Abstract
Hematopoietic cell transplantation (HCT) survivors face a multitude of short- and long-term health complications in the years after treatment. One important health complication that is associated with significant morbidity is metabolic syndrome (MetSyn). This constellation of findings, which includes obesity, glucose and lipid dysmetabolism, and hypertension, places affected individuals at increased risk for type 2 diabetes mellitus, cardiovascular complications, and stroke. Previous studies have linked MetSyn in HCT survivors to prior treatment; however, few studies have addressed the potential roles of systemic inflammation and immune system dysfunction after HCT. Within this review, we address the recent advances in the understanding of adipose tissue biology, immune, and inflammatory mechanisms involved in MetSyn in non-HCT patients, and lastly, we discuss potential novel mechanisms that may play a role in MetSyn development after HCT, such as hematopoietic stem cell source, inflammatory status of the stem cell donor, and microbiome composition, all of which represent potential new directions for post-HCT MetSyn research.
Keywords: Hematopoietic cell, transplantation, Metabolic syndrome, Survivor, Late effects
INTRODUCTION
More than 19,000 hematopoietic cell transplantations are performed annually and since the 1980s, the cumulative number of transplantations now exceeds 320,000 [1]. Because of improvements in donor selection, graft-versus-host disease (GVHD) prophylaxis, and other supportive care measures, survival after allogeneic hematopoietic cell transplantation (HCT) has increased dramatically over the last 3 decades [2]. As well, there have been an expanding number of nonmalignant diseases deemed treatable using HCT, all of which have led to a growing population of long-term HCT survivors. As the number of long-term survivors grows, it is becoming increasingly apparent that they face significant health challenges affecting multiple organ and hormonal systems [3]. One specific complication that leads to long-term morbidity is metabolic syndrome (MetSyn). MetSyn represents a constellation of clinical findings, including hypertension, obesity, dyslipidemia, and altered glucose metabolism, which places individuals at increased risk for premature cardiovascular disease and type 2 diabetes mellitus [4,5] (Table 1). The costs, both in terms of morbidity and in actual health care dollars spent, in the non-HCT population are high and increase as the number of MetSyn components present in an individual increases. Costs for individuals with the constellation of obesity, diabetes, hyperlipidemia, and hypertension are more than double that of individuals with obesity and hypertension alone [6]. The financial costs for MetSyn care among survivors of HCT are likely to be even greater, making this an important priority topic in HCT survivor care. Current management principles are outlined in Table 2.
Table 1.
Metabolic Syndrome Diagnostic Criteria [4]
| Criterion | Men | Women |
|---|---|---|
| Waist circumference | >102 cm | >88 cm |
| Blood pressure* | ≥130/85 mmHg | ≥130/85 mmHg |
| HDL* | <40 mg/dL | <50 mg/dL |
| Triglycerides* | ≥150 mg/dL | ≥150 mg/dL |
| Fasting glucose* | ≥100 mg/dL | ≥100 mg/dL |
HDL indicates high-density lipoprotein.
An individual must have 3 of 5 findings to meet CMS criteria.
Medication management qualifies as having finding.
Table 2.
Current Management Recommendations of Metabolic Syndrome [5]
| Metabolic Abnormality | Recommended Management |
|---|---|
| Obesity • Increased waist circumference • Increased BMI |
Increase physical activity, goal ≥30 min moderate intensity activity most days of the week Decrease caloric intake; specifically, decrease saturated fat, trans fat and cholesterol intake |
| Dyslipidemia • Decreased HDL • Increased triglycerides |
Increase physical activity, goal level as above Make dietary modifications, decrease saturated fat, trans fat, and cholesterol intake Introduce lipid lowering drugs, as appropriate |
| Hypertension | Increase physical activity, goal level as above Alcohol use in moderation Make dietary modifications, including sodium reduction Introduce antihypertensive drugs, as appropriate |
| Glucose dysmetabolism • Impaired glucose tolerance • Type 2 diabetes mellitus |
Increase physical activity; goal ≥30 min moderate intensity activity most days of the week Decrease caloric intake; monitor carbohydrate intake Introduce pharmacotherapy to achieve hemoglobin A1C < 7% |
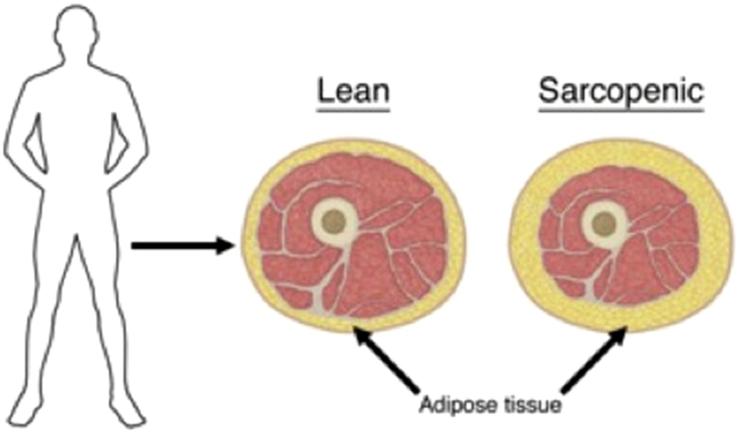
Although MetSyn is a significant health issue among the general population of the United States, affecting approximately 23% of the adult population [7] and 6% of the pediatric population [8], it is even more prevalent among the HCT-survivor population. HCT survivors have increased rates of MetSyn, as well as its individual components, compared with the general population in both pediatric and adult survivors [9-16] (Table 3). For pediatric survivors, the incidence of MetSyn varies significantly from 7% to 32% at a median of 4 to 15 years after HCT [10,11,15,18,19]. There is not consensus regarding a MetSyn definition in children, likely contributing to this variability. Similarly, among mixed cohorts of adult autologous and allogeneic HCT survivors, the estimates have ranged widely from 25% to 49%, anywhere from a median of 3 to 9 years after HCT [9,20,21]. Some investigators suggest that these clinical findings can present quite early after HCT. McMillen et al. reported 40% of their adult allogeneic HCT patients met modified criteria for MetSyn at 1 year after transplantation, increased from 34% at baseline, although longer follow-up data were not included to show whether characteristics persisted over time [22]. Strikingly, HCT survivors display decreased lean tissue mass and increased fat mass, leading to normal body mass index or waist circumference measures despite unhealthy proportions of adipose tissue [10,14]. Thus, it is likely that the degree of adiposity is underestimated in HCT survivors (Figure 1).
