Abstract
Background
The prevalence of pseudoresistant hypertension (HTN) due to inaccurate BP measurement remains unknown.
Methods
Triage BP measurements and measurements obtained at the same clinic visit by trained physicians were compared in consecutive adult patients referred for uncontrolled resistant HTN (RHTN). Triage BP measurements were taken by the clinic staff during normal intake procedures. BP measurements were obtained by trained physicians using the BpTRU device. The prevalence of uncontrolled RHTN and differences in BP measurements were compared.
Results
Of 130 patients with uncontrolled RHTN, 33.1% (n=43) were falsely identified as having uncontrolled RHTN based on triage BP measurements. The median (IQR) of differences in systolic BP between pseudoresistant and true resistant groups were 23 (17 – 33) mm Hg and 13 (6 – 21) mm Hg, respectively (P=0.0001). The median (IQR) of differences in diastolic BP between the two groups were 12 (7 – 18) mm Hg and 8 (4 – 11) mm Hg, respectively (P=0.001).
Conclusion
Triage BP technique overestimated the prevalence of uncontrolled RHTN in approximately 33% of the patients emphasizing the importance of obtaining accurate BP measurements.
Keywords: Pseudoresistant hypertension, blood pressure measurement technique, resistant hypertension, blood pressure
Introduction
Uncontrolled RHTN is defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg with use of at least 3 antihypertensive medications.1–3 Falsely elevated BP levels contribute to pseudoresistant HTN, that is, BP levels that appear uncontrolled, but actually are not. The prevalence of pseudoresistance among persons with apparent RHTN is estimated to be as high as 50%.3,4 The most common causes of pseudoresistance are inaccurate BP measurement technique, medication non-adherence, under-treatment, and white coat HTN.2,3 Of these, only pseudoresistance secondary to inaccurate BP measurement has not been previously quantified.
Proper technique is essential to accurately measure BP. However, during delivery of routine health care, guidelines for accurate BP measurement are rarely followed.5 Errors commonly made during routine BP measurements include: use of an incorrectly sized cuff; placing the cuff over clothes; relying on standing or supine BP measurements; deflating BP cuff too fast; omitting 3–5 minutes of rest before BP measurement; taking the BP simultaneously with other ongoing activities like completing forms or answering questions; and introduction of operator biases associated with non-automated devices.6–13 The combined effects of these errors in technique may over or under estimate BP levels resulting in the misdiagnosis and mismanagement of uncontrolled RHTN.
Quantification of the degree of error in measurement related to inaccurate BP technique has not been previously reported. In the current study, we determined the prevalence of pseudoresistance attributed to measurement error during routine BP measurement in consecutive patients referred for uncontrolled apparent RHTN to our hypertension specialty clinic. We further attempted to identify patient characteristics more commonly associated with pseudoresistant HTN due to inaccurate BP measurement.
Methods
Participants and Study Design
Consecutive adult patients referred to the University of Alabama at Birmingham (UAB) HTN Clinic for evaluation and treatment of RHTN between June 2013 – November 2015 were retrospectively analyzed. Patients with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg obtained during routine intake measurement while prescribed 3 or more antihypertensive medications at the time of their initial visit were considered as having uncontrolled apparent RHTN. For the purposes of this study, routine intake or triage BP measurements obtained by clinic staff were compared to BP measurements obtained in the same patient at the same visit by trained physician examiners. Falsely elevated or peudoresistant HTN was identified if the triage BP was elevated (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mmHg) but controlled (SBP <140 mm Hg and DBP <90 mmHg) when measured by trained physicians. True uncontrolled RHTN was identified if the triage BP was concordant with the trained physician measurement (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg). Data on patient demographics, medication use, cardiovascular risk factors and BP measurements during the initial and subsequent clinic visits were collected. The protocol was approved by UAB’s Institutional Review Board.
