharide, which has been linked to heightened production of proinflammatory cytokines and a greater incidence of lethal systemic inflammation observed in males [9]. The existence of gender bias in the immune response to infectious diseases is further supported by numerous in vivo studies focused on Mycobacterium marinum [10], Streptococcus pneumoniae [11], Streptococcus pyogenus [12], Plasmodium chabaudi [13], and Mycoplasma pulmonis [14]. A similar tendency has been seen in humans against numerous pathogens including: Mycobacterium tuberculosis [15], Influenza virus [16] and community-acquired pneumonia [17] in which men are more susceptible than women.
In contrast to the above, the impact of sex on vaccine-induced protection has received substantially less attention and is thus less clear. This lack of clarity is also exacerbated by inconsistencies between the limited numbers of investigations completed. For example, in several studies, women appeared to exhibit better responses to vaccination than men. This was the case for influenza, hepatitis A and B, and herpes simplex (HSV)-2 vaccines [18,19] [20]. In addition, when using the 23-valent pneumococcal polysaccharide vaccine (PPV23), vaccine efficacy was higher in females versus males [21]. In contrast to the above studies, men demonstrated superior Ab responses to diphtheria, measles, and smallpox vaccines when compared to women [19,22]. Men were also better protected against diphtheria and tetanus than their female counterparts [23,24].
Thus, while a great deal is known regarding the impact of gender on primary infection, the impact of gender on protection following vaccination is substantially less clear. Furthermore, based on the limited number of studies that have been done in this regard, results suggest the infectious agent itself may also influence the role gender plays in vaccine-induced immunity and protection [5].
Francisella tularensis (Ft), the causative agent of tularemia, is a gram negative, intracellular pathogen. Ft has also been used as bio warfare agent due primarily to its high virulence and ability to be aerosolized [25–27]. Most notably however, clinical incidence due to primary infection and progression of tularemia in endemic areas is significantly higher in males than in females. While this may reflect differences in pathogen exposure through hunting and outdoors professional activities (CDC – http://www.cdc.gov/tularemia/statistics/agesex.html), [28], gender differences could also be a contributing factor. In addition, there is no approved tularemia vaccine and thus substantial efforts are underway to develop one. Therefore, we sought to fill a critical knowledge gap in tularemia vaccine development and investigate the impact of gender on tularemia vaccine efficacy. We demonstrate for the first time that while we observe no difference in the susceptibility of naïve male versus female mice to Ft challenge, female mice, which are first vaccinated with either inactivated or attenuated Ft vaccine are more resistant to infection as compared to their male counterparts. Results of experiments examining humoral and cellular immune responses following vaccination of male versus female mice are also support this conclusion.
2. Materials and methods
2.1. Mice
Pathogen-free, 6-to-8-week-old male and female C57BL/6Tac mice were purchased from Taconic Farms. Mice were housed in sterile microisolator cages in the animal biosafety level 2 (ABSL-2) and ABSL-3 facilities at the Albany Medical Center (AMC). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Albany Medical College.
2.2. Ft organisms
Ft LVS and Ft SchuS4 were cultured aerobically at 37 °C in modified Mueller-Hinton broth (MHB) or agar (Becton Dickinson, Sparks, MD) supplemented with ferric pyrophosphate and Iso-Vitalex (Becton Dickinson, Sparks, MD). Ft LVS SodB mutant was grown in Brain-Heart Infusion (BHI) medium and the active mid-log phase bacteria were harvested and used for immunization.
2.3. Generation of iFt immunogen
Ft LVS grown in MHB was inactivated using paraformaldehyde as previously described [29,30]. Inactivation was verified by plating a 100 μl sample (1 × 109 iFt organisms) on chocolate agar plates (Becton, Sparks, MD) for 7 days. The protein concentration of iFt was estimated by Lowry's method, the iFt preparations were stored at −20 °C in PBS.
2.4. Immunization and challenge studies
Prior to immunization, each mouse was anesthetized by intraperitoneal (i.p.) injection of 20% ketamine plus 5% xylazine. In the case of iFt vaccination, mice were subsequently administered intranasally (i.n.) either 20 μl of PBS (control) or iFt (1500 ng) in 20 μl of PBS. Unless, otherwise indicated, mice were immunized on day 0 and boosted on day 21. Immunized mice were then challenged on day 35 i.n. using 1–10 × LD50 of Ft LVS. In this case, 1 × LD50 is equivalent to 800 CFU of Ft LVS administered i.n. In the case of immunizations using live attenuated vaccine, an attenuated Ft LVS SodB mutant organism was utilized as the vaccine. Specifically, 1 × 103 CFU of Ft LVS SodB in 50 μl of PBS were administered intradermally (i.d.) followed by an i.n. boost with 1 × 103 CFU in 20 μl of PBS on day 21 post-primary immunization. Mice were then challenged i.n. with 20–30 CFU of Ft SchuS4 in 20 μl of PBS on day 42 post-primary immunization. The challenged mice were subsequently monitored for survival for a minimum of 25 days using death as an endpoint.
2.5. Quantification of bacterial burden
Following immunization and challenge, mice were euthanized at various time intervals as indicated in the individual figures and bacterial burden in the lungs, liver and spleen of infected mice was monitored as previously described [29].