Table 3.
Review of Studies Examining Metabolic Syndrome in Patients Treated with HCT
| Study | Year | n | Age | Source (n) | Median Time after HCT, yr | Treated with TBI, % | MetSyn, % | Other |
|---|---|---|---|---|---|---|---|---|
| Taskinen M et al. [13] | 2000 | 23 | 10-32 | Allo | 10.8 | 78 | 39 | |
| Shalitin et al. [16] | 2006 | 91 | 4-32 | Allo (45), auto (46) | 6.2 (mean) | 16 | ND | 27.9% of tested patients with dyslipidemia |
| Taskinen M et al. [17] | 2007 | 31 | 7-34 | Allo | 6 | 90 | 39 | 48% developed GH deficiency (75% of individuals with MetSyn) |
| Chow EJ et al. [11] | 2010 | 26 | 8-21 | Allo | 6 | 100 | 23 | 38.5% treated with cranial radiation, 50% developed GH deficiency |
| Oudin C et al. [15] | 2011 | 60 | 18-41 | Allo (39), auto (21) | 15.4 | 72 | 15 | |
| Bajwa R et al. [10] | 2012 | 160 | 5-28 | Allo (99) Auto (70) | 7 | 37 | 7.5 | 17% developed GH deficiency |
| Frisk P et al. [12] | 2012 | 18 | 17-37 | Allo (3), auto (15) | 18.2 | 100 | 17 | 39% treated with cranial radiation |
| Paris C et al. [18] | 2012 | 69 | 6-25 | Allo (59), auto (10) | 4 | 55 | 32 | Low HDL most common component. Corticosteroid use before or after post-HCT was most significant risk factor for MetSyn. |
| Bizzarri C et al. [14] | 2015 | 45 | 13.9 ± 4.8 | Allo (40), auto (5) | 4-6.9 (mean) | 47 | 0 | |
| Oudin C et al. [19] | 2015 | 170 | 24.8 ± 5.4 | Allo (124), auto (46) | 14.5 (mean) | 73 | 17 | 9% treated with cranial/craniospinal radiation; GH deficiency associated with increased MetSyn risk |
| Higgins K et al. [20] | 2005 | 16 | 25-54 | Allo (13), auto (3) | 6 (mean) | 93 | 25 | Hypertriglyceridemia most common |
| Annaloro C et al. [9] | 2008 | 85 | 26-63 | Allo (39), auto (46) | 9 | 78 | 34 | Hypertriglyceridemia most common |
| Majhail NS et al. [21] | 2009 | 86 | 21-71 | Allo | 3 | 77 | 49 | Hypertriglyceridemia most common |
| McMillen KK et al. [22] | 2014 | 785 | 18-74 | Allo | 48 | 34% pre-HCT 40% modified MetSyn at 1 yr |
Hypertriglyceridemia most common |
Allo indicates allogeneic; auto, autologous; ND, not defined; GH, growth hormone.
Figure 1.
Sarcopenic obesity. Increased adipose tissue present compared to lean individual, despite similar body mass. Frequently observed after hematopoietic cell transplantation.
Similar metabolic sequelae occur in cancer survivors not treated with HCT, but generally, reported rates and magnitude of risk are higher among HCT survivors [11,13,23]. Studies of MetSyn in HCT recipients can be difficult to interpret because many do not report pre-HCT MetSyn traits or prevalence. Many also fail to describe conditioning intensity, or they include heterogeneously treated patients. It is currently unclear whether autologous transplantation survivors share the same level of risk for MetSyn or its individual components compared with allogeneic transplantation recipients [9,10,24]. Despite an abundance of descriptive data on MetSyn in various subgroups of HCT survivors, questions regarding the underlying mechanism of this process remain unanswered. Is post-HCT MetSyn due to the intensity of delivered therapy, different therapeutic exposures either during the transplantation or before it, or is the increased incidence of MetSyn after HCT due to immune alterations and inflammation associated with chemotherapy and made worse by an allogeneic stem cell source and immune suppression?