Routine intake BP measurements
Triage BP measurements were taken as part of intake procedures by clinic staff using an automated BP device (Welch Allyn, New York, USA) with adult small, regular and large sized cuffs available. Typically a single BP reading is obtained as part of the electronically documented vital signs for the visit. The clinic staff had not had any special training in BP technique beyond what that they learned during their respective professional education and on-the-job instruction from fellow staff members and no standardized BP measurement protocol was utilized. The BP is generally measured with patients sitting in a common, often busy, intake area and often while being asked questions related to their current medical history and medication use. The BP measurement is usually taken almost immediately after having the patient sit adjacent to the nurses’ station. Blood pressure cuffs are variably placed directly on the skin or over clothes.
BP measurements by trained physicians
BP measurements were taken by physicians following AHA recommendations.5 These measurements were obtained using an oscillometric device BpTRU (VSM Med Tech Ltd. Coquitlam, Canada) that automatically obtains serial BP measurements. BpTRU is a validated device which has passed the standards of the British Society of HTN and the Association of Advancement of Medical Instrumentation and is more consistent with the ambulatory BP readings compared with single office visit measurements.14–16 The BpTRU device takes 6 consecutive BP measurements one minute apart providing an average based on the last 5 measurements. BP measurements were taken following a standard protocol: having patients sit for 5 minutes in a quiet room, back supported, feet on the floor, with the arm used for measurement supported at chest level and placing the appropriately sized cuff (adult small, regular or large) directly on the skin on the non-dominant arm. Once the device was appropriately in place, the physician left the room and allowing for unobserved BP measurements.
Statistical Analysis
Summary statistics were obtained and non-normally distributed data displayed as median and IQR. The prevalence of pseudoresistant HTN was calculated for the initial and follow-up visits in patients with uncontrolled BP levels as measured by BpTRU (primary analysis). The differences between SBP and DBP measurements taken at triage intake and by trained physicians were obtained in this group. Mann-Whitney test was used to compare BPs between pseudoresistant and true resistant groups as determined by BP measurement technique with p<0.05 considered significant. Individual associations of age, sex and race with differences in SBP and DBP between the two measurement techniques were assessed. A secondary analysis including the entire study cohort was conducted to compare the difference between triage and BpTRU SBP across different triage SBP categories. All statistical calculations were performed using SPSS (IBM, USA) software.
Results
Of 192 patients prescribed 3 or more medications during their initial clinic visit, 58 patients with controlled triage and expertly obtained BP measurements, and 4 patients with controlled triage and uncontrolled expertly obtained BP measurements were excluded. As a result, 130 patients with uncontrolled apparent RHTN were included in the primary analysis. Of those, 115 and 68 patients had second and third follow-up visits, respectively. Overall, patients referred for uncontrolled RHTN were more likely female and obese with a median age of 59 years and with a median use of 4 antihypertensive medications, most commonly angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Table 1 shows the baseline characteristics of the entire cohort and the pseudoresistant and true resistant groups.
Table 1.
