Abstract
Water disinfection greatly reduced the incidence of waterborne diseases, but the reaction between disinfectants and natural organic matter in water leads to the formation of drinking water disinfection by-products (DBPs). DBPs have been shown to be toxic, but their effects on the ovary are not well defined. This study tested the hypothesis that monohalogenated DBPs (chloroacetic acid, CAA; bromoacetic acid, BAA; iodoacetic acid, IAA) inhibit antral follicle growth and steroidogenesis in mouse ovarian follicles. Antral follicles were isolated and cultured with either vehicle or DBPs (0.25–1.00 mM of CAA; 2–15 µM of BAA or IAA) for 48 and 96 h. Follicle growth was measured every 24 h and the media were analyzed for estradiol levels at 96 h. Exposure to DBPs significantly inhibited antral follicle growth and reduced estradiol levels compared to controls. These data demonstrate that DBP exposure caused ovarian toxicity in vitro.
Keywords: disinfection by-products, haloacetic acids, ovary, folliculogenesis, steroidogenesis
Introduction
The treatment of water using disinfectants such as chlorine, ozone, chloramines, chlorine dioxide, and ultraviolet radiation greatly reduced the incidence of waterborne diseases, including cholera and typhoid and is considered a major public health achievement of the 20th century.1 However, the reaction between disinfectants and natural organic as well as inorganic matter in the source water can lead to an unintended consequence, which is the formation of drinking water disinfection by-products (DBPs).2, 3 Trihalomethanes were the first DBP chemical class discovered in 1974.4, 5 To date, more than 600 DBPs have been identified in finished drinking waters.3 In 2006, the U.S. EPA issued the Stage 2 Disinfectants (D)/DBP Rule to control the maximum contaminant levels of 11 DBPs, including four trihalomethanes, five haloacetic acids (HAAs), bromate (BrO3−), and chlorite (ClO2−).6 The five regulated HAAs include chloroacetic acid (CAA), dichloroacetic acid, trichloroacetic acid, bromoacetic acid (BAA), and dibromoacetic acid at a maximum contaminant level of 60 µg/L for their sum.6 Unregulated HAAs include iodoacetic acid (IAA), bromochloroacetic acid, tribromoacetic acid, bromodichloroacetic acid, and chlorodibromoacetic acid.7
Many DBPs are cytotoxic, genotoxic, mutagenic, and teratogenic.8 Epidemiological studies demonstrate an association between lifetime exposures to DBPs and increased risk of cancers.9–12 Further, some studies show an association between DBP exposure and adverse pregnancy outcomes such as low birth weight, small-for-gestational age, still birth, and birth defects.13–20 Because the HAAs are the most regulated chemical class of DBPs, many studies focused on their toxicological impacts both in vitro and in vivo. The monohaloacetic acids (monoHAAs) modulate gene expression involved in the stress response to DNA damage, cell cycle regulation, the induction of reactive oxygen species, and apoptosis in non-transformed human intestinal cells.21–23 IAA exposure induces malignant transformation of NIH/3T3 xenografts in Balb/c nude mice that progress to highly aggressive fibrosarcomas.24 MonoHAAs affect CD-1 mouse embryos and induce dysmorphogenesis in neural tube and eye development and produce anomalies in heart development.25 Several dihaloacetic acids alter intestinal microbial populations and their metabolism, which could lead to bioactivation of promutagens or procarcinogens in rats.26 Dibromoacetic acid alters spermatogenesis and disrupts testicular steroidogenesis in male rats.27, 28 In addition, dibromoacetic acid disrupts estrous cyclicity and suppresses estradiol catabolism, which leads to alterations in steroid production in female rats.29, 30
Although the previous studies indicate that DBPs are toxicants in many systems, the effects of the monoHAAs on the ovary are largely unknown. The ovarian follicle is the functional unit of the ovary that is responsible for growth of the oocyte and the production of sex steroid hormones. Antral follicles are the most mature type of mammalian ovarian follicles and are the major source of sex steroid hormone production. Because antral follicles are the only follicle type capable of ovulation and the major producers of estradiol (E2), alterations in proper growth or function of the antral follicle may result in reduced fertility or abnormal steroidogenesis. Therefore, this study tested the hypothesis that the monoHAAs (CAA, BAA, and IAA) directly affect follicle growth and E2 production in mouse antral follicles in vitro.
Material and Methods
Chemicals
The monoHAAs (CAA, BAA, and IAA) were purchased from Sigma-Aldrich (St. Louis, MO). The sources and purities of the monoHAAs used in this research are listed in Table 1. Stock solutions of the monoHAAs (1 M) were dissolved in dimethylsulfoxide (DMSO) and stored in sealed sterile glass vials (Supelco, Bellefonte, PA) at −20 °C. Each stock solution was diluted in α-minimal essential medium (α-MEM, Life Technologies, Grand Island, NY) on the day of each experiment. Final concentrations in culture were 0.25, 0.50, 0.75, and 1.00 mM of CAA; and 2, 5, 10 and 15 µM of BAA and IAA.
Table 1.