TREATMENT-RELATED RISK FACTORS
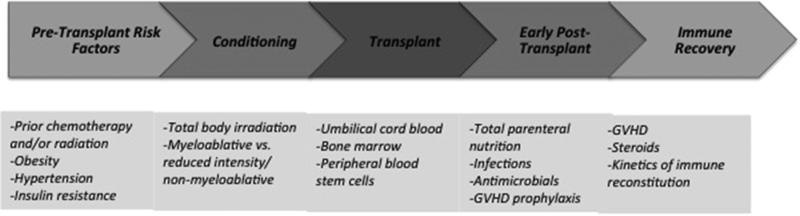
Hypotheses on the underlying mechanism of how MetSyn evolves after HCT have primarily focused on treatment-related exposures experienced by patients. Before, during, and after the transplantation process, HCT recipients are faced with numerous therapeutic insults, which may trigger or contribute to MetSyn (Figure 2). These include initial chemotherapies and therapeutic radiation delivered before HCT and the conditioning regimen, which commonly consists of high-dose chemotherapy (myeloablative or nonmyeloablative/reduced intensity), with or without total body irradiation (TBI). Recipients of allogeneic transplants are commonly treated with calcineurin inhibitors and possibly corticosteroids as post-transplantation GVHD prophylaxis or treatment. HCT recipients receive many other supportive therapies, such as antimicrobials and total parenteral nutrition, all of which may indirectly be implicated in the pathogenesis of MetSyn.
Figure 2.
Timeline for potential metabolic syndrome risk factors throughout the hematopoietic cell transplantation process.
Some investigators have speculated that TBI plays a key role in MetSyn after HCT given the well-established associations of therapeutic radiation exposure and MetSyn components in childhood cancer survivors, particularly in childhood acute lymphoblastic leukemia or central nervous system–tumor survivors previously treated with cranial or craniospinal radiation [25,26]. Although a role for TBI has been reported in some studies [10,24,27,28], others have found no difference compared with chemotherapy-based conditioning regimens [19]. Among childhood cancer survivors not treated with HCT, the link between MetSyn and therapeutic radiation has been attributed to growth hormone insufficiency [29]; similarly, growth hormone insufficiency and subsequent MetSyn have been identified in allogeneic HCT survivors, as well [17,19].
High-dose corticosteroids have not been directly linked to MetSyn risk in HCT recipients; however, such a link seems logical, given the known associations between corticosteroids and insulin resistance, hypertension, and obesity in childhood acute lymphoblastic leukemia survivors [30,31]. Allogeneic HCT recipients are at high risk for both acute and chronic GVHD, for which high-dose corticosteroids are considered first-line treatment. Steroid treatment often causes hypertension, weight gain, hyperglycemia, and hypertriglyceridemia, all of which are similarly observed in MetSyn; however, many of the steroid-related effects will partially resolve with cessation of therapy. Similarly, immune suppressants, such as calcineurin inhibitors and mTor inhibitors, can cause hyperlipidemia, hypertension, and insulin resistance, although, the association of these agents with subsequent MetSyn among HCT survivors has not been reported. Within the solid-organ transplantation populations, the associations between calcineurin inhibitor–driven insulin resistance, hyperlipidemia, and hypertension are well documented [32]; however, the duration of exposure and the doses used differ between the solid-organ and HCT populations, as do the numerous other therapeutic exposures and the immune statuses of recipients, making comparisons between these groups difficult. To date, there has not been a comprehensive analysis of the role of immunosuppressive agents in the development of MetSyn in HCT survivors.