2.6. Serum lactate dehydrogenase (LDH) assay
Serum concentrations of LDH were measured using a lactate dehydrogenase activity assay kit (Sigma–Aldrich, St. Louis, MO). Standards and serum samples were diluted according to manufacturers protocol and 50 μl/well were added to plates along with NAD substrate. Plates were incubated at 37 °C and read at 450 nm using a microplate reader (VersaMax, Molecular Devices, Sunnyvale, CA). LDH activity was determined using following equation: LDH activity = B × sample dilution factor/(reaction time) × V, where B = amount of NADH generated (nmole) and V = sample volume (mL) added to the well.
2.7. Histopathology
Lung, liver and spleen from iFt-vaccinated and Ft LVS-challenged mice were excised and processed for histology as previously described [29]. Disease severity in the tissues was then assessed based upon cellular infiltration, thickening of alveolar septa, and airway passage congestion.
2.8. Cytokine quantification in tissues and broncho-alveolar lavage (BAL) fluid
Tissue homogenates were obtained as indicated above when measuring bacterial burdens. Supernatants were then collected and stored at −20 °C for cytokine analysis. Luminex assay was performed to determine in vivo cytokine levels of interferon-gamma (IFN-γ), tumor necrosis factor-α(TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17 (IL-17), and monocyte chemoattractant protein-1 (MCP-1) to assess inflammation.
2.9. Assessment of humoral immune responses
Anti-Ft Ab production in response to immunization and/or Ft infection in iFt-immunized mice was measured by enzyme linked immunosorbent assay (ELISA) as previously described [29,30].
2.10. Statistical analysis
Statistical data for bacterial clearance and cytokine production was generated using analysis of variances by two-way ANOVA or Mann–Whitney two-tailed test on post-challenge day 7, which is the peak of infection. In the case of survival, significance was determined using a log-rank (Montel-Cox) test. The data are expressed as mean ± standard deviation (SD). Differences between the experimental groups were considered statistically significance at a P < 0.05. The data were analyzed using GraphPad prism (v6.0) software (GraphPad Software, San Diego, CA).
3. Results
3.1. Naive male and female mice are equally susceptible to primary infection with Ft
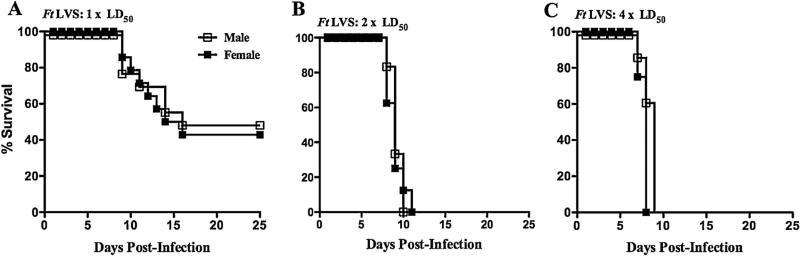
Initially, we sought to determine if there was a difference in susceptibility of naïve male versus female mice to challenge with Ft LVS. In this case, infection of naïve mice with 1 × LD50 (Fig. 1A), 2 × LD50 (Fig. 1B) or 4 × LD50 (Fig. 1C) of Ft LVS resulted in no significant differences in survival between male and female mice.
Fig. 1.
Naive male and female mice are equally susceptible to Ft infection. Naive C57BL/6Tac male and female mice were challenged i.n. with 1 × LD50 (A), 2 × LD50 (B), or 4 × LD50 (C) of Ft LVS and subsequently monitored for 25 days for survival. The survival data represent combined data from two separate experiments (a combined total of 8–14 mice/group).
3.2. Increased survival of female versus male mice is observed following challenge of vaccinated mice with Ft LVS
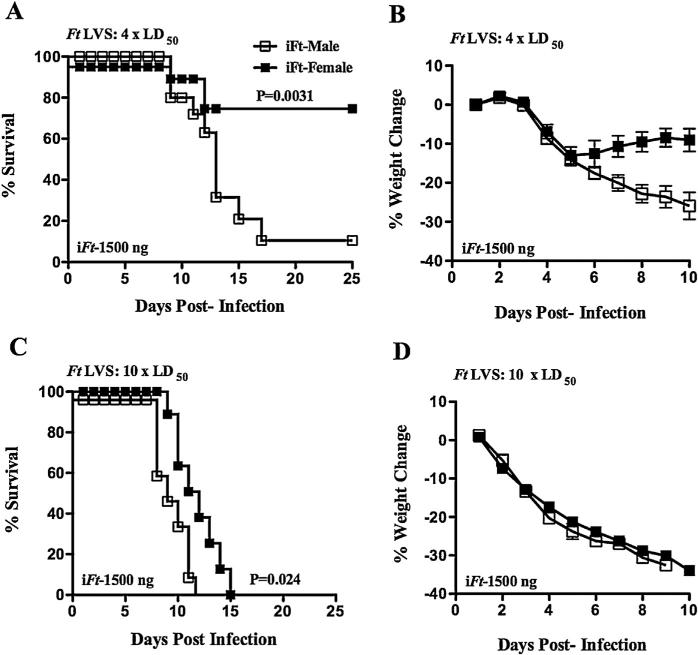
Given the relatively short time to death of 7–10 days following Ft LVS challenge (Fig. 1), it was possible any impact of gender on the adaptive immune response, and thus survival, may not be evident when challenging naïve mice with Ft. Thus, to address this possibility, male and female C57BL/6Tac mice were vaccinated i.n. with iFt and subsequently challenged i.n. with Ft LVS. Similar to naïve mice, both male and female PBS-immunized mice were equally susceptible to Ft LVS infection, succumbing to infection by day 10. However, a marked difference in susceptibility to infection was observed among male and female mice following vaccination and subsequent challenge with 4 × LD50. Male mice were significantly more susceptible to Ft LVS challenge than their female counterparts, as evidenced by their decreased survival and increased weight loss (Fig. 2A and B). This difference was also evident in terms of extended survival, but not weight loss, when challenging with 10 × LD50, in that vaccinated female mice had an extended median time to death (MTD) (12 days), which was significantly higher than that of their male counterparts (9.5 days) (Fig. 2C and D).