Monohaloacetic acid sources and purities
| HAAa | CASN | MW (g/mol) | Source | Purity (%) |
|---|---|---|---|---|
| CAA | 79-11-8 | 94.5 | Fluka | >99 |
| BAA | 79-08-3 | 138.95 | Fluka | >99 |
| IAA | 64-69-7 | 185.95 | Sigma-Aldrich | >99 |
Abbreviations: HAA, haloacetic acids; CAA, chloroacetic acid; BAA, bromoacetic acid; IAA, iodoacetic acid
These culture concentrations were based on previous studies in other cell types.31–33 Specifically, Plewa et al.33 conducted a comparative systematic analysis of chronic cytotoxicity and acute genomic DNA damaging capacity of 12 individual HAAs in mammalian cells. Interestingly, IAA and BAA showed the highest chronic cytotoxicity and the rank order for genotoxicity was IAA > BAA > CAA, followed by other di- and trihaloacetic acids. These results supported the hypothesis that monoHAAs may pose a public health concern and various follow-up studies were conducted to investigate the molecular mechanisms of monoHAA toxicity.31–33 Even though CAA was less cytotoxic compared to BAA and IAA, comparing all three different halogen substitutes can provide better insights on the impact of type of halogen on structure-activity relationship. Therefore, we decided to test the selected three monoHAAs for this study. The concentrations of monoHAAs were chosen from previous studies showing that they induced genomic DNA damage in Chinese hamster ovary (CHO) cells within the concentration range that expressed above 70% cell viability.31–33 In a previous study conducted by Plewa et al.33, the lowest toxic concentrations for CAA, BAA and IAA were shown to be 0.25 mM, 2 µM, and 0.5 µM, respectively. Further, Dad et al.31 determined that the lowest non-cytotoxic concentrations of CAA, BAA and IAA that induced a significant reduction in ATP levels as compared to each concurrent negative control were 1 mM, 6 µM, and 3 µM, respectively. Using these values as guidance, we conducted a rough concentration range-finder assay for antral follicles to optimize the selected monoHAA concentrations.
The lowest concentrations of CAA, BAA and IAA used in this study correspond to 23,625 µg/L, 277.9 µg/L and 371.9 µg/L, respectively. These levels are relatively higher than the average HAA levels found in drinking water facilities. For example, in the U.S. EPA’s information collection rule (ICR) effort, which involved 500 large drinking water facilities, HAAs ranged from <1.0 to 170 µg/L individually.34 In another occurrence study, HAAs ranged from 5 to 130 µg/L, with a median of 34 µg/L.35 Even though the levels of monoHAAs used in this study are higher than the HAA levels found in finished drinking water, it should be noted that the human population gets exposed to DBPs not only by ingestion, but also by inhalation and dermal exposure through showering or bathing, resulting in higher accumulated exposure levels.6
Animals
Cycling female CD-1 mice (postnatal days 28–30) were purchased from Charles River Laboratories (Charles River, CA). The mice were housed at the University of Illinois at Urbana-Champaign, College of Veterinary Medicine Animal Facility. Mice were housed under 12-hour light-dark cycles at 22 ± 1 °C and were provided food and water ad libitum. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
Mouse Antral Follicle Culture
Cycling female CD-1 mice were euthanized on postnatal days 31–38 and their antral follicles were mechanically isolated from the ovary based on size (200 – 450 µm). Interstitial tissues were removed from the isolated follicles using watchmaker’s forceps.36–38 Ovaries from 2–3 mice were used for each experiment and approximately 25–30 antral follicles were obtained per mouse per experiment. Isolated antral follicles were individually placed in wells of a 96-well culture microplate with unsupplemented α-MEM prior to treatment. Treatment groups included either DMSO vehicle control or different concentrations of the selected monoHAAs (CAA: 0.25, 0.50, 0.75, or 1.00 mM; BAA and IAA: 2, 5, 10 or 15 µM), which were prepared in supplemented α-MEM. Supplemented α-MEM contained unsupplemented α-MEM, 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium, Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin (Sigma-Aldrich, St. Louis, MO), 100 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO), 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (FCS; Atlanta Biological, Lawrenceville, GA).39 An equal volume of DMSO or monoHAAs (0.75 µl/ml media) was added for each treatment to obtain constant vehicle concentration. Follicles were incubated for 96 hours at 37 °C, 5% CO2 in 150 µl of supplemented α-MEM medium. During culture, follicle growth was measured every 24 hours as described below. At 96 hours, media were collected and subjected to E2 measurements as described below. Each separate experiment contained 8–12 follicles per treatment group, and each experiment was repeated at least 3 times.
For recovery experiments, media containing monoHAAs were removed from the culture wells after 48 hours and replaced with fresh supplemented α-MEM. The cultures were incubated for an additional 48 hours. Follicle growth was measured every 24 hours and E2 levels were measured at 48 hours and 96 hours as described below. Each experiment was conducted on at least three separate occasions.
Analysis of Antral Follicle Growth
Antral follicle growth was examined every 24 hours by measuring follicle diameters on perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameters were averaged and presented as percent change over time (0 hour, 100%) for each treatment group.