THE IMMUNE SYSTEM AS A MEDIATOR OF METABOLIC SYNDROME
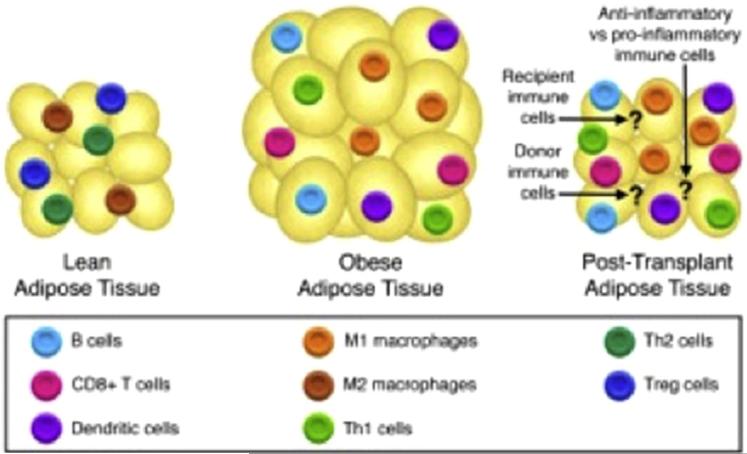
Outside of the cancer and HCT survivor populations, there has been extensive research investigating the inflammatory properties of adipose cells, particularly within the visceral or white adipose tissue (WAT). Obesity is now recognized as a chronic inflammatory state that drives the development of insulin resistance and MetSyn. Body mass index and the degree of abdominal adiposity are positively correlated with serum inflammatory markers, including C-reactive protein, TNF-α, and IL-6. Formerly recognized as excess energy storage depots, adipocytes are now known to serve critical endocrine and immune functions [33-37]. Multiple immune cell populations accumulate within adipose tissue and these vary based on body composition (Table 4). Within lean individuals, adipose tissue is predominantly populated by regulatory T cells (Tregs), Th 2 cells, and M2 macrophages, whereas, in obese individuals, adipose tissue contains Th 1, M1 macrophages, CD8+ T cells, B cells, and dendritic cells [38] (Figure 3). The importance of the immune cells in these metabolic processes is particularly intriguing in the setting of HCT, where significant tissue injury, inflammation, and perturbations in immune cell number and function occur. As well, the kinetics of immune cell turnover in the adipose tissue, from recipient to donor cells, is not known and may impact the timing of MetSyn onset.
Table 4.
Immune Cells Involved in Metabolic Syndrome
| Immune Cell Type | Role in MS |
|---|---|
| Monocytes and macrophages | |
| M1 | Increased in obese adipose tissue, produces IL-6, TNF-α, and IL-1β antagonizing insulin signaling and driving insulin resistance |
| M2 | Decreased in obese adipose tissue, produce IL-10, IL-1Ra |
| T cells | |
| CD4+ | Increased in obese adipose tissue and peripheral blood |
| Treg | Decreased in obese adipose tissue and peripheral blood, anti-inflammatory effect |
| Th 1 | Increased in obese adipose tissue, secrete IFN-γ, which stimulates monocytes to differentiate into M1 macrophages |
| Th 2 | Decreased in obese adipose tissue and peripheral blood, associated with M2 macrophage activation |
| Th 17 | Increased in obese adipose tissue, secrete IL-17, which enhances proinflammatory macrophage function. |
| CD8+ | Increased in obese adipose tissue, precede accumulation of macrophages and play a role in macrophage differentiation |
| NKT | Increased in obese adipose tissue in mice, unclear how they impact MS risk and inflammation |
| B cells | Increased in obese adipose tissue, promotes M1 macrophage polarization |
| NK | Produce IFN-γ, which stimulates monocytes to differentiate into M1 macrophages |
| ILC2s | Decreased in obese adipose tissue, promote accumulation of eosinophils and M2 macrophages in adipose tissue |
| Dendritic cells | Increased in obese adipose tissue, promote Th 17 cell generation and inflammation in mice. |
| Neutrophils | Help recruit macrophages to adipose tissue |
| Eosinophils | Decreased in obese adipose tissue, help improve glucose metabolism by maintaining abundance of M2 macrophages |
| Mast cells | Increased in obese adipose tissue, produce IL-6 and IFN-β |
Figure 3.
Immune cells and adipose tissue. Anti-inflammatory immune cells present in lean adipose tissue, proinflammatory immune cells present in obese adipose tissue. Unknown immune cells present in adipose tissue after hematopoietic cell transplantation.
Monocytes and Macrophages
Although adipose tissue contains multiple immune cell types, macrophages play a central role in obesity-associated inflammation and in the development of subsequent complications, such as insulin resistance [39]. Obese individuals have increased numbers and proportions of peripheral blood monocytes [40], which led to the discovery that these blood findings were accompanied by increases of macrophages in the WAT compared with that of lean individuals. Furthermore, adipose tissue macrophages and peripheral blood monocytes in obese individuals are more likely to be polarized away from a M2 cell type and more toward a proinflammatory, or M1, cell type [34], a process that is driven, at least in part, by increased concentrations of IFN-γ and lipopolysaccharide [41]. M1 macrophages produce proinflammatory cytokines, including IL-6, TNF-α, and IL-1β [42]. These cytokines antagonize insulin signaling, leading to insulin resistance [39]. Conversely, M2 macrophages produce tolerogenic cytokines and other molecules that dampen inflammation, including IL-10 and IL-1Ra.
Within the setting of cancer and HCT, macrophages may play a role in mitigating or propagating malignant disease progression, depending on their polarization. In studies of tumor-associated macrophages in both solid tumors and hematologic disease, M2-polarized macrophages promote tumor growth and metastasis, whereas, M1 macrophages drive antitumor activity [43,44]. Potentially, even outside of the tumor microenvironment, increased M1 macrophages after HCT may have an early protective antitumor effect, with later negative consequences on metabolic health.