Fig. 2.
Improved survival of female versus male mice challenged with Ft LVS following iFt vaccination. C57BL/6Tac male and female mice were immunized i.n. with either 20 μl of vehicle (PBS), 20 μl of PBS containing 1500 ng of iFt on day 0 and were then boosted with the same iFt preparations on day 21. Mice were then challenged i.n. on day 35 with 4 × LD50 (A and B) or 10 × LD50 (C and D) Ft LVS, and subsequently monitored for 25 days for survival. The mice were also weighed at the indicated times post-infection to monitor the progression of infection (B and D). The survival data represent combined data from two independent experiments (a combined total of 12–16 mice/group).
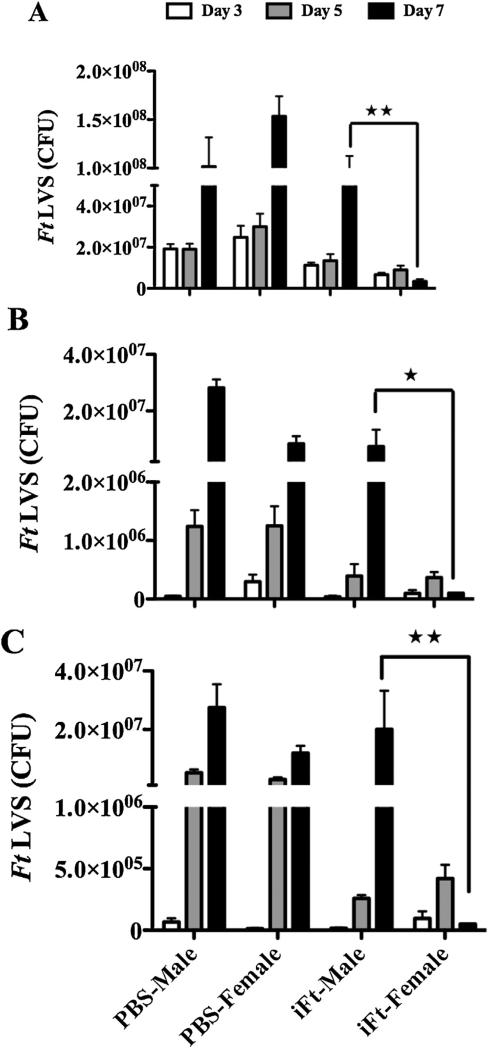
3.3. Decreased bacterial burden is observed in vaccinated female versus male mice
The above differences in body weight loss and survival influenced by gender prompted us to also examine bacterial burden as a reflection of bacterial control. Specifically, we assessed bacterial burden in iFt-vaccinated male versus female mice challenged with a sublethal dose (1 × LD50) of Ft LVS. Consistent with survival studies, mice of both sexes receiving PBS i.n. exhibited significantly higher bacterial numbers in the lungs, liver, and spleen compared to vaccinated mice (Fig. 3). However, bacterial numbers recovered from the lungs, livers, and spleens of vaccinated female mice were substantially lower than those observed in the same tissues of vaccinated male mice particularly on day 7 post-challenge. Collectively, vaccinated female mice not only exhibited a reduced bacterial burden as compared to their male counterparts, but also exhibited more rapid clearance of bacteria from the above tissues compared to the vaccinated males (Fig. 3).
Fig. 3.
Lower bacterial burden is observed in vaccinated female versus male mice. C57BL/6Tac male and female mice were immunized i.n. with 20 μL of PBS (vehicle) or 20 μL of PBS containing 1500 ng iFt on day 0 and subsequently boosted with the same on day 21. On day 35 mice were given a sublethal challenge i.n. with Ft LVS [850 CFU (~1 × LD50)]. Bacterial burdens from lung (A), liver (B), and spleen (C) were determined on days 3, 5, and 7 post-challenge. The data presented represent the average bacterial count of 3 mice sacrificed at each time point ± SD and are from a single experiment. Similar results were obtained in two independent experiments. *P < 0.05, **P < 0.01.