Analysis of E2 Levels
For continuous monoHAA exposure groups, media from each treatment group were collected after 96 hours of incubation and stored at −80 °C until all experiments were conducted. For recovery experiments, two sets of media were collected. The first set of media containing monoHAAs was collected from the culture wells after 48 hours and the second set of media, which were freshly replaced at 48 hours, was collected after an additional 48 hours of incubation and then stored at −80 °C. Collected media were diluted 1:5 and subjected to measurements of E2 levels using enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s guidelines (DRG International, Mountainside, NJ). All samples were run in duplicate and mean values of each sample were used for the statistical analysis. The analytical sensitivity of the ELISA was 9.714 pg/ml and both intra- and inter-assay coefficients of variation were below 10%. E2 levels were averaged and presented as percent change compared to the DMSO control.
Statistical Analysis
Data were expressed as the mean ± SEM and comparisons among experimental groups were performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc comparison. Statistical significance was assigned at p < 0.05.
Results
Effect of MonoHAA Treatments on Antral Follicle Growth
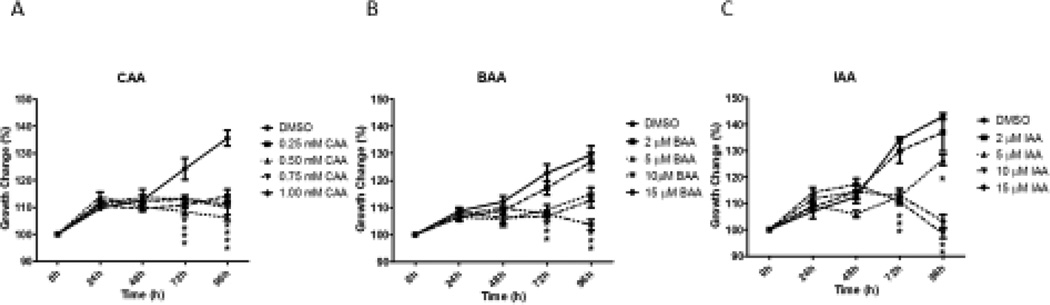
All three monoHAAs (96 hour exposures) inhibited growth of antral follicles.. CAA (0.25, 0.5, 0.75 and 1.0 mM) inhibited antral follicle growth compared to DMSO controls, beginning at 72 hours and continuing through 96 hours of culture (Figure 1A). BAA or IAA exposure (5, 10 and 15 µM) inhibited antral follicle growth compared to controls, beginning at the 72 exposure time and continuing through 96 hours of culture (Figure 1B and Figure 1C).
Figure 1. Effect of CAA, BAA or IAA Treatment on Antral Follicle Growth.
Mechanically isolated antral follicles were cultured with CAA, BAA, or IAA for 96 hours. Growth of follicles was recorded in micrometers every 24 hours and reported as percent change compared to the follicle size at the beginning of treatment (0 hour = 100%). DMSO = dimethylsulfoxide, CAA = chloroacetic acid, BAA = bromoacetic acid, IAA = iodoacetic acid. Data represent means ± SEM from at least three separate experiments. Asterisks (*) represent significant differences from DMSO control at each time point (p ≤ 0.05).
Effect of MonoHAA Treatments on Estradiol Levels
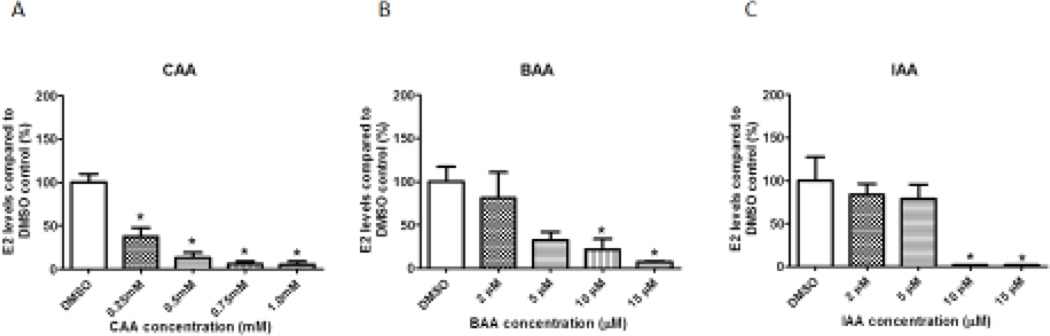
All monoHAAs reduced E2 levels in the media compared to controls (Figure 2). Treatment with 0.25, 0.50, 0.75 or 1.00 mM CAA for 96 hours decreased E2 levels compared to the DMSO control (Figure 2A). Similarly, exposure treatment with 10 or 15 µM BAA or 10 or 15 µM IAA for 96 hours decreased E2 levels compared to the controls (Figure 2B and Figure 2C).
Figure 2. Effect of CAA, BAA or IAA Treatment on Estradiol Levels.
Antral follicles were cultured with CAA, BAA or IAA for 96 hours. After culture, media were collected and analyzed for estradiol levels. DMSO = dimethylsulfoxide, CAA = chloroacetic acid, BAA = bromoacetic acid, IAA = iodoacetic acid. Data represent means ± SEM from at least three separate experiments. Asterisks (*) represent significant differences from DMSO control (p ≤ 0.05).