Cytokines
Along with the proinflammatory M1 macrophage-derived cytokines IL-6, TNF-α, and IL-1β, additional soluble mediators regulate the inflammatory properties of adipose tissue. The IL-36 cytokines, which include IL-36α, IL-36β, IL-36γ, and IL-36 receptor antagonist, are subgroup of the IL-1 family cytokines expressed by monocytes/macrophages and may play a role in obesity. Specifically, IL-36α is produced by adipose tissue–resident macrophages and can drive inflammatory gene expression in adipocytes [45]. In contrast, the IL-36 receptor antagonist antagonizes inflammatory signaling pathways, is expressed in pre-adipocytes, and is down-regulated by TNF-α [46]. Further investigation of this subgroup of cytokines in obesity and in post-HCT MetSyn is warranted. Another member of the IL-1 family is IL-33, a cytokine that binds to its receptor, ST2, and drives Th 2-type cytokine production. ST2 is expressed by type 2 innate lymphoid cells (ILC2s) in adipose tissue. IL-33 regulates adipose tissue function and homeostasis, in part by increasing ILC2 production of the Th 2 cytokines IL-13 and IL-5, which reduces adipose inflammatory cytokine production [47]. This process serves to maintain a critical balance between Th 1 and Th 2 cells within the adipose tissues. ILC2s also maintain WAT eosinophils and M2 macrophages [48,49], which is crucial in the development of beige cells within WAT [47], a subgroup of adipocytes linked to obesity resistance.
Interestingly, ST2 has become an important biomarker for development of steroid-refractory GVHD and mortality, and the ST2/IL-33 pathway appears to be integral in propagating the inflammatory response in acute GVHD [50,51]. Pretransplantation plasma ST2 levels were higher in obese transplant recipients, as were subsequent nonrelapse mortality rates, most commonly from acute and chronic GVHD [52], indicating a possible connection between the inflammatory processes associated with obesity/MetSyn and GVHD.
Other Immune Cells
Although macrophages are thought to be the most functionally important immune cell in adipose tissue, T cells play a role in macrophage polarization and cytokine profile. In both murine obesity models and in humans, T cells, particularly CD4+ cells, are increased in adipose tissue and this accumulation appears to precede that of macrophages [53]. Within the circulating peripheral blood T cell compartment, Wagner and colleagues reported that in morbidly obese individuals, total CD4+ T cells are increased but Tregs are reduced [54]; however, this has not been consistently shown [55]. Interestingly, within experimental models, Tregs play a protective role in atherosclerosis, which is a known complication of MetSyn [56]. The immune suppressive function of Tregs may also be linked to myeloid-derived suppressor cells (MDSCs). MDSCs enhance M2 macrophage polarization, in part through IL-10 production [57]. To date, there are no data documenting a relationship between MDSCs and MetSyn, although, similar to Tregs, increased MDSCs after HCT is associated with less GVHD [58], possibly suggesting protective, anti-inflammatory effects in MetSyn. Dendritic cells also play an important role in maintaining immune tolerance. Recent work in mice has shown that a subpopulation of dendritic cells that express perforin are protective against MetSyn and autoimmunity, in part by preventing inflammatory processes in the adipose tissue by depleting inflammatory T cells [59].
Natural killer (NK) cells are key producers of IFN-γ, which as above, drives M1 macrophage polarization in adipose tissue. In mice fed a high-fat diet, the numbers of WAT NK cells were increased by 3 to 5 fold, and in NK-deficient mice, fat-associated macrophages were decreased, with the greatest impact on M1 macrophages. Notably, NK-deficient mice fed a high-fat diet gained weight but could maintain insulin sensitivity, thereby linking NK cell–derived IFN-γ to inflammation-induced insulin resistance [36]. Interestingly, NK cells recover rapidly after HCT and, along with having potent graft-versus-leukemia properties, they are also protective against GVHD [60]. Potentially, these beneficial early effects of NK cells may have a later adverse impact on HCT survivors by contributing to MetSyn, although, this has not been formally investigated.
NK T (NKT) cells are a specialized group of innate T cells that recognize glycolipids in the context of CD1d and rapidly produce cytokines upon activation. These cells are increased in the WAT of mice fed a high-fat diet fed mice [61], but their role in insulin resistance is not clear. Murine studies have shown that NKT cells play an important role in the evolution of insulin resistance [61,62]; however, other studies show that they prevent development of insulin resistance [63], perhaps reflecting the functional heterogeneity of NKT cell subtypes. Considering that NKT cells are observed at different frequencies in humans than they are in mice, this remains an area for further investigation in human MetSyn. This will be particularly interesting within the post-HCT survivor population, where NKT cells are, similar to many of the immune cell types above, associated with reduced rates of GVHD [64].