3.4. Vaccinated female mice exhibit a reduced inflammatory response than male mice following Ft LVS challenge
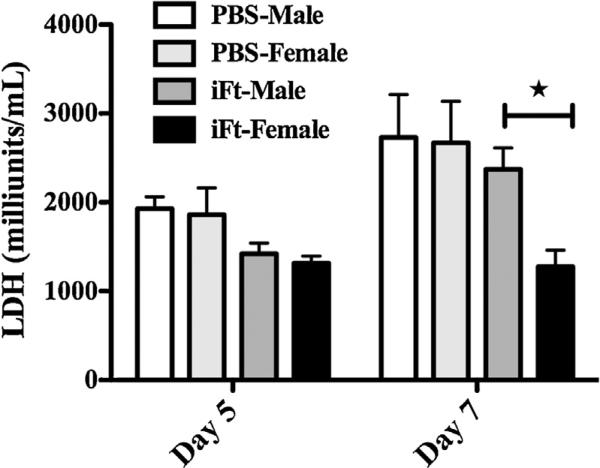
To provide further support for the above observed gender differences in response to Ft LVS challenge following vaccination, tissue inflammation was assessed in iFt-vaccinated male and female mice by measuring the serum concentration of LDH, which provides a means of quantitation for tissue destruction and inflammation. Serum samples for LDH measurements were taken on day 5 and 7 post-challenge with sublethal dose of Ft LVS. While no significant differences in the serum LDH concentration between the vaccinated and unvaccinated groups were observed on day 5, on day 7 male and female mice in the PBS-immunized groups, along with iFt-vaccinated males, exhibited significantly higher levels of LDH in serum versus vaccinated female mice, indicating more severe inflammation and tissue destruction in the PBS and vaccinated male mice as compared to the iFt-vaccinated female mice (Fig. 4). Furthermore, histological examination of lung tissues from both PBS and iFt-vaccinated male and female mice with a sublethal dose (1 × LD50) of Ft LVS exhibited more severe inflammation in PBS-immunized male and female mice as well as iFt-vaccinated male mice as compared to iFt-vaccinated female mice on day 7 post-challenge (Supplemental Fig. 1B–E). Specifically, tissue damage and necrotic lesions were observed in the lung tissue of iFt-vaccinated males. In contrast, in iFt-vaccinated female mice, mild inflamma-tory foci in the lungs were observed, with histopathology being less severe overall versus iFt-vaccinated male mice.
Fig. 4.
Vaccinated female mice exhibit less inflammation than male mice as indicated by reduced lactate dehydrogenase release in serum following Ft LVS infection. C57BL/6Tac male and female mice were immunized and challenged as described in Fig. 3. Serum concentrations of LDH were determined at the indicated time points post-infection. The data are depicted as mean ± SD (4 mice per group) and are representative of two independent experiments. *P < 0.05.
Supplemental Fig. 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.04.054.
3.5. Proinflammatory cytokine levels are reduced in female versus male mice, following iFt-vaccination and Ft LVS challenge
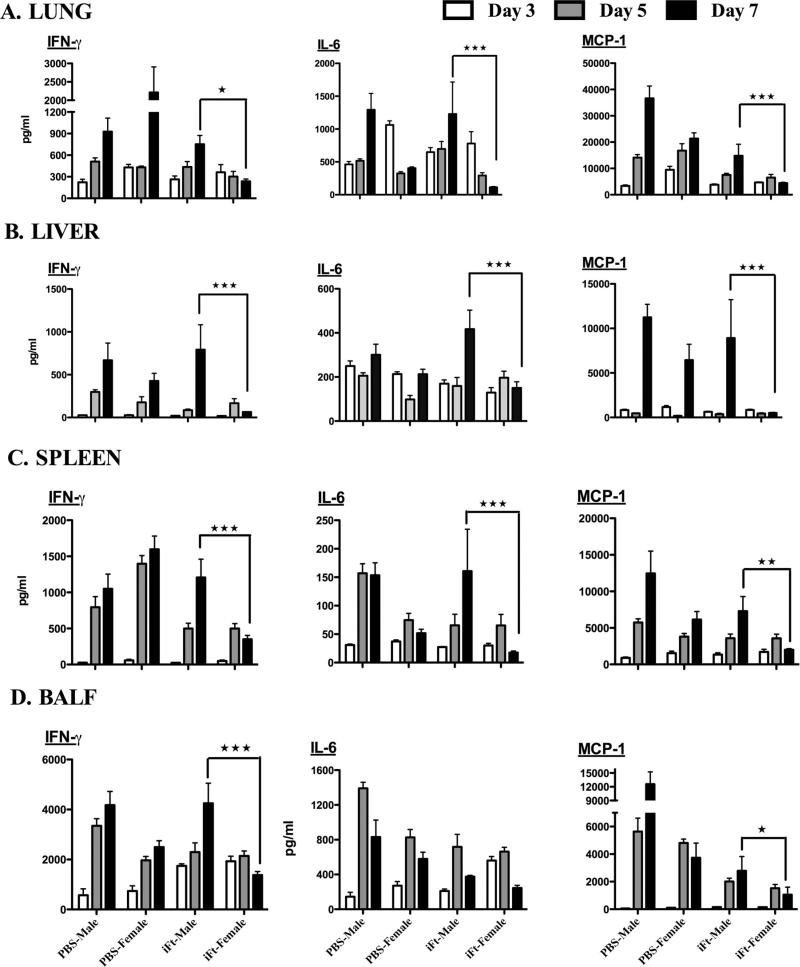
Several studies have demonstrated that male mice produce significantly higher levels of proinflammatory cytokines as compared to female mice following in vivo LPS exposure [31,32]. We have previously observed that regulated IFN-γ production strongly correlates with increased protection during Ft infection [29]. Thus we also evaluated the proinflammatory cytokine responses including that of IFN-γ in lung homogenates of PBS and iFt-vaccinated male versus female mice prior to and following Ft LVS challenge. For the most part, there were no significant differences in cytokine profiles between vaccinated male and female mice pre-challenge (Supplemental Fig. 2). However, while early in the infection post-challenge both iFt-vaccinated male and female mice showed similar levels of cytokine production, at 7 days post-challenge, the levels of IFN-γ, IL-6, and MCP-1 were significantly elevated in iFt-vaccinated male mice. In contrast, iFt-vaccinated female mice displayed regulated production of proinflammatory cytokines in tissues and BAL fluid (Fig. 5A and D). Furthermore, proinflammatory cytokines measured in spleen and liver homogenates exhibited similar differences to that of the lungs (Fig. 5B and C).