Baseline characteristics of patients with uncontrolled RHTN
| Variables | Overall cohort | Pseudoresistant | True resistant |
|---|---|---|---|
| Number of patients (%) | 130 (100) | 43 (33.1) | 87 (66.9) |
| Age (years), Median (IQR) | 59 (51 – 70) | 63 (52–72) | 58 (48 – 67) |
| Body mass index (kg/m2), Median (IQR) |
32.9 (27.1 – 38.1) | 32.8 (27 – 39) | 33.3 (28.7 – 38.0) |
| Female (%)* | 95 (73.1) | 17 (39.5) | 78 (89.6) |
| African American (%) | 59 (45.4) | 18 (41.8) | 41 (47.1) |
| Comorbidities | |||
| Coronary artery disease | 12 (9.2) | 6 (13.9) | 6 (6.9) |
| Diabetes mellitus* | 48 (36.9) | 9 (20.9) | 39 (44.8) |
| Hypercholesterolemia | 50 (38.4) | 18 (41.8) | 32 (36.7) |
| Smoking | 46 (35.4) | 17 (39.5) | 29 (33.3) |
| History of stroke/Transient ischemic attack |
7 (5.4) | 3 (6.9) | 4 (4.6) |
| Estimated glomerular filtration rate ≥ 60 (ml/min/1.73m2) |
84 (64.6) | 31 (72.1) | 53 (60.9) |
| Medication use | |||
| Number of antihypertensive medications, Median (IQR) |
4 (3 – 5) | 3 (3 – 4) | 4 (4 – 5) |
| Diuretics* | 86 (66.1) | 23 (53.5) | 63 (72.4) |
| Mineralocorticoid antagonists |
29 (22.3) | 7 (16.2) | 22 (25.3) |
| Alpha blockers | 50 (38.4) | 12 (27.9) | 38 (43.7) |
| Beta blockers | 93 (71.5) | 27 (62.8) | 66 (75.8) |
| Vasodilators | 39 (30) | 10 (23.2) | 29 (33.3) |
| Centrally acting agents | 51 (39.2) | 12 (27.9) | 39 (44.8) |
| Angiotensin converting enzyme inhibitors/Angiotensin receptor blockers |
109 (83.8) | 38 (88.4) | 71 (81.6) |
| Calcium channel blockers | 83 (63.8) | 28 (65.1) | 55 (63.2) |
| Aspirin | 63 (48.4) | 22 (51.2) | 41 (47.1) |
| Statin | 51 (39.2) | 20 (46.5) | 31 (35.6) |
IQR = inter-quartile range,
p < 0.05
At the initial visit, 43 out of 130 patients referred for uncontrolled RHTN were misdiagnosed as uncontrolled based on a falsely elevated BP reading during triage, when compared to the expertly obtained BP reading. This translates into prevalence of pseudoresistant HTN of 33.1% attributable to poor BP technique. The prevalence of pseudoresistant HTN during the second and third follow-up visits in the same patients was 30.1% and 29.6%, respectively. For patients in the true resistant group, the median (IQR) of routine intake SBP and DBP measurements were 171 (157 – 184) mmHg and 92 (81 – 103) mmHg, respectively and the median (IQR) of expertly obtained SBP and DBP were 159 (151 – 173) mmHg and 89 (78 – 96) mmHg respectively. For patients in pseudoresistant group the median (IQR) of routine intake SBP and DBP measurements were 155 (145 – 163) mmHg and 85 (77 – 93) mmHg, respectively and the median (IQR) of expertly obtained SBP and DBP were 129 (122 – 135) mmHg and 75 (67 – 81) mmHg, respectively.
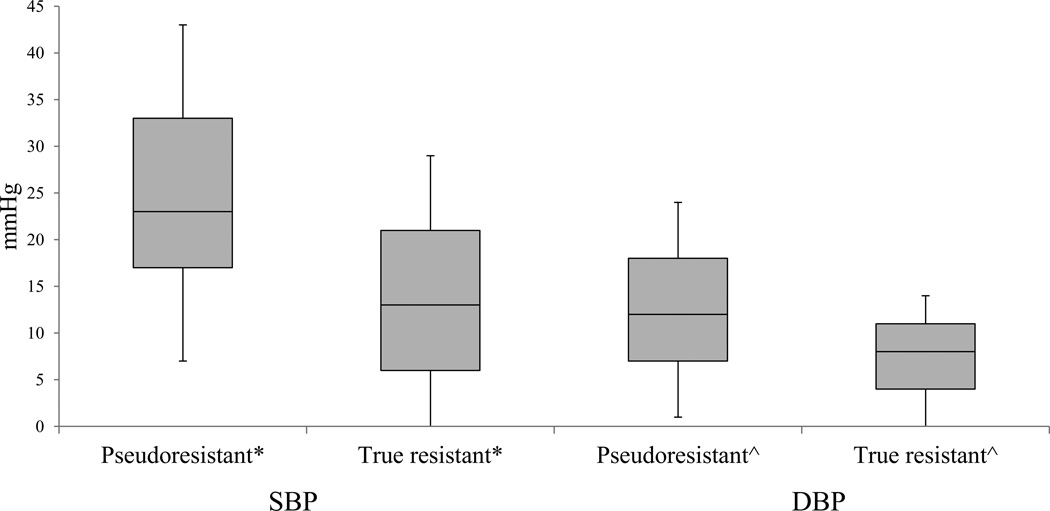
At the initial clinic visit the median (IQR) of differences in SBP between routine intake and expertly obtained measurements among pseudoresistant and true resistant groups was 23 (17 – 33) mmHg and 13 (6 – 21) mmHg, respectively (P=0.0001). The median (IQR) of differences in diastolic BP between routine intake and expertly obtained measurements among the two groups was 12 (7 – 18) mmHg and 8 (4 – 11) mmHg, respectively (P=0.001) (Figure 1). Race, sex, age and follow-up visits had no impact on the observed differences in SBP and DBP measurements taken during triage and by trained physicians (data not displayed). Differences in SBP when comparing triage with the trained physician readings were larger in patients with higher initial triage BP readings (Table 2).