Adipokines
Along with the presence of M1 macrophages and other proinflammatory immune cells that drive obesity-induced inflammation, adipose tissue produces adipokines. The 2 best characterized adipokines are leptin and adiponectin, which play key functions in regulating metabolism and immunity. Leptin serum concentrations are proportional to body fat and regulate appetite and energy expenditure. Additionally, and perhaps most pertinent to the HCT setting, is the role of leptin in modulating immune cells and inflammation. Leptin decreases the proliferative capacity of Tregs; increases circulating granulocytes, NK cells, and monocytes; and induces T cell differentiation toward a Th 1 phenotype, all of which further enhances inflammatory cytokine production [65]. Despite this, there are limited data on leptin serum concentrations after transplantation and whether they are associated with MetSyn or other adverse complications [11]. In contrast to leptin, adiponectin is decreased in obesity and type 2 diabetes mellitus [66] and has anti-inflammatory effects on endothelial cells, smooth muscle cells, and macrophages, in part by inhibiting the effects of TNF-α and IL-6 on these tissues, as well as through inhibition of NF-kB activation [67] and IL-10 and IL-1Ra production [68]. Adiponectin also influences immune cell development and function, and it inhibits myelomonocytic progenitor proliferation as well as certain macrophage functions, such as phagocytosis and inflammatory cytokine production [67]. As well, adiponectin receptors are expressed at higher levels on M2 macrophages, as compared to M1 macrophages [69]. Again, whether serum adiponectin concentration is associated with reduced post-transplantation MetSyn has not been investigated.
INFLAMMATION AFTER HCT: PROPOSED HYPOTHESES AND FUTURE DIRECTIONS
Despite the rapidly growing literature addressing the importance of immune cells and cytokines in MetSyn progression and pathophysiology, much of the work has been performed in murine models. Recent intriguing mouse studies show successful treatment of type 2 diabetes with HCT [70], directly linking immune-mediated inflammation to metabolic processes. To date, investigation of MetSyn in HCT survivors remains sparse. Thus, there are many questions regarding the mechanisms and risk factors for post-HCT MetSyn. Considering that patients with post-HCT MetSyn have both similarities and differences to nontransplantation individuals and that transplant recipients have profound perturbations in immune function, the immunological changes in these patients must be carefully considered. It is not clear, particularly in humans, what initiating events lead the immune system to switch from maintaining adipose tissue homeostasis to development of the proinflammatory process outlined above and whether these are exacerbated by aspects of HCT, including therapeutic radiation, chemotherapy, immune suppression, GVHD, or graft characteristics.
The data supporting treatment-related effects on MetSyn are compelling but not fully explanatory, nor are the precise mechanisms of those effects fully understood. Given the immune perturbations that occur with HCT, including immune reconstitution and GVHD, transplantation-associated immune dysregulation may result in proinflammatory changes that impact metabolic health in survivors, similar to what has been shown in non-HCT patients. As above, multiple similarities in the immune cells and cytokines that drive GVHD after HCT are also observed in MetSyn. Studying these processes in a longitudinal fashion among HCT recipients, with close attention to the role of circulating and adipose-resident immune cells, may lend important insight into the pathophysiology of both processes. It is also feasible that high-dose chemotherapy and radiation therapy cause significant DNA damage, particularly within the mitochondria, which leads to mitochondrial dysfunction and resultant fat storage and hyperlipidemia [71].
A compelling argument could be made that the stem cell source may also affect risk for MetSyn in survivors based on the variability in inflammatory properties across stem cells sources, including bone marrow, peripheral blood, and the more naïve umbilical cord blood. In support of this, 2 months after immune-deficient mice were engrafted with CD34+ cells from either adult bone marrow or fetal bone marrow or liver, mice had different circulating lymphocyte populations, with the latter differentiating into more tolerogenic cells (Tregs and Th 2 cells) that would be expected to protect against MetSyn [72]. Similar observations have been made in the gene expression and inflammatory potential of fetal and adult monocytes, with the latter being more inflammatory [73]. It is currently not known whether the inflammatory status of the stem cell donor impacts the inflammatory behavior of the graft within the recipient, or rather whether an inflammatory condition, such as obesity in the donor, increases risk for MetSyn or other inflammatory conditions in the recipient. Obesity is associated with DNA methylation modifications in the promoter regions of genes involved in lipid metabolism, immune and inflammatory response, and cytokine signaling [74], but the effect of these modifications in HCT donors has not been investigated. Using a mouse model, bone marrow stem cells from obese mice give rise to more inflammatory macrophages, even after serial transplantations into nonobese mice, suggesting that obesity induces inflammatory-heritable changes at the level of the hematopoietic stem cell and that this is transferrable to transplant recipients [75]. These questions have yet to be addressed in the human HCT patient population, but they warrant attention as research into the pathophysiology of MetSyn in HCT survivors advances. Such data could potentially have bearing on choice of stem cell source, as well as selection of individual transplant donors. In instances where changes could not be made, this information could have bearing on post-transplantation monitoring or treatment.