Effects of Acute (48 hour) MonoHAA Treatments on Recovery of Antral Follicle Recovery Growth
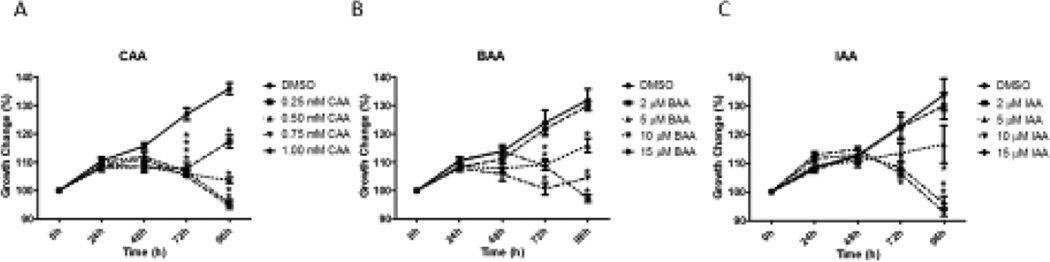
Exposure to monoHAAs for 48 hours was sufficient to inhibit antral follicle growth at 96 hours compared to controls (Figure 3). Exposure to CAA (0.25, 0.5, 0.75 or 1.0 mM) for 48 hours inhibited antral follicle growth compared with DMSO controls beginning at 72 hours and continuing through 96 hours of culture (Figure 3A; n = 4, p < 0.05). Exposure to 5, 10 or 15 µM BAA for 48 hours inhibited antral follicle growth beginning at 72 hours and continued through 96 hours of culture (Figure 3B). Exposure to 5 or 15 µM IAA for 48 hours inhibited antral follicle growth at 96 hours, whereas exposure to 10 µM IAA for 48 hours inhibited antral follicle growth starting at 72 hours and continuing for 96 hours of culture (Figure 3C).
Figure 3. Effect of Acute 48 hour-Exposure of CAA, BAA or IAA on the Recovery of Antral Follicle Growth.
Mechanically isolated antral follicles were cultured with CAA, BAA or IAA. Chemicals were removed after 48 hours and follicles were cultured for an additional 48 hours with fresh supplemented α-MEM (total 96 hours of culture). Growth of follicles was recorded in micrometers every 24 hours and reported as percent change compared to the follicle size at the beginning of culture. DMSO = dimethylsulfoxide, CAA = chloroacetic acid, BAA = bromoacetic acid, IAA = iodoacetic acid. Data represent means ± SEM from at least three separate experiments. Asterisks (*) represent significant differences from DMSO control at each time-point (p ≤ 0.05).
Effects of Acute 48 hour-MonoHAA Treatments on Estradiol Levels
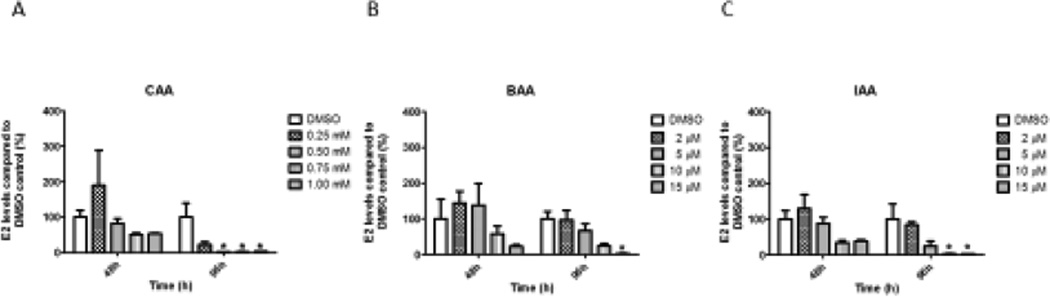
Exposure to three monoHAAs for 48 hours followed by recovery induced significant decreases in E2 levels in the media at 96 hours (Figure 4). Specifically, treatment with 0.5, 0.75 and 1.0 mM CAA for 48 hours decreased E2 levels compared to the DMSO control at 96 hours (Figure 4A). Similar to the continuous 96-hour exposure with monoHAAs, 15 µM BAA, 10 µM IAA, or 15 µM IAA for 48 hours inhibited E2 levels at 96 hours compared to the controls (Figure 4B and Figure 4C).
Figure 4. Effect of Acute 48 hour-Exposure of CAA, BAA or IAA With and Without Recovery on Estradiol Levels.
Antral follicles were cultured with CAA, BAA or IAA. Chemicals were removed after 48 hours and follicles were cultured for an additional 48 hours with fresh supplemented α-MEM (total 96 hours of culture). Media were collected at 48 and 96 hours and analyzed for estradiol levels. DMSO = dimethylsulfoxide, CAA = chloroacetic acid, BAA = bromoacetic acid, IAA = iodoacetic acid. Data represent means ± SEM from at least three separate experiments. Asterisks (*) represent significant differences from DMSO control (p ≤ 0.05).
Discussion
These studies demonstrate that both continuous (96 hour) and acute (48 hour) exposure followed by 48 hours of recovery to three monoHAAs inhibited growth of antral follicles and decreased E2 levels in vitro. To our knowledge, this is the first study to examine the direct effects of monoHAAs on mouse antral follicles.