Figure 1.
Box-Whisker plot of median (inter-quartile range) for differences between triage and expertly obtained blood pressure for pseudoresistant and true resistant groups.
Differences in both systolic blood pressure (SBP) and diastolic blood pressures (DBP) reported. *P = 0.0001, ^P = 0.001.
Table 2.
Differences in SBP between triage and BpTRU measurement techniques
| Triage SBP levels (n = 192) | Median (IQR) mmHg of difference in SBP |
|---|---|
| < 120 mmHg (13) | 8 (4.5 – 11.5) |
| 120 – 129 mmHg (24) | 11 (5.3 – 14.8) |
| 130 – 139 mmHg (25) | 17 (12 – 20)* |
| 140 – 149 mmHg (26) | 16 (7 – 19.3) |
| 150 – 159 mmHg (25) | 17 (7.5 – 22)* |
| ≥ 160 mmHg (79) | 20 (12 – 28)* |
SBP – Systolic blood pressure. All groups were compared with the reference group < 120 mmHg.
p < 0.05
Discussion
The current study provides the first estimate of the prevalence of pseudoresistant HTN based on routine vs. expert BP measurement in patients with apparent RHTN. The prevalence of pseudoresistant HTN among patients referred to a HTN specialty clinic at the time of the initial visit attributable to poor BP technique was 33.1%. The prevalence of pseudoresistance did not change significantly for as many as 2 follow-up visits. Therefore, approximately 1 in 3 patients referred to a HTN specialty clinic for uncontrolled RHTN may have pseudoresistance due to inaccurate BP measurement.
Thus far, studies investigating the prevalence of pseudoresistant HTN have focused on determining the contribution from white coat HTN, under treatment, and medication non-adherence. Using data from Spanish ambulatory BP monitoring registry, De la Sierra et al. reported a prevalence of white coat HTN of 37.5% among 8295 persons with seemingly uncontrolled RHTN.4 Similarly, in a prospective study of Brazilian cohort with RHTN, the prevalence of white coat HTN was 39%.17 Under treatment of RHTN has been quantified in outpatient community clinics, where only 22,189 out of 44,684 persons (49.7%) with apparent treatment resistant HTN were treated optimally with effective antihypertensive treatment regimens based on using a diuretic and at least 50% of the recommended maximum dose of all agents.18 Grigoryan et al. found that among persons with uncontrolled RHTN, none were prescribed a mineralocorticoid antagonist and 91% were prescribed a suboptimal dose of diuretic.19. The prevalence of medication non-adherence among persons with RHTN has been estimated at 36% using a pill count method and 53% using urine drug metabolite measurements.20,21 Adding to these other causes of pseudoresistance, the current study indicates that approximately 33% of patients referred to a HTN specialty clinic for RHTN are falsely identified as having uncontrolled RHTN based on routine clinic BP measurements.