Likewise, there is emerging evidence for the role of the gut microbiome in altering metabolic health, and it is likely that through the multiple toxicities and prolonged antibiotic use during the HCT course, there is derangement in the normal gut flora of HCT recipients [76]. There is intriguing evidence in murine models that fecal transplantation from an obese to a lean donor can induce obesity or ameliorate development of MetSyn [77]. The relative abundance and types of bacteria are altered in MetSyn; specifically, the ratio between Firmicutes and Bacteroidetes shifts in the setting of obesity and MetSyn, so that a greater proportion of Firmicutes is observed [77,78]. There is extensive crosstalk between the gut microbiota and the immune system, especially during HCT, including alterations in microbial composition related to specific post-HCT complications, such as GVHD [79]. Increased Bacteroidetes abundance before transplantation appears to protect against acute GVHD after HCT, possibly in part because of increased propionate production, which promotes Treg production and homing to the gut [80]. Thus, the interaction between the microbiome, the donor, as well as the recipient and any transplantation-associated complications, may have bearing on the subsequent development of MetSyn after allogeneic HCT.
CONCLUSIONS
MetSyn is a common and severe complication after HCT, but the causes are not well defined. There have been exciting discoveries and improvements in understanding of the pathophysiology of MetSyn over the last decade. The immune system appears to play a key role in the propagation of this disorder. As the underlying pathophysiology of Met-Syn in the general population is better understood, opportunities exist to translate those findings to the HCT-survivor population. However, there may also be distinct factors that drive post-HCT MetSyn, given the very unique nature of HCT. Gaining a greater understanding of the biology of this morbid process, through increased research on the impacts of immune reconstitution, stem cell source, donor/recipient interactions, and the post-transplantation microbiome on MetSyn outcomes, may guide future alterations in HCT management and supportive care. These alterations could ultimately lead to decreased proportions of survivors experiencing late cardiovascular complications or type 2 diabetes mellitus. As the number of survivors continues to grow, there must be increased focus on the prevention and management of long-term health consequences, as well as decreasing early and late toxicities.
Highlights.
Survivors of hematopoietic cell transplantation are at increased risk of metabolic syndrome
Immune system perturbations may be implicated in subsequent development of metabolic syndrome
Additional novel mechanisms for metabolic syndrome warrant further investigation
ACKNOWLEDGMENTS
This work was supported in part by Institutional Research Grant number 124166-IRG-58-001-55-IRG22 from the American Cancer Society (L.M.T.), Hyundai Hope on Wheels Young Investigator Clinical Grant (L.M.T.), the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (L.M.T,), and 5R01AI100879-03 (M.R.V.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial disclosures statement: None.
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org2013.
- 2.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau DM, Malone DC, Raebel MA, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Synd Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 7.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. Adolescents, 1999-2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 9.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 10.Bajwa R, Skeens M, Garee A, et al. Metabolic syndrome and endocrine dysfunctions after HSCT in children. Pediatr Transplant. 2012;16:872–878. doi: 10.1111/petr.12002. [DOI] [PubMed] [Google Scholar]
- 11.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16:1674–1681. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisk P, Arvidson J, Larsson M, Naessen T. Risk factors for cardiovascular disease are increased in young adults treated with stem cell transplantation during childhood. Pediatr Transplant. 2012;16:385–391. doi: 10.1111/j.1399-3046.2012.01693.x. [DOI] [PubMed] [Google Scholar]
- 13.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 14.Bizzarri C, Pinto RM, Ciccone S, et al. Early and progressive insulin resistance in young, non-obese cancer survivors treated with hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2015;62:1650–1655. doi: 10.1002/pbc.25603. [DOI] [PubMed] [Google Scholar]
- 15.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117:4442–4448. doi: 10.1182/blood-2010-09-304899. [DOI] [PubMed] [Google Scholar]
- 16.Shalitin S, Phillip M, Stein J, et al. Endocrine dysfunction and parameters of the metabolic syndrome after bone marrow transplantation during childhood and adolescence. Bone Marrow Transplant. 2006;37:1109–1117. doi: 10.1038/sj.bmt.1705374. [DOI] [PubMed] [Google Scholar]
- 17.Taskinen M, Lipsanen-Nyman M, Tiitinen A, et al. Insufficient growth hormone secretion is associated with metabolic syndrome after allogeneic stem cell transplantation in childhood. J Pediatr Hematol Oncol. 2007;29:529–534. doi: 10.1097/MPH.0b013e3180f61b67. [DOI] [PubMed] [Google Scholar]
- 18.Paris C, Yates L, Lama P, et al. Evaluation of metabolic syndrome after hematopoietic stem cell transplantation in children and adolescents. Pediatr Blood Cancer. 2012;59:306–310. doi: 10.1002/pbc.24104. [DOI] [PubMed] [Google Scholar]
- 19.Oudin C, Auquier P, Bertrand Y, et al. Metabolic syndrome in adults who received hematopoietic stem cell transplantation for acute childhood leukemia: an LEA study. Bone Marrow Transplant. 2015;50:1438–1444. doi: 10.1038/bmt.2015.167. [DOI] [PubMed] [Google Scholar]
- 20.Higgins K, Noon C, Davies M, et al. Features of the metabolic syndrome present in survivors of bone marrow transplantation in adulthood. Bone Marrow Transplant. 2005;36:279–280. doi: 10.1038/sj.bmt.1705060. author reply 280. [DOI] [PubMed] [Google Scholar]
- 21.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43:49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillen KK, Schmidt EM, Storer BE, Bar M. Metabolic syndrome appears early after hematopoietic cell transplantation. Metab Synd Relat Disord. 2014;12:367–371. doi: 10.1089/met.2014.0051. [DOI] [PubMed] [Google Scholar]