Fig. 5.
Proinflammatory cytokine levels are reduced in female versus male mice, following iFt-vaccination and Ft LVS challenge. C57BL/6Tac male and female mice were immunized and challenged as described in Fig. 3. The levels of proinflammatory cytokines from tissue homogenates and BAL fluid were determined on days 3, 5, and 7 post-challenge using Luminex assay. All the cytokines measured are listed in Section 2 and were all detected in these studies. In this figure however, only cytokines levels for which differences between male and female mice were observed are presented and include: IL-6, IFN-γ, and MCP-1. Cytokine levels in the lungs (A), liver (B), spleen (C), and BAL fluid (D) are presented. The data are presented as mean ± SD (4 mice per group) and are representative of two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Supplemental Fig. 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.04.054.
3.6. iFt-Vaccinated female mice exhibit elevated anti-Ft Ab titers as compared to their male counterparts following Ft LVS challenge
Given that iFt-vaccinated female mice exhibit better protection, lower bacterial burden, and reduced histopathology, as compared to their male counterparts, we hypothesized that the humoral immune response, which, in some cases, can mediate protection against Ft infection [29,33], may also be superior to that of male mice. Accordingly, we observed that iFt vaccination generated elevated levels of Ft-specific Abs in female mice as compared to their male counterparts both prior to (Supplemental Fig. 3A) and post-Ft LVS challenge. Specifically, vaccinated female mice produced higher titers of Ft-specific IgA and IgG2c in serum as well as in BALF by day 7 post-challenge, as compared to the vaccinated male mice (Supplemental Fig. 3B and C).
Supplemental Fig. 3 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.04.054.
3.7. Gender-based protection is also observed in mice vaccinated with a live attenuated Ft vaccine (SodB mutant) and subsequently challenged with the highly virulent Ft SchuS4 organism
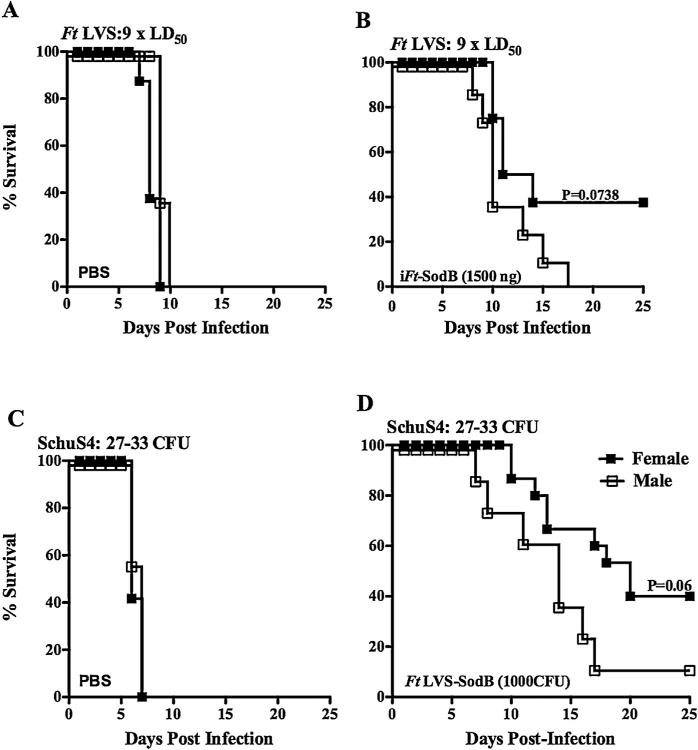
Having observed differences in survival in male versus female mice following iFt vaccination and subsequent Ft LVS challenge, we sought to address the question as to whether a gender-based difference would also be observed when immunizing and challenging with Ft SchuS4. Unlike Ft LVS, Ft SchuS4 is highly virulent for humans and therefore is a model organism against which most Ft vaccines are being tested. Importantly, the iFt immunogen used to immunize mice in Ft LVS challenge experiments does not induce protection against Ft SchuS4 challenge. Thus, to address the above question, it was necessary to choose a vaccination model, which had been previously shown to generate protection against infection with Ft SchuS4. Thus, in this case, mice were immunized with a live attenuated vaccine (SodB mutant derived from Ft LVS), which has been shown to provide clearly measurable, although incomplete, protection against Ft SchuS4 infection in C57BL/6J mice [34]. To first verify that the Ft SodB organism can function similar to iFt generated from Ft LVS, Ft SodB was similarly inactivated and used as immunogen in an Ft LVS challenge experiment. In fact, the use of iFt generated from Ft SodB not only generated improved survival in female versus male mice, but also the level of protection was superior to that of iFt generated from wildtype Ft LVS (Fig. 6A and B). Most importantly however, in the case of Ft SchuS4 challenge, female mice vaccinated with live attenuated Ft SodB mutant showed increased survival versus their male counterparts, exhibiting a higher MTD of 20 days as compared to the male mice, with an MTD of 14 days (Fig. 6C and D).