Many studies have investigated the direct effects of other environmental toxicants such as pesticides and environmental endocrine disruptors on ovarian follicles.36, 38, 40 However, little is known about the potential toxic effects of DBPs, which are ubiquitous in finished drinking water, on the ovary. This study demonstrated that the monoHAAs express similar adverse effects on folliculogenesis as well-known environmental reproductive toxicants, including methoxychlor, bisphenol A, and phthalates.36–41 It is possible that ovarian toxicity is affected by exposure time and that follicles could recover after the removal of some toxicant from the biological systems. However, our study demonstrated that 48 hours of monoHAA exposure permanently inhibited antral follicle growth and E2 production.
One possible mechanism of monoHAA-induced antral follicle growth inhibition is the generation of reactive oxygen species (ROS). IAA induces toxicity in hippocampal neuronal cells by inhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a major enzyme involved in glycolysis, leading to hypoglycemia and the generation of ROS.42 In CHO cells, monoHAAs inhibit GAPDH activity and reduce pyruvate, a substrate for the tricarboxylic acid cycle.32 This lack of pyruvate causes mitochondrial stress, leading to the generation of ROS, a reduction in cellular ATP levels, and induced cytotoxicity and genotoxicity.23, 31 In addition, exposure to each monoHAA alters the transcription levels of multiple oxidative stress responsive genes and induces the antioxidant response element.23 Interestingly, previous studies showed that other toxicants that inhibit follicle growth and steroidogenesis cause oxidative stress in mouse antral follicles. Di(2-ethylhexyl) phthalate and its metabolite mono-(2-ethylhexyl) phthalate induce oxidative stress and inhibit mouse antral follicle growth.43, 44 These studies further support the hypothesis that monoHAAs may cause ovarian toxicity through ROS production.
HAAs are alkylating agents that undergo SN2 reactions to form covalent bonds with nucleophiles in living systems. The SN2 reactivity of monoHAAs is dependent on the α-carbon-halide bond length and bond dissociation energy and follows the pattern of C-I > C-Br >> C-Cl. This relative SN2 reactivity correlates with cytotoxicity and genotoxicity in CHO cells.33 The inhibition kinetics of GAPDH correlates with the alkylating potential of monoHAAs and correlates with a wide range of adverse biological effects among a diversity of bioassay systems.32 In this study, IAA and BAA inhibited folliculogenesis and E2 production at lower concentrations compared to CAA, following the reported pattern of monoHAA toxic potency.32, 33 Compared to previous studies where the distinct toxicity rank order of IAA > BAA > CAA was observed,33 IAA and BAA showed similar toxic potencies in this study. These results could be due the complexity of the antral follicle structure compared to cultured cells. It is possible that IAA loses its iodo substituent before it exerts follicles toxicity. The iodo substituent is a better leaving group than the bromo substituent. Thus, IAA has a better chance of losing its α-carbon-halide reactive site than BAA.
Adverse reproductive outcomes such as infertility are significant health problems worldwide.45 The causes of infertility and other adverse reproductive outcomes are often unclear, but it is known that environmental toxicants may alter female reproduction.46 Therefore, understanding the potential toxic effects of agents that we consume on a daily basis over a lifetime is important. Although our in vitro data indicate that direct exposure to monoHAAs causes ovarian toxicity, it is still unknown whether there is a strong correlation between DBP exposure and fertility outcomes in women. While investigating the toxicity of a single chemical is important, the toxicological effects of a complex DBP mixture should be considered carefully because the complex mixture represents the composition of finished water that people consume in daily life. Therefore, there is a need for toxicology research to examine DBP mixtures using quantitative biological analyses. This study focused on the toxicity of three monoHAAs on the ovarian follicle and clearly showed their different toxic potencies. Therefore, we anticipate that this follicle assay may be a useful tool for investigating and analyzing the adverse effects of DBP mixtures on ovarian follicles. Such experiments will provide information that fills the gaps between individual DBP studies in vitro and in vivo and eventually help provide guidelines to estimate the health risks of DBP exposure.
Compared to the epidemiology studies on cancer risk, epidemiology studies on adverse reproductive outcomes have broader endpoints and tend to have lower odds-ratios.19, 47, 48 Several points need to be considered in advance to support a reasonable conclusion. Exposure assessment in epidemiology studies is challenging. The DBP exposure route includes ingestion, inhalation, and absorption through the skin through showering and bathing.49 Therefore, a detailed questionnaire or interview is needed for estimations of exposure, and blood sampling may improve the accuracy of actual DBP exposure levels. Even though trihalomethanes and HAAs are the most abundant DBP classes found in drinking water, evidence demonstrates that other DBPs are more cytotoxic and genotoxic than trihalomethanes or HAAs.7, 50–52 In addition, studies suggest that adverse pregnancy outcomes may result from the toxicity of DBP mixtures and not from individual DBP compounds.53, 54 Therefore, efforts are needed to monitor and determine the levels and toxicity of other DBP chemical classes in finished drinking water. Haloacetonitriles (another DBP class) reduces fertility and increases early implantation failure in rats.55 Maternal exposure to trichloroacetonitrile results in fetal cardiovascular and urogenital anomalies.56 Gestational exposure to mixtures of regulated trihalomethanes and HAAs results in pregnancy loss and eye malformation in rats.57 However, a recent study evaluated the toxicity of a DBP mixture in a multigenerational bioassay in rats and observed no adverse effects on fertility, pregnancy maintenance, prenatal or postnatal survival, and birth weights.58 Collectively, these data highlight the need for in depth studies on reproductive toxicity of DBPs and their biological mechanisms.