- 23.Baker KS, Chow EJ, Goodman PJ, et al. Impact of treatment exposures on cardiovascular risk and insulin resistance in childhood cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:1954–1963. doi: 10.1158/1055-9965.EPI-13-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nottage KA, Ness KK, Li C, et al. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia -From the St. Jude Lifetime Cohort. Br J Haematol. 2014;165:364–374. doi: 10.1111/bjh.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietila S, Makipernaa A, Sievanen H, et al. Obesity and metabolic changes are common in young childhood brain tumor survivors. Pediatr Blood Cancer. 2009;52:853–859. doi: 10.1002/pbc.21936. [DOI] [PubMed] [Google Scholar]
- 27.Rajendran R, Abu E, Fadl A, Byrne CD. Late effects of childhood cancer treatment: severe hypertriglyceridaemia, central obesity, non alcoholic fatty liver disease and diabetes as complications of childhood total body irradiation. Diabet Med. 2013;30:e239–e242. doi: 10.1111/dme.12234. [DOI] [PubMed] [Google Scholar]
- 28.Mayson SE, Parker VE, Schutta MH, et al. Severe insulin resistance and hypertriglyceridemia after childhood total body irradiation. Endocr Pract. 2013;19:51–58. doi: 10.4158/EP12115.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 30.Chow EJ, Pihoker C, Friedman DL, et al. Glucocorticoids and insulin resistance in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:621–626. doi: 10.1002/pbc.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 33.Haase J, Weyer U, Immig K, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562–571. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 34.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wensveen FM, Jelencic V, Valentic S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 37.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adi-pose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 38.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 40.Zaldivar F, McMurray RG, Nemet D, et al. Body fat and circulating leukocytes in children. Int J Obes (Lond) 2006;30:906–911. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- 41.Tedesco S, Bolego C, Toniolo A, et al. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology. 2015;220:545–554. doi: 10.1016/j.imbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 43.Sousa S, Brion R, Lintunen M, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101. doi: 10.1186/s13058-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panchabhai S, Kelemen K, Ahmann G, et al. Tumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myeloma. Leukemia. 2015 doi: 10.1038/leu.2015.191. http://dx.doi.org/10.1038/leu.2015.191 [Epub ahead of print] [DOI] [PubMed]
- 45.van Asseldonk EJ, Stienstra R, Koenen TB, et al. The effect of the interleukin-1 cytokine family members IL-1F6 and IL-1F8 on adipocyte differentiation. Obesity. 2010;18:2234–2236. doi: 10.1038/oby.2010.55. [DOI] [PubMed] [Google Scholar]
- 46.Towne JE, Renshaw BR, Douangpanya J, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichenbach DK, Schwarze V, Matta BM, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125:3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–539. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gleimer M, Li Y, Chang L, et al. Baseline body mass index among children and adults undergoing allogeneic hematopoietic cell transplantation: clinical characteristics and outcomes. Bone Marrow Transplant. 2015;50:402–410. doi: 10.1038/bmt.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 54.Wagner NM, Brandhorst G, Czepluch F, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity. 2013;21:461–468. doi: 10.1002/oby.20087. [DOI] [PubMed] [Google Scholar]
- 55.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th 2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng H, Chi H. The interplay between regulatory T cells and metabolism in immune regulation. Oncoimmunology. 2013;2:e26586. doi: 10.4161/onci.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia S, Sha H, Yang L, et al. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by inter-leukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, et al. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43:776–787. doi: 10.1016/j.immuni.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Olson JA, Leveson-Gower DB, Gill S, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Parekh VV, Gabriel CL, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schipper HS, Rakhshandehroo M, van de Graaf SF, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaidos A, Patterson S, Szydlo R, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:5030–5036. doi: 10.1182/blood-2011-11-389304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adiposespecific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 67.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 68.Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 69.Barnes MA, Carson MJ, Nair MG. Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine. 2015;72:210–219. doi: 10.1016/j.cyto.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flaquer M, Franquesa M, Barquinero J, et al. Bone marrow transplantation induces normoglycemia in a type 2 diabetes mellitus murine model. Transplant Proc. 2009;41:2282–2285. doi: 10.1016/j.transproceed.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 71.Mercer JR, Cheng KK, Figg N, et al. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ Res. 2010;107:1021–1031. doi: 10.1161/CIRCRESAHA.110.218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krow-Lucal ER, Kim CC, Burt TD, McCune JM. Distinct functional programming of human fetal and adult monocytes. Blood. 2014;123:1897–1904. doi: 10.1182/blood-2013-11-536094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demerath EW, Guan W, Grove ML, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24:4464–4479. doi: 10.1093/hmg/ddv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3:664–675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 78.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:640–645. doi: 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biagi E, Zama D, Nastasi C, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015;50:992–998. doi: 10.1038/bmt.2015.16. [DOI] [PubMed] [Google Scholar]