Fig. 6.
Gender-based protection is also observed in mice vaccinated with a live attenuated Ft vaccine (SodB mutant) and subsequently challenged with the highly virulent Ft SchuS4 organism. Upper panel – C57BL/6Tac male and female mice were immunized i.n. with either 20 μl of vehicle (PBS) or 20 μl of 1500 ng of iFt-SodB on day 0, and boosted on day 21. Mice were then challenged i.n. on day 35 with 9 × LD50 CFU Ft LVS (A and B) and subsequently monitored for 25 days for survival. Lower panel – C57BL/6Tac male and female mice were immunized i.d. with either 50 μl of vehicle (PBS) or 50 μl of 1 × 103 CFU of attenuated Ft LVS SodB mutant (C and D) on day 0 and boosted i.n. on day 21 with either 20 μl of vehicle (PBS) or 20 μl of 1 × 103 CFU of attenuated Ft LVS SodB mutant. Mice were then challenged i.n. on day 42 i.n. with 27–33 (~15 to 25 × LD50) CFU of Ft SchuS4 and subsequently monitored for 25 days for survival. Panels A and B represent one experiment containing 8 mice/group, while panels C and D are combined results from two independent experiments with a total of 16 mice/group.
4. Discussion
Multiple studies by a number of research groups have reported gender-based susceptibility to numerous pathogens and infectious diseases. However, studies on sex bias in tularemia infection have not been published. In general, males of many species are more susceptible than females to primary bacterial, viral, and fungal infections [11,35,36]. In contrast, in this study we show for the first time that both naïve male and female C57BL/6Tac mice are equally susceptible to Ft LVS tularemia (Fig. 1). However, prior mucosal immunizations with inactivated or live Ft vaccine results in a gender-based protection in the case of both Ft LVS and Ft SchuS4 challenge. Specifically, vaccinated male mice develop severe clinical disease and a significantly higher mortality rate, which correlates with increased weight loss as compared to immunized female mice (Fig. 2). Importantly, this implies tularemia vaccine efficacy will vary based on gender, which has been observed in clinical trials involving other infectious agents. Specifically, the efficacy of HSV-2 vaccination was 73% in women and only 11% in men [20]. Similarly, another study using PPV23 to prevent hospitalization due to S. pneumoniae demonstrated vaccine efficacy to be 68% in females and 34% in males [21]. However, in contrast to the above, vaccinated men are better protected against diphtheria and tetanus, which correlates with higher levels of toxin-specific Ab, which is required for protection in both cases [23,24]. The above differences in protection based on gender may reflect, in part, the type of immune response required for protection, suggesting that not just the individual's gender, but also the type of infectious agent involved are important factors in determining which gender is best protected against a given infection following vaccination. For example, when Ab alone is required for protection, males seem to be better protected than females. While this paradigm does not hold true in the case of HSV-2 and S. pneumoniae, where Ab is also known to play an important role in protection, the mechanism of protection is more complex, in that both Ab and cellular immune components (phagocytic cells) are required to resolve infection [37,38]. Thus, in the case of vaccination, further investigation will be required to more clearly define correlations between the specific gender protected and the immune component(s) required for protection against specific infections.