In conclusion, the purpose of this study was to test the hypothesis that CAA, BAA, and IAA inhibit antral follicle growth and E2 levels in cultured mouse ovarian antral follicles. The present study was designed to compare the effects of acute and continuous monoHAA exposure on ovarian follicles. The three selected monoHAAs significantly inhibited the growth of antral follicles and reduced E2 levels compared to controls in a dose-response manner. After an initial monoHAA exposure, follicles did not recover from exposure in terms of growth or E2 levels. This study demonstrates that monoHAAs are ovarian toxicants in vitro.
Future studies should determine the mechanisms by which the selected monoHAAs inhibit follicle growth and reduce estradiol levels. Such studies could examine if the selected monoHAAs cause slow follicle growth by inducing atresia or oxidative stress and/or by inhibiting cell cycle regulators. Such studies could determine if the selected monoHAAs reduce estradiol production by inhibiting steroidogenic enzyme activity in the estradiol biosynthesis pathway or by increasing the ability of antral follicles to metabolize estradiol.
Highlights.
MonoHAAs inhibit mouse antral follicle growth in vitro.
Continuous monoHAA exposure reduces the levels of E2 in media.
Acute monoHAA-exposure inhibits follicle growth and decreases E2 levels.
MonoHAAs may be ovarian toxicants.
Acknowledgments
This work was supported by grant numbers NIH R01ES019178 (JAF) NIH R01 DK 093690 (WAR), NIH T32 ES 007326 (CJ), and NIH T32 ES 007015 (CJ).
Abbreviations
- BAA
bromoacetic acid
- CAA
chloroacetic acid
- CHO
Chinese hamster ovary
- DBP
disinfection by-product
- DMSO
dimethylsulfoxide
- E2
estradiol
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IAA
iodoactic acid
- α-MEM
α-minimal essential medium
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler D, Miller G. The role of public health improvements in health advances: the twentieth-century United States. Demography. 2005;42(1):1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 2.Krasner SW. The formation and control of emerging disinfection by-products of health concern. Philos. Transact. A Math. Phys. Eng. Sci. 2009;367(1904):4077–4095. doi: 10.1098/rsta.2009.0108. [DOI] [PubMed] [Google Scholar]
- 3.Richardson SD, Postigo C. Formation of DBPs: State of the Science. Recent Advances in Disinfection by-Products. 2015;1190:189–214. [Google Scholar]
- 4.Bellar TA, Lichtenberg JJ, Kroner RC. Occurrence of Organohalides in Chlorinated Drinking Waters. J Am Water Works Ass. 1974;66(12):703–706. [Google Scholar]
- 5.Rook JJ. Formation of haloforms during chlorination of natural waters. J. Soc. Water Treat. Exam. 1974;23:234–243. [Google Scholar]
- 6.U. S. Environmental Protection Agency. National primary drinking water regulations: Stage 2 disinfectants and disinfection byproducts rule. Fed. Reg. 2006;71(2):387–493. [Google Scholar]
- 7.Plewa MJ, Wagner ED. Charting a New Path To Resolve the Adverse Health Effects of DBPs. In: Karanfil T, Mitch W, Westerhoff P, Xie Y, editors. Occurrence, Formation, Health Effects, and Control of Disinfection By-Products. Vol. 1190. Washington, DC: 2015. pp. 3–23. Am. Chem Soc. [Google Scholar]
- 8.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. 2007;636(1–3):178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Costet N, Villanueva CM, Jaakkola JJ, Kogevinas M, Cantor KP, King WD, Lynch CF, Nieuwenhuijsen MJ, Cordier S. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case-control studies. Occup. Environ. Med. 2011;68(5):379–385. doi: 10.1136/oem.2010.062703. [DOI] [PubMed] [Google Scholar]
- 10.Kogevinas M. Epidemiological approaches in the investigation of environmental causes of cancer: the case of dioxins and water disinfection by-products. Environ. Health. 2011;1(Suppl 1):S3. doi: 10.1186/1476-069X-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva CM, Cantor KP, Cordier S, Jaakkola JJ, King WD, Lynch CF, Porru S, Kogevinas M. Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology. 2004;15(3):357–367. doi: 10.1097/01.ede.0000121380.02594.fc. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castano-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am. J. Epidemiol. 2007;165(2):148–156. doi: 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- 13.Aschengrau A, Zierler S, Cohen A. Quality of community drinking water and the occurrence of late adverse pregnancy outcomes. Arch. Environ. Health. 1993;48(2):105–113. doi: 10.1080/00039896.1993.9938403. [DOI] [PubMed] [Google Scholar]
- 14.Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE. Public drinking water contamination and birth outcomes. Am. J. Epidemiol. 1995;141(9):850–862. doi: 10.1093/oxfordjournals.aje.a117521. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm K, Cook A, Bower C, Weinstein P. Risk of birth defects in Australian communities with high levels of brominated disinfection by-products. Environ. Health Perspect. 2008;116(9):1267–1273. doi: 10.1289/ehp.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang BF, Jaakkola JJ, Guo HR. Water disinfection by-products and the risk of specific birth defects: a population-based cross-sectional study in Taiwan. Environ. Health. 2008;7:23. doi: 10.1186/1476-069X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang BF, Magnus P, Jaakkola JJ. Risk of specific birth defects in relation to chlorination and the amount of natural organic matter in the water supply. Am. J. Epidemiol. 2002;156(4):374–382. doi: 10.1093/aje/kwf038. [DOI] [PubMed] [Google Scholar]