Myers et al., in several studies of general hypertensive cohorts, have reported that BP measurements obtained using the BpTRU device are usually lower than expertly obtained measurements using manual sphygmomanometer (155 ± 5/88 ± 2 mm Hg vs 174 ± 5/92 ± 2 mm Hg, respectively) and correlate closely with the ambulatory BP monitor (ABPM) readings (155 ± 5/88 ± 2 mm Hg vs 146 ± 3/82 ± 2 mm Hg).22,23 BpTRU measurements seemingly avoid overestimation of BP resulting from single office measurements (136 ± 17/78 ± 11 mm Hg vs 141 ± 15/80 ± 10 mm Hg).24 In the current study, the differences in triage and expert BP measurements were even larger than the above reports, suggesting that falsely elevated office BP readings may be even more prominent in patients with apparent resistant hypertension. Although the BpTRU device was used in the current study, the observed discrepancies between routine and expertly obtained BP measurements are likely related to important differences in the entire BP measuring process including having the patient sit in a quiet area for 3–5 minutes before beginning the BP assessment, use of properly sized cuffs, avoiding any interaction with the patient during BP measurements, correct positioning of the body and arm, and application of the cuff directly over the exposed arm. Undersizing of BP cuff, sitting without a back support, crossing the legs, placement of the arm with the BP cuff above or below the level of right atrium or holding the arm up against gravity can cause errors in BP measurement5,7,8,10,25,26 In the current study, in contrast to triage measurements, the expert measurements were obtained using a standard protocol in accordance with the AHA guidelines ensuring proper body position and the placement of correct size cuff.5
Background noise, patient anxiety in the presence of a provider, and patient-observer conversation contribute to falsely elevated BP measurements.27–30 In the current study, the routine intake BP was measured in a common intake area with surrounding noise and distracting activities. Commonly, the BP measurements were taken while questions being asked to patients regarding their current medical issues and medication use. In addition to having a 5 minute resting period prior to BP measurement, use of the BpTRU device by physicians allowed patients to sit quietly during BP measurements in the absence of a health care provider to reduce anxiety and minimize the effects of background noise and conversation. While BpTRU measurements with or without a 5 minute rest period have been reported to be lower compared to routine clinic measurements,31 some studies have reported that a 5 minute rest period prior to using the BpTRU device results in BP readings even lower than daytime readings obtained with ABPM.32,33 The current analysis was based on having included the 5 minute rest period prior to use of the BpTRU device per current AHA guidelines. Whether guidelines should be revised to not have a 5 minute rest period with use of devises that take serial readings is an important methodological question for subsequent studies.
Manual sphygmomanometers are still often used in clinic or office settings. Observer variability in auscultatory technique, digit preference, ability to detect auscultatory gap and Korotkoff sounds and rate of cuff deflation significantly impact the accuracy of manual BP measurements.34–37 Use of automated BP devices eliminates the aforementioned errors. Since the routine intake measurements in our clinic were taken using an automated device, it is likely that the prevalence of pseudoresistant HTN may be even higher in clinics that rely on manual BP measurements. The impact of gender, race and aging on BP disparities resulting from inaccurate BP measurement has not been previously reported. We stratified race as African Americans and non-African Americans and age as ≥ 70 years or < 70 years and found no association between race, gender or age and the differences in BP measurements taken by routine and expert techniques. In addition, 4 patients had controlled BP measurements at triage, but uncontrolled during expertly obtained measurements. Although speculative, a reactive rise in BP due to repeated cuff inflations is a possible explanation for this discordance.38
Strengths of the current study include comparing routine BP measurement with expert technique by physicians trained per AHA recommendations in the same patient only minutes apart. All physician obtained BP readings were done using the same standardized procedure and with use of a validated BP device. Study limitations include findings based on a retrospective analysis. Also, the routine intake BP measurements were consistently taken 5–10 minutes prior to expert measurements in all patients. It has been shown that order of measurement affects BP, with subsequent measurements having lower values.39 Both the order of measurement as well as the 5–10 minute interval between each of the two measurement techniques, could have contributed to the observed differences in BP. In addition, BpTRU disregards the first reading and provides an average of five subsequent readings. An average of multiple measurements could also explain a portion of the observed BP discrepancies between the two techniques; perhaps by regression to the mean.34 A prospective trial with randomization to the timing and number of BP measurements would be needed to differentiate between these effects. Such a trial should include ABPM and outcome measures in order to corroborate that accurate BP measurement better predicts target organ damage or hard outcomes. It remains unknown to what extent the triage BP technique at UAB ’s clinic reflects practices in other clinics in the community.
