Abstract
Developmental exposure to endocrine-disrupting compounds (EDCs) alters reproduction and energy homeostasis, both of which are regulated by the arcuate nucleus (ARC). Little is known about the effects of EDC on ARC gene expression. In Experiment #1, pregnant dams were treated with either two doses of bisphenol A (BPA) or oil from embryonic day (E)18-21. Neonates were injected from postnatal day (PND)0-7. Vaginal opening, body weights, and ARC gene expression were measured. Chrm3 (muscarinic receptor 3) and Adipor1 (adiponectin receptor 1) were decreased by BPA. Bdnf (brain-derived neurotropic factor), Igf1 (insulin-like growth factor 1), Htr2c (5-hydroxytryptamine receptor), and Cck2r (cholescystokinin 2 receptor) were impacted. In Experiment #2, females were exposed to BPA, diethylstilbestrol (DES), di(2-ethylhexyl)phthalate, or methoxychlor (MXC) during E11-PND7. MXC and DES advanced the age of vaginal opening and ARC gene expression was impacted. These data indicate that EDCs alter ARC genes involved in reproduction and energy homeostasis in females.
Keywords: arcuate nucleus, bisphenol A, diethylstilbestrol, phthalates, methoxychlor, energy homeostasis, puberty, endocrine-disrupting compounds
1. Introduction
Recent research has begun to elucidate the perturbations of central nervous system regulators of homeostatic functions including reproduction and energy homeostasis by endocrine-disrupting compounds (EDCs). These two functions are controlled, in part, through the hypothalamus, which is regarded as a key brain region integrating hormonal control of energy homeostasis and reproduction [1;2]. Many hypothalamic nuclei are involved in this integration include the arcuate nucleus (ARC), the paraventricular nucleus (PVH), the lateral hypothalamus (LH), and the ventromedial nucleus (VMH) [3]. Of these, the ARC is near an area of local permeability in the blood brain barrier and is readily exposed to circulating steroids, hormones, and nutrients including insulin, leptin, and glucose [4]. ARC neurons, in particular proopiomelanocortin (POMC), neuropeptide Y (NPY), and KNDy (Kisspeptin-Neurokinin B-Dynorphin) neurons, respond to both peripheral signals, reflecting the energy status of the body, and to inputs from the hindbrain to integrate sensory attributes, emotional states, motivated behaviors [4-6].
The effect on energy homeostasis from developmental exposures to common EDCs has potential relevance in the increase in childhood and adulthood obesity in the industrialized world. Evidence suggests that EDCs, especially bisphenol A (BPA), may affect normal energy homeostasis by altering homeostatic functions, glucose homeostasis, and peripheral gene expression [7-9]. Recent evidence also suggests that perinatal exposure to BPA alters hypothalamic melanocortin neurocircuitry and ARC neuropeptide gene expression in response to high fat diets [10]. The mechanisms behind these effects are unknown but potentially involve organizational and epigenetic effects in the hypothalamus during development, which potentially is mediated by the classical estrogen receptors (ER) α/β. Little is known about the effects of perinatal exposure to other estrogenic and non-estrogenic EDC on expression of ARC genes involved in energy homeostasis.
Developmental exposures to estrogenic EDCs alter many neuroendocrine reproductive functions [11;12]. BPA and other estrogenic EDCs (diethylstilbestrol (DES), methoxychlor (MXC)) are reported to alter the timing of puberty onset [11-15], however not consistently [16;17]. Phthalates are typically considered anti-androgenic and are known to alter male reproduction including puberty [18;19], but there is limited data with mixed results on phthalates (e.g., di(2-ethylhexyl)phthalate, dibutyl phthalate) in females. A few studies showed that the exposure advanced pubertal onset [20;21], and other studies suggested the exposure delayed puberty [22]. Differences in the timing of exposure may explain, in part, the contradictory results with phthalates. Puberty and the control of reproduction are initiated by interactions between peripheral energy signals (leptin, insulin, adiponectin, etc.) with hypothalamic neurons, in particular, NPY/AgRP [23] and KNDy [24]. Activity of neurotransmitters [25-28] and expression of growth factors (IGF-1) [29] in the hypothalamus are associated with the onset of puberty and the reproductive cycle in females. However, little is known about the effects of perinatal exposure to EDCs on expression of the genes associated with these signals in the hypothalamus and especially in the ARC.