In regard to Ft infection, it is known that both cellular and humoral immunity can play important roles in resolving this infection [29,30,39], similar to the cases with HSV-2 and S. pneumoniae. Specifically, it has been established that cell-mediated immunity (CMI) plays a critical role in protection against tularemia [39]. For example, both CD4 and CD8T cells can proliferate and produce IFN-γ in response to a number of Ft proteins [40]. Additionally, depletion of CD4 T-cells, CD8 T-cells, or IFN-γ abolishes vaccine-induced immunity against type A Ft (SchuS4) infection [41]. Thus the production of IFN-γ by Th1 cells is thought to be key to developing protective immunity against Ft. In fact, in the case of IFN-γ production, we do show a difference between males and females (Fig. 5), which may help explain our observed differences in protection between genders. In contrast to cellular immunity, the role of humoral immunity in the resolution of Ft infection and protection remains controversial, although a number of investigations have demonstrated that humoral immunity can play a role in protection against tularemia, in particular Ft LVS (type B) infection. The latter is consistent with the observation that Ft has been shown to have an extracellular phase [33,42]. In addition, this controversy may be partially explained by studies from our laboratory, which demonstrated that while both Ab and IFN-γ can be critical for vaccine-induced protection [29], the need for Ab can be overcome, when IFN-γ levels are sufficiently high [30]. In regard to the generation of humoral immunity to Ft in these studies, we did observe elevated levels of Ft-specific IgA IgG and IgG2c in iFt-vaccinated female mice in response to iFt vaccination (Supplemental Fig. 3A), as well as Ft LVS infection on day 7 post-Ft LVS challenge (Supplemental Fig. 3B and C), which, as discussed above, may or may not be a significant contributor to the enhanced protection that we observed in female mice. In addition to the above, vaccinated female mice showed significantly less bacterial burden in the lung, liver, and spleen versus their vaccinated male counterparts (Fig. 3). Improved control of bacterial replication and early clearance of Ft LVS in vaccinated female mice was also reflected in the extent of tissue inflammation, as measured by serum LDH, and tissue pathology. In regard to the latter, more extensive cellular infiltration and associated cell death was apparent in the lungs of vaccinated male mice versus vaccinated female mice (Fig. 4 and Supplemental Fig. 1). More specifically, the increased bacterial load at day 7 observed in male mice likely contributes to the increased cytokine levels and inflammation observed. The latter also being reflected in LDH values obtained. Importantly, our laboratory as well as other laboratories, have demonstrated that non-protected mice (in this case male mice, Fig. 5) generally exhibit elevated levels of proinflammatory cytokines later in infection (5–7 days post-challenge), which we also observed in these studies [29,30,34,43]. Furthermore, the improved survival of vaccinated female mice following challenge with the highly virulent Ft SchuS4 strain (Fig. 6) indicates that the gender differences in disease susceptibility are not unique to Ft LVS, suggesting that the increased susceptibility of male mice to tularemia following vaccination is not Ft strain specific.
In regard to the broader mechanism(s) by which gender bias in protection against infection is mediated, it is firmly established that disparities in immune responses between males and females to infectious disease challenge are, in part, the result of the actions of reproductive hormones. Both in vitro and in vivo studies have revealed that testosterone, a male hormone, can be immunosuppressive and down-regulate immunoglobulin and cytokine production [44]. In contrast, estrogen, a female hormone, restores cellular immunity in injured male mice via suppression of interleukin-6 production [45]. Estrogen also suppresses MCP1 expression in murine macrophages, thus exerting anti-inflammatory effects potentially via prevention of macrophage accumulation [46]. Numerous studies suggest that mice succumb to tularemia infection due to excessive inflammation [29,30,47]. If estrogen limits and testosterone exacerbates such inflammation, this may explain why immunized female mice are less susceptible than immunized male mice to tularemia challenge in our studies.
In summary, we demonstrate for the first time that gender differences impact disease outcome in the case of lethal respiratory tularemia following mucosal vaccination. Specifically, female mice generate a more robust humoral immune response following vaccination. In turn, female mice are also better protected than their male counterparts against Ft challenge, where a key mechanism for protection requires both humoral and cellular immunity can play key roles in protection [30,39]. Taken together, the results presented in these studies demonstrate for the first time significant differences between males and females in susceptibility to Ft, reinforcing the importance of gender consideration in vaccine development against Ft and most likely other pathogens as well.
Supplementary Material
Acknowledgements
The authors thank the CIMD Immunology core as well as Animal Care Facility at Albany Medical College for expert technical assistance during this work. The National Institutes of Health (P01 AI056320 and RO1 AI100138) funded these studies.
Abbreviations
- Ft
F. tularensis
- iFt
inactivated Ft
- SodB
superoxide dismutase B
- Ab
antibody
- mAb
monoclonal Ab
- Ag
antigen
- i.n.
intranasal
- i.d.
intradermal
- BAL
bronchial alveolar lavage fluid
- MTD
median time to death
Footnotes
Conflict of interest statement: None of the authors have conflicts of interest to disclose.