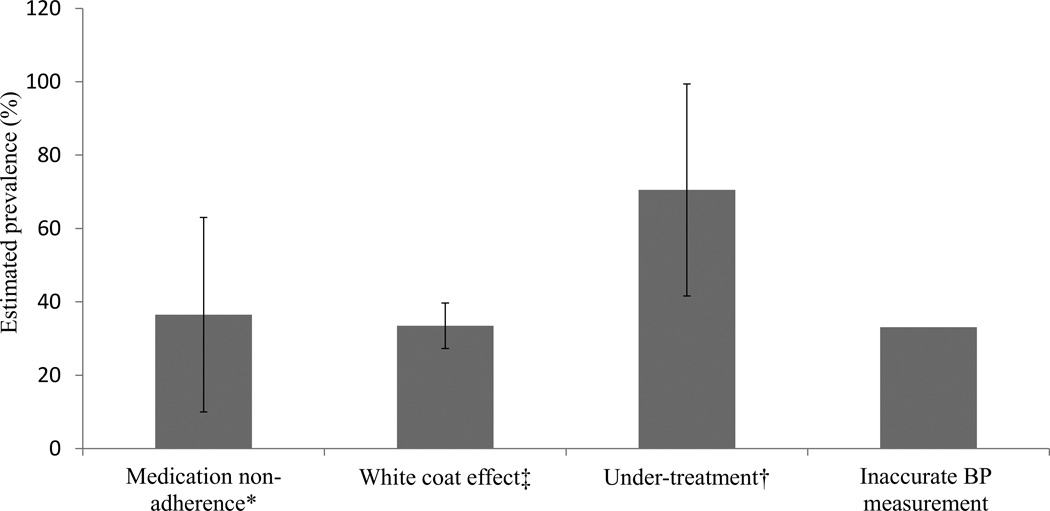
The current study adds an estimate of inaccurate BP measurement to the reported causes of pseudoresistant HTN in addition to medication non-adherence, white coat effect, and under-treatment as shown in Figure 2. The current analysis demonstrates that routinely obtained BP measurements overestimate the prevalence of uncontrolled RHTN by approximately 33% when compared to measurements done by hypertension specialists. These results highlight the importance of adhering to guideline recommended BP technique to avoid overestimation of BP and thus minimize potentially unnecessary diagnostic testing and excessive medical therapies.
Figure 2.
Estimated prevalence (mean, standard deviation) of each of the causes of pseudoresistant hypertension.
*references 20–21; ‡ references 4,18,40,41; † references 19,20.
Highlights.
Poor BP measurement technique contributes to pseudoresistant hypertension (HTN).
The prevalence of pseudoresistant HTN due to inaccurate BP measurement was 33%.
Routine clinic BP rarely underestimates the true BP in resistant HTN.