There are numerous ARC genes that have been characterized for their role in controlling reproduction and energy homeostasis. These genes include receptors for hormones and neurotransmitters, signalling molecules, transcription factors, and neuropeptides (reviewed in [6;30;31]. Peripheral hormonal receptors, including members of G-protein coupled receptor (GPCR) and cytokine receptor families, are vital for the hypothalamic sensing of the body’s energy status, which is then communicated to other hypothalamic neurons to control reproduction, especially during puberty. GPCR are common receptors for neurotransmitters involved in reproduction and energy homeostasis including serotonin receptors. ARC neuropeptides (e.g., POMC, NPY, AgRP, Kisspeptin) are integral to the hypothalamic control of feeding, energy expenditure, and puberty and are modulated by both peripheral and central signals of energy homeostasis. Therefore, to address the question of whether these genes are altered in the ARC by developmental exposures to EDCs, we designed a Taqman Low-Density Array (TLDA) to analyze relative gene expression.
In order to determine the organizational effects of EDCs, dams and neonates were treated during fetal and neonatal periods when steroid receptor expression and the development of the melanocortin neurocircuitry in the rodent hypothalamus occur (E10-P14) [32-37]. In addition, EDCs alters hypothalamic gene expression [38], including steroid hormone receptor expression (ERα/β and AR) [7;39] during similar developmental windows. Furthermore, this exposure window encompasses critical gonadal differentiation events that start mid-gestation (i.e., E11) and subsequent early ovarian developmental events, including oocyte nest breakdown and follicular assembly in rats (i.e., E18-PD7), which can influence reproductive health later in life [40].
Therefore, the current study sought to determine if developmental (gestational and neonatal) exposures to a selection of known EDCs will alter puberty (vaginal opening), body weight, and ARC gene expression in adult female rats. As an initial EDC screen, we exposed dams and neonate females to two doses of BPA, a known disruptor of reproduction and energy homeostasis [10;14] and analyzed for gene expression using the TLDA. In the subsequent experiment, dams and neonate females were exposed to either BPA, MXC, di(2-ethylhexyl)phthalate (DEHP), or DES. We hypothesize that important genes involved in reproduction and energy homeostasis would be differentially regulated in the ARC by developmental exposures to these compounds, which may contribute to the dysregulation of reproduction and energy homeostasis in juveniles and adults.
2. Materials and Methods
2.1. Animals
Eight- to twelve-week-old Fischer CDF female rats (Charles River, Wilmington, MA) were maintained on a 14-hour light/10-hour dark cycle and fed, ad libitum. A reduced isoflavone diet was provided in order to minimize possible effects of phytoestrogens (Purina, 5V01, Brentwood, MO). The estrous cycles of the rats were followed daily, and individual females were mated with untreated males starting on the day of proestrus. A sperm-positive vaginal smear was designated embryonic day 0 (E0). All animal care and treatment protocols were carried out in accordance with institutional guidelines and were approved by Rutgers University Institutional Animal Care and Use Committee.
2.2. Chemicals
All chemicals (BPA, MXC, DES, DEHP, dimethyl sulfoxide (DMSO), sesame oil, and corn oil) were purchased from Sigma-Aldrich.
2.3. Experimental Design and Treatments
In Experiment #1, the timed-pregnant females received one of two different treatment dosages of BPA: 50 µg/kg/day (Lo-BPA; n = 3 litters; 10 pups) and 50 mg/kg/day (Hi-BPA; n = 4 litters; 8 pups) in 1 ml vehicle/kg BW. Control animals (n = 5 litters; 12 pups) received an emulsion of 10% absolute ethanol (EtOH) and 90% corn oil. Daily treatments were administered (i.p.) to the pregnant dams beginning on E18 and sub-cutaneously (in the folds of the neck) to the neonates within 8 hours of birth (considered PND 0) and continued until PND 7. Starting PND 28, females were followed for their vaginal opening as an indication of pubertal age. Vaginal opening was followed to determine if developmental exposures to EDCs alters the age of puberty [41-43]. After vaginal opening, the stages of estrous cycles of all females were followed by vaginal cytology, and 8 randomly selected females from each treatment were weighed and euthanized between PND 50 and 60 on proestrous day after their third estrous cycle for hypothalamic tissue isolation and gene expression analysis.