- 18.Magnus P, Jaakkola JJ, Skrondal A, Alexander J, Becher G, Krogh T, Dybing E. Water chlorination and birth defects. Epidemiology. 1999;10(5):513–517. [PubMed] [Google Scholar]
- 19.Rivera-Nunez Z, Wright JM. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J. Occup. Environ. Med. 2013;55(10):1125–1134. doi: 10.1097/JOM.0b013e3182a4ffe4. [DOI] [PubMed] [Google Scholar]
- 20.Wright JM, Schwartz J, Dockery DW. Effect of trihalomethane exposure on fetal development. Occup. Environ. Med. 2003;60(3):173–180. doi: 10.1136/oem.60.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attene-Ramos MS, Wagner ED, Plewa MJ. Comparative human cell toxicogenomic analysis of monohaloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol. 2010;44(19):7206–7212. doi: 10.1021/es1000193. [DOI] [PubMed] [Google Scholar]
- 22.Muellner MG, Attene-Ramos MS, Hudson ME, Wagner ED, Plewa MJ. Human cell toxicogenomic analysis of bromoacetic acid: a regulated drinking water disinfection by-product. Environ. Mol. Mutagen. 2010;51:205–214. doi: 10.1002/em.20530. [DOI] [PubMed] [Google Scholar]
- 23.Pals J, Attene-Ramos MS, Xia M, Wagner ED, Plewa MJ. Human cell toxicogenomic analysis linking reactive oxygen species to the toxicity of monohaloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol. 2013;47(21):12514–12523. doi: 10.1021/es403171b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X, Wang S, Zheng W, Wang X, Liu X, Jiang S, He G, Zheng Y, Qu W. Tumorigenicity of drinking water disinfection byproduct iodoacetic acid in NIH3T3 cells. Envion. Sci. Technol. 2013;47(11):5913–5920. doi: 10.1021/es304786b. [DOI] [PubMed] [Google Scholar]
- 25.Hunter ES, Rogers EH, Schmid JE, Richard A. Comparative effects of haloacetic acids in whole embryo culture. Teratology. 1996;54(2):57–64. doi: 10.1002/(SICI)1096-9926(199606)54:2<57::AID-TERA1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.George SE, Nelson GM, Swank AE, Brooks LR, Bailey K, George M, DeAngelo A. The disinfection by-products dichloro-, dibromo-, and bromochloroacetic acid impact intestinal microflora and metabolism in Fischer 344 rats upon exposure in drinking water. Toxicol. Sci. 2000;56(2):282–289. doi: 10.1093/toxsci/56.2.282. [DOI] [PubMed] [Google Scholar]
- 27.Carr TL, Ciurlionis R, Milicic I, Whitney K, Ligouri MJ, Warder SE, Strakhova MI, Blomme EAG. Role of cytochrome P450c17 alpha in dibromoacetic acid-induced testicular toxicity in rats. Arch. Toxicol. 2011;85(5):513–523. doi: 10.1007/s00204-010-0600-2. [DOI] [PubMed] [Google Scholar]
- 28.Linder RE, Klinefelter GR, Strader LF, Suarez JD, Roberts NL, Dyer CJ. Spermatotoxicity of dibromoacetic acid in rats after 14 daily exposures. Reprod. Toxicol. 1994;8(3):251–259. doi: 10.1016/0890-6238(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 29.Balchak SK, Hedge JM, Murr AS, Mole ML, Goldman JM. Influence of the drinking water disinfection by-product dibromoacetic acid on rat estrous cyclicity and ovarian follicular steroid release in vitro. Reprod. Toxicol. 2000;14(6):533–539. doi: 10.1016/s0890-6238(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 30.Goldman JM, Murr AS. Dibromoacetic acid-induced elevations in circulating estradiol: effects in both cycling and ovariectomized/steroid-primed female rats. Reprod. Toxicol. 2003;17(5):585–592. doi: 10.1016/s0890-6238(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 31.Dad A, Jeong CH, Pals JA, Wagner ED, Plewa MJ. Pyruvate remediation of cell stress and genotoxicity induced by haloacetic acid drinking water disinfection by-products. Environ. Mol. Mutagen. 2013;54(8):629–637. doi: 10.1002/em.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pals JA, Ang JK, Wagner ED, Plewa MJ. Biological mechanism for the toxicity of haloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol. 2011;45(13):5791–5797. doi: 10.1021/es2008159. [DOI] [PubMed] [Google Scholar]
- 33.Plewa MJ, Simmons JE, Richardson SD, Wagner ED. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ. Mol. Mutagen. 2010;51(8–9):871–878. doi: 10.1002/em.20585. [DOI] [PubMed] [Google Scholar]
- 34.McGuire MJ, McLain JL, Obolensky A. Information Collection Rute Data Analysis. Denver, CO: AwwaRF and AWWA; 2002. [Google Scholar]