Acknowledgments
Funding source: This work was support by funding from the National Heart, Lung, and Blood Institute, T32-HL007457 and R01 HL113004.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures or conflict of interest: None
References
- 1.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM American Heart Association Professional Education Committee. Resistant HTN: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 3.Judd E, Calhoun DA. Apparent and true resistant HTN: definition, prevalence and outcomes. J Hum Hypertens. 2014;28(8):463–468. doi: 10.1038/jhh.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant HTN classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 5.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 6.Wingfield D, Freeman GK, Bulpitt CJ Gerneral Practice HTN Study Group (GPHSG) Selective recording in blood pressure readings may increase subsequent mortality. QJM. 2002;95:571–577. doi: 10.1093/qjmed/95.9.571. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Radford T, Dasgupta I. Unvalidated blood pressure devices with small cuffs are being used in hospitals. BMJ. 2001;323:398. [PMC free article] [PubMed] [Google Scholar]
- 8.Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation. 1983;68:763–766. doi: 10.1161/01.cir.68.4.763. [DOI] [PubMed] [Google Scholar]
- 9.Terent A, Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood Press. 1994;3:156–163. doi: 10.3109/08037059409102246. [DOI] [PubMed] [Google Scholar]
- 10.Cushman WC, Cooper KM, Horne RA, Meydrech EF. Effect of back support and stethoscope head on seated blood pressure determinations. Am J Hypertens. 1990;3:240–241. doi: 10.1093/ajh/3.3.240. [DOI] [PubMed] [Google Scholar]
- 11.Netea RT, Lenders JW, Smits P, Thien T. Arm position is important for blood pressure measurement. J Hum Hypertens. 1999;13:105–109. doi: 10.1038/sj.jhh.1000720. [DOI] [PubMed] [Google Scholar]
- 12.Linfors EW, Feussner JR, Blessing CL, Starmer CF, Neelon FA, McKee PA. Spurious HTN in the obese patient. Effect of sphygmomanometer cuff size on prevalence of HTN. Arch Intern Med. 1984;144:1482–1485. [PubMed] [Google Scholar]
- 13.Bovet P, Hungerbuler P, Quilindo J, Grettve ML, Waeber B, Burnand B. Systematic difference between blood pressure readings caused by cuff type. HTN. 1994;24:786–792. doi: 10.1161/01.hyp.24.6.786. [erratum in: HTN. 1995:25(4 Pt 1)660] [DOI] [PubMed] [Google Scholar]
- 14.Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 h ambulatory blood pressure monitoring in the assessment of blood pressure in patients with HTN. BMC Cardiovasc Disord. 2005;5:18–24. doi: 10.1186/1471-2261-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JM, Mattu GS, Perry TL, Jr, Gelferc ME, Strange KD, Zorn A, Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood Press Monit. 2001 Jun;6(3):161–165. doi: 10.1097/00126097-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Mattu GS, Heran BS, Wright JM. Overall accuracy of the BpTRU--an automated electronic blood pressure device. Blood Press Monit. 2004;9(1):47–52. doi: 10.1097/00126097-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 18.Egan BM, Zhao Y, Li J, Brzezinski AW, Todoran TM, Brook RD, Calhoun DA. Prevalence of optimal treatment regimens in patients with apparent treatment resistant hypertension based on office blood pressure in a community based practice network. Hypertension. 2013;62:691–697. doi: 10.1161/HYPERTENSIONAHA.113.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigoryan L, Pavlik VN, Hyman DJ. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J Am Soc Hypertens. 2013;7(6):471–476. doi: 10.1016/j.jash.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza WA, Sabha M, de Faveri Favero F, Bergste-Mendes G, Yugar-Toledo JC, Moreno H, et al. Intensive monitoring of adherence to treatment helps to identify ‘true’ resistant hypertension. J Clin Hypertens (Greenwich) 2009;11(4):183–191. doi: 10.1111/j.1751-7176.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31(4):766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- 22.Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the "white coat effect" in routine practice. Am J Hypertens. 2003 Jun;16(6):494–497. doi: 10.1016/s0895-7061(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009 Feb;27(2):280–286. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- 24.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, Kaczorowski J. Conventional versus automated measurement of blood pressure in primary care patients with systolichypertension: randomised parallel design controlled trial. BMJ. 2011 Feb 7;342:d286. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97–101. [PubMed] [Google Scholar]