In Experiment #2, the timed-pregnant females were divided into groups with each group receiving one of 4 different EDCs or two control vehicles. The EDCs and doses were as follows: BPA (50 mg/kg/day; n = 8 litters, 26 pups); DES (0.1 µg/kg/day; n = 6 litters, 20 pups); and DEHP (500 mg/kg/day; n = 5 litters, 18 pups) in a vehicle of EtOH:oil (1:9) (1 ml vehicle/kg BW volume). Another group received MXC (75 mg/kg/day; n = 8 litters, 16 pups) in a vehicle of dimethylsulfoxide (DMSO): sesame oil (1:2). There were two sub-groups of control animals receiving either the EtOH:corn oil vehicle (n = 5 litters, 15 pups) or the DMSO:sesame oil vehicle (n = 5 litters, 13 pups). Daily treatments were administered (i.p.) to the pregnant dams beginning on E11 and sub-cutaneously (in the folds of the neck) to the neonates within 8 hours of birth (considered PND 0) and continued until PND 7. The vaginal opening of all female offspring and the stages of estrous cycle were followed, similar to Experiment #1. One to two females from each dam (n = litter) were randomly chosen, weighed, and euthanized on proestrous day between PND 70 and 90 for isolation of hypothalamic tissues and analysis of gene expression. The age of sacrifice was determined by estrous cyclicity and due to perturbations in estrous cycles by EDC treatments, the range in ages is ~20 days. See Table 1 for a list of the samples sizes (litters) and number of total pups for each experiment, treatment, and endpoint.
Table 1.
Samples sizes (litters) and number of pups for each experiment, treatment, and endpoint.
| Vaginal Opening | Body Weight | QPCR | ||||
|---|---|---|---|---|---|---|
| # of Litter | # of Pups | # of Litter | # of Pups | # of Litter | # of Pups | |
| Experiment #1 | ||||||
| Control | 5 | 12 | 5 | 12 | 3 | 8 |
| 50 μg BPA | 3 | 10 | 3 | 10 | 3 | 8 |
| 50 mg BPA | 4 | 8 | 4 | 8 | 4 | 8 |
| Experiment #2 | ||||||
| ETOH | 5 | 15 | 3 | 5 | 3 | 5 |
| DES | 6 | 20 | 6 | 10 | 6 | 10 |
| DEHP | 5 | 18 | 4 | 7 | 4 | 7 |
| DMSO | 5 | 13 | 4 | 4 | 4 | 4 |
| MXC | 8 | 16 | 5 | 8 | 3 | 5 |
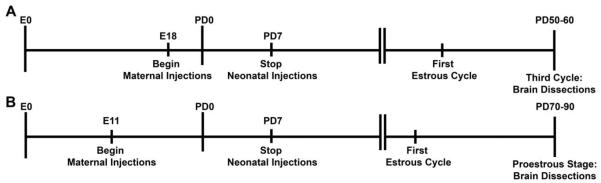
Doses of compounds were chosen from previous experimentation demonstrating reproductive effects or from the literature. BPA doses (high (50 mg/kg/day) and low (50 µg/kg/day)) represent the EPA LOAEL and reference doses, respectively [7;39]. DEHP was administered at 500 mg/kg/day, which is higher than the highest dose that human infants are exposed in neonatal intensive care units (~23 mg/kg/day) [44;45], but was shown to be effective at inducing transgenerational effects in male mice [46]. The environmental levels of MXC range from 160 mg/l in waters down-stream of MXC-sprayed areas to 0.1 ng/kg/day, the FDA's calculated average daily intake of MXC in general adult population. In addition, the 75 mg/kg/day is within the dose range that is used in many studies and has been previously shown to be effective in producing reproductive effects without inducing complete sterility in females [47]. DES (0.1 µg) is a lower dose than what is used in previous studies on pregnant women [48], but is effective in rodents as a model estrogenic EDC [49]. In addition, while oral exposure is considered more common for some EDCs (e.g., MXC or BPA), for certain cases parenteral exposure is more relevant (e.g., DES and exposure to DEHP via medical devices). Thus, to be consistent between EDCs, and to precisely control the doses of the chemicals that were administered, we choose to use a parenteral route. See Figure 1 for a timeline of Experiment #1 and #2.