References
- 1.Owens IP. Ecology and evolution. Sex differences in mortality rate. Science. 2002;297:2008–9. doi: 10.1126/science.1076813. [DOI] [PubMed] [Google Scholar]
- 2.Gordon HS, Rosenthal GE. The relationship of gender and in-hospital death: increased risk of death in men. Med Care. 1999;37:318–24. doi: 10.1097/00005650-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 3.van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. J Infect Dis. 2014;209(Suppl. 3):S79–80. doi: 10.1093/infdis/jiu159. [DOI] [PubMed] [Google Scholar]
- 4.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–8. doi: 10.1001/archsurg.134.9.935. discussion 8-40. [DOI] [PubMed] [Google Scholar]
- 5.Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–21. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, Hermann C. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res. 2006;26:887–92. doi: 10.1089/jir.2006.26.887. [DOI] [PubMed] [Google Scholar]
- 7.Lamason R, Zhao P, Rawat R, Davis A, Hall JC, Chae JJ, et al. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunol. 2006;7:2. doi: 10.1186/1471-2172-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12–27. doi: 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun. 1991;59:4089–96. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadioglu A, Cuppone AM, Trappetti C, List T, Spreafico A, Pozzi G, et al. Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J Infect Dis. 2011;204:1971–9. doi: 10.1093/infdis/jir657. [DOI] [PubMed] [Google Scholar]
- 12.Medina E, Goldmann O, Rohde M, Lengeling A, Chhatwal GS. Genetic control of susceptibility to group A streptococcal infection in mice. J Infect Dis. 2001;184:846–52. doi: 10.1086/323292. [DOI] [PubMed] [Google Scholar]
- 13.Benten WP, Wunderlich F, Mossmann H. Testosterone-induced suppression of self-healing Plasmodium chabaudi malaria: an effect not mediated by androgen receptors. J Endocrinol. 1992;135:407–13. doi: 10.1677/joe.0.1350407. [DOI] [PubMed] [Google Scholar]
- 14.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect Immun. 2001;69:2865–71. doi: 10.1128/IAI.69.5.2865-2871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(Suppl. 3):S100–6. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis. 2014;209(Suppl. 3):S93–9. doi: 10.1093/infdis/jiu020. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53:166–74. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–74. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32:2198–203. doi: 10.1016/j.vaccine.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Troy JD, Hill HR, Ewell MG, Frey SE. Sex difference in immune response to vaccination: a participant-level meta-analysis of randomized trials of IMVAMUNE((R)) smallpox vaccine. Vaccine. 2015;33:5425–31. doi: 10.1016/j.vaccine.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–6. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]
- 24.Volzke H, Kloker KM, Kramer A, Guertler L, Doren M, Baumeister SE, et al. Susceptibility to diphtheria in adults: prevalence and relationship to gender and social variables. Clin Microbiol Infect. 2006;12:961–7. doi: 10.1111/j.1469-0691.2006.01477.x. [DOI] [PubMed] [Google Scholar]
- 25.Harris S. Japanese biological warfare research on humans: a case study of micro-biology and ethics. Ann N Y Acad Sci. 1992;666:21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x. [DOI] [PubMed] [Google Scholar]
- 26.Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM., Jr Biological warfare. A historical perspective. JAMA. 1997;278:412–7. [PubMed] [Google Scholar]
- 27.Alibek K. The Soviet Union's anti-agricultural biological weapons. Ann N Y Acad Sci. 1999;894:18–9. doi: 10.1111/j.1749-6632.1999.tb08038.x. [DOI] [PubMed] [Google Scholar]
- 28.Rohrbach BW, Westerman E, Istre GR. Epidemiology and clinical characteristics of tularemia in Oklahoma, 1979 to 1985. South Med J. 1991;84:1091–6. doi: 10.1097/00007611-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–57. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bitsaktsis C, Rawool DB, Li Y, Kurkure NV, Iglesias B, Gosselin EJ. Differential requirements for protection against mucosal challenge with Francisella tularensis in the presence versus absence of cholera toxin B and inactivated F. tularensis. J Immunol. 2009;182:4899–909. doi: 10.4049/jimmunol.0803242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, et al. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–8. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Moxley G, Stern AG, Carlson P, Estrada E, Han J, Benson LL. Premenopausal sexual dimorphism in lipopolysaccharide-stimulated production and secretion of tumor necrosis factor. J Rheumatol. 2004;31:686–94. [PubMed] [Google Scholar]
- 33.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 34.Bakshi CS, Malik M, Mahawar M, Kirimanjeswara GS, Hazlett KR, Palmer LE, et al. An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine. 2008;26:5276–88. doi: 10.1016/j.vaccine.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, et al. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–47. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Shankar J, Restrepo A, Clemons KV, Stevens DA. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev. 2011;24:296–313. doi: 10.1128/CMR.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–53. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 38.Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8:627–39. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev. 2008;225:244–55. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjostedt A, Eriksson M, Sandstrom G, Tarnvik A. Various membrane proteins of Francisella tularensis induce interferon-gamma production in both CD4+ and CD8+ T cells of primed humans. Immunology. 1992;76:584–92. [PMC free article] [PubMed] [Google Scholar]
- 41.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–54. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, et al. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis. 2007;196:134–7. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 43.Kubelkova K, Krocova Z, Balonova L, Pejchal J, Stulik J, Macela A. Specific antibodies protect gamma-irradiated mice against Francisella tularensis infection. Microb Pathogen. 2012;53:259–68. doi: 10.1016/j.micpath.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 45.Messingham KA, Heinrich SA, Kovacs EJ. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J Leukoc Biol. 2001;70:887–95. [PubMed] [Google Scholar]
- 46.Frazier-Jessen MR, Kovacs EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995;154:1838–45. [PubMed] [Google Scholar]
- 47.Sharma J, Mares CA, Li Q, Morris EG, Teale JM. Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain. Microb Pathogen. 2011;51:39–47. doi: 10.1016/j.micpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.