- 26.Mitchell PL, Parlin RW, Blackburn H. Effect of vertical displacement of the arm on indirect blood-pressure measurement. N Engl J Med. 1964;271:72–74. doi: 10.1056/NEJM196407092710204. [DOI] [PubMed] [Google Scholar]
- 27.Lusk SL, Gillespie B, Hagerty BM, Ziemba RA. Acute effects of noise on blood pressure and heart rate. Arch Environ Health. 2004;59(8):392–3999. doi: 10.3200/AEOH.59.8.392-399. [DOI] [PubMed] [Google Scholar]
- 28.Zheng D, Giovannini R, Murray A. Effect of respiration, talking and small body movements on blood pressure measurement. J Hum Hypertens. 2012;26(7):458–462. doi: 10.1038/jhh.2011.53. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JJ, Long JM, Thomas SA, Malinow KL, Katcher AH. The effects of talking on the blood pressure of hypertensive and normotensive individuals. Psychosom Med. 1981;43(1):25–33. doi: 10.1097/00006842-198102000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Lynch JJ, Thomas SA, Long JM, Malinow KL, Chickadonz G, Katcher AH. Human speech and blood pressure. J Nerv Ment Dis. 1980;168(9):526–534. doi: 10.1097/00005053-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Brothwell S, Dutton M, Ferro C, Stringer S, Cockwell P. Optimising the accuracy of blood pressure monitoring in chronic kidney disease: the utility of BpTRU. BMC Nephrol. 2013 Oct 10;14:218. doi: 10.1186/1471-2369-14-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamarre-Cliche M, Cheong NN, Larochelle P. Comparative assessment of four blood pressure measurement methods in hypertensives. Can J Cardiol. 2011 Jul-Aug;27(4):455–460. doi: 10.1016/j.cjca.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens (Greenwich) 2014 Feb;16(2):83–86. doi: 10.1111/jch.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handler J, Zhao Y, Egan BM. Impact of the Number of Blood Pressure Measurements on Blood Pressure Classification in U.S. Adults: NHANES 1999–2008. Journal of clinical hypertension (Greenwich, Conn) 2012;14(11):751–759. doi: 10.1111/jch.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28(4):571–586. doi: 10.1016/j.ccl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen PE, Janniche H. The accuracy of auscultatory measurement of arm blood pressure in very obese subjects. Acta Med Scand. 1974;195:403. doi: 10.1111/j.0954-6820.1974.tb08160.x. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y, Abe K, Sasaki S, Minami N, Munakata M, Sakuma H, Hashimoto J, Sekino H, Imai K, Yoshinaga K. Clinical evaluation of semiautomatic and automatic devices for home blood pressure measurement: comparison between cuff-oscillometric and microphone methods. Journal of HTN. 1989;7:983–990. doi: 10.1097/00004872-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Charmoy A, Wurzner G, Ruffieux C, Hasler C, Cachat F, Waeber B, Burnier M. Reactive rise in blood pressure upon cuff inflation: cuff inflation at the arm causes a greater rise in pressure than at the wrist in hypertensive patients. Blood Press Monit. 2007 Oct;12(5):275–280. doi: 10.1097/MBP.0b013e3282c9ac9a. [DOI] [PubMed] [Google Scholar]
- 39.Myers MG, McInnis NH, Fodor GJ, Leenen FH. Comparison between an automated and manual sphygmomanometer in a population survey. Am J Hypertens. 2008 Mar;21(3):280–283. doi: 10.1038/ajh.2007.54. [DOI] [PubMed] [Google Scholar]
- 40.Muxfeldt ES, Bloch KV, Nogueira Ada R, Salles GF. True resistant hypertension: is it possible to be recognized in the office? Am J Hypertens. 2005;18:1534–1540. doi: 10.1016/j.amjhyper.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14(12):1263–1269. doi: 10.1016/s0895-7061(01)02193-8. [DOI] [PubMed] [Google Scholar]