- 35.Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad GD, Thruston AD., Jr The occurrence of a new generation of disinfection by-products. Environ. Sci. Technol. 2006;40(23):7175–7185. doi: 10.1021/es060353j. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006;93(2):382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 37.Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol. Sci. 2005;88(1):213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 38.Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 2011;119(1):209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 2015;92(5):120. doi: 10.1095/biolreprod.115.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015;284(1):42–53. doi: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziv-Gal A, Craig ZR, Wang W, Flaws JA. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod. Toxicol. 2013;42:58–67. doi: 10.1016/j.reprotox.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Fonseca K, Cardenas-Rodriguez N, Pedraza-Chaverri J, Massieu L. Calcium-dependent production of reactive oxygen species is involved in neuronal damage induced during glycolysis inhibition in cultured hippocampal neurons. J. Neurosci. Res. 2008;86(8):1768–1780. doi: 10.1002/jnr.21634. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol. Appl. Pharmacol. 2012;258(2):288–295. doi: 10.1016/j.taap.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod. 2012;87(6):152. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum. Reprod. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 46.Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol. Reprod. 2015;93(1):20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinckley AF, Bachand AM, Reif JS. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ. Health Perspect. 2005;113(12):1808–1813. doi: 10.1289/ehp.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CY, Xiao ZP, Ho SC, Wu TN, Tsai SS. Association between trihalomethane concentrations in drinking water and adverse pregnancy outcome in Taiwan. Environ. Res. 2007;104(3):390–395. doi: 10.1016/j.envres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Arbuckle TE, Hrudey SE, Krasner SW, Nuckols JR, Richardson SD, Singer P, Mendola P, Dodds L, Weisel C, Ashley DL, Froese KL, Pegram RA, Schultz IR, Reif J, Bachand AM, Benoit FM, Lynberg M, Poole C, Waller K. Assessing exposure in epidemiologic studies to disinfection by-products in drinking water: Report from an international workshop. Environ. Health Perspect. 2002;110:53–60. doi: 10.1289/ehp.02110s153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong CH, Wagner ED, Siebert VR, Anduri S, Richardson SD, Daiber EJ, McKague AB, Kogevinas M, Villanueva CM, Goslan EH, Luo W, Isabelle LM, Pankow JF, Grazuleviciene R, Cordier S, Edwards SC, Righi E, Nieuwenhuijsen MJ, Plewa MJ. Occurrence and toxicity of disinfection byproducts in European drinking waters in relation with the HIWATE epidemiology study. Environ. Sci. Technol. 2012;46(21):12120–12128. doi: 10.1021/es3024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plewa MJ, Wagner ED. Mammalian Cell Cytotoxicity and Genotoxicity of Disinfection By-Products. Denver, CO: Water Research Foundation; 2009. p. 134. [Google Scholar]
- 52.Richardson SD, Fasano F, Ellington JJ, Crumley FG, Buettner KM, Evans JJ, Blount BC, Silva LK, Waite TJ, Luther GW, McKague AB, Miltner RJ, Wagner ED, Plewa MJ. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ. Sci. Technol. 2008;42(22):8330–8338. doi: 10.1021/es801169k. [DOI] [PubMed] [Google Scholar]
- 53.Simmons JE, Richardson SD, Speth TF, Miltner RJ, Rice G, Schenck KM, Hunter ES, 3rd, Teuschler LK. Development of a research strategy for integrated technology-based toxicological and chemical evaluation of complex mixtures of drinking water disinfection byproducts. Environ. Health Perspect. 2002;110(Suppl 6):1013–1024. doi: 10.1289/ehp.02110s61013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons JE, Teuschler LK, Gennings C, Speth TF, Richardson SD, Miltner RJ, Narotsky MG, Schenck KD, Hunter ES, 3rd, Hertzberg RC, Rice G. Component-based and whole-mixture techniques for addressing the toxicity of drinking-water disinfection by-product mixtures. J. Toxicol. Environ. Health A. 2004;67(8–10):741–754. doi: 10.1080/15287390490428215. [DOI] [PubMed] [Google Scholar]
- 55.Smith MK, George EL, Zenick H, Manson JM, Stober JA. Developmental toxicity of halogenated acetonitriles: drinking water by-products of chlorine disinfection. Toxicology. 1987;46(1):83–93. doi: 10.1016/0300-483x(87)90140-5. [DOI] [PubMed] [Google Scholar]
- 56.Smith MK, Randall JL, Tocco DR, York RG, Stober JA, Read EJ. Teratogenic effects of trichloroacetonitrile in the Long-Evans rat. Teratology. 1988;38(2):113–120. doi: 10.1002/tera.1420380203. [DOI] [PubMed] [Google Scholar]
- 57.Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES, 3rd, Simmons JE. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reprod. Toxicol. 2011;31(1):59–65. doi: 10.1016/j.reprotox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Narotsky MG, Klinefelter GR, Goldman JM, DeAngelo AB, Best DS, McDonald A, Strader LF, Murr AS, Suarez JD, George MH, Hunter ES, Simmons JE. Reproductive toxicity of a mixture of regulated drinking-water disinfection by-products in a multigenerational rat bioassay. Environ. Health Perspect. 2015;123(6):564–570. doi: 10.1289/ehp.1408579. [DOI] [PMC free article] [PubMed] [Google Scholar]