Figure 1.
A timeline illustrating the experimental protocol for Experiment #1 and #2. Pregnant dams were injected with the EDC or vehicle from embryonic day (E) 18 (Exp. #1, A) or E11 (Exp. #2, B) and the neonates were injected until postnatal day (PND) 7. Estrous cycles were monitored daily after puberty. Females were sacrificed in proestrus after the third estrous cycle in Experiment #1 or in proestrus in Experiment #2.
2.4. Tissue Dissection and RNA Extraction
Vehicle- or EDC-treated adult females were decapitated quickly and the brain was extracted and transferred to ice-cold Sorensen’s Buffer. Hypothalamic nuclei were microdissected for RNA extraction and quantitative real-time analysis. The basal hypothalamus was cut using a brain slicer (Ted Pella, Redding, CA) into 1-mm thick coronal rostral and caudal blocks. The slices corresponded to Nissl-stained figures in Chemoarchitectonic Atlas of The Rat Brain by Paxinos and coauthors [50] using pages 183–232. The tissue blocks were placed in RNAlater for 24 h (Life Technologies, Inc.), and the rostral and caudal parts of the ARC were dissected from the rostral and caudal blocks, respectively, using a dissecting microscope. Dissected tissue was stored at −80 ºC. From the combined nuclei (rostral and caudal ARC), total RNA was extracted and DNase I-treated using Ambion RNAqueous Micro Kits (Life Technologies, Inc.) according to the manufacturer’s protocol. RNA samples were tested for quantity and purity using the NanoDrop ND-2000 spectrophotometer (ThermoFisher, Waltham, MA). RNA quality was assessed by applying 1 µl of each RNA sample to a channel of an Agilent Nanochip and analyzing on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). Only samples with RIN > 6 were used in the experiments.
2.5. Reverse Transcription and Quantitative Real-Time PCR
Complementary DNA (cDNA) was synthesized from 200 ng total RNA using Superscript III (Life Technologies, Inc.), 4 μl 5x buffer, 25 mM MgCl2, 10 mM dNTP, 100 ng random hexamer primers (Promega), 40 U/μl Rnasin (Promega), and 100 mM dithiothreitol (DTT) in diethylpyrocarbonate (DEPC)-water (GeneMate) in total volume of 20 μl. RT was conducted using the following protocol: 5 min at 25 °C, 60 min at 50 °C, 15 min at 70 °C. The cDNA was diluted to 1:20 with Nuclease-free water (GeneMate) for a final cDNA concentration of 0.5 ng/μl and stored at −20 °C. Basal hypothalamic (BH) test tissue RNA was used for positive and negative controls (no reverse transcriptase) and processed simultaneously with the experimental samples.
For qPCR, 5 μl cDNA template (an equivalent of 2.5 ng total RNA) was amplified using Taqman® Gene Expression Master Mix (Life Technologies) on a StepOnePlus™ Real-Time PCR system. For Experiment #1, samples were analyzed using a Taqman® Custom Low-Density Gene Array (TLDA) of 40 genes selected for their roles in energy homeostasis, reproduction, and hypothalamic neuronal activity. Genes and assays identification numbers are presented in Table 2. The gene assays were arrayed in duplicate on each plate along with endogenous controls (Actb, 3 wells; 18s, 1 well). One of the Actb wells was used for a sample of pooled controls to determine inter-plate variation. One experimental sample was run per plate including an untreated male sample used as calibrator for determining relative gene expression. The amplification protocol for the plates was as follows: 95 °C for 10 min (initial denaturing) followed by 40 cycles of amplification at 94 °C for 15 sec (denaturing), 60 °C for 60 sec (annealing). The relative mRNA expression data were analyzed using the ΔΔCT method, where CT is cycle threshold [51].
Table 2.
Taqman® Low Density Array Genes.
| Gene Name | Gene | Assay ID |
|---|---|---|
| 5HT2c Receptor | Htr2c | Rn00562748_m1 |
| Adiponectin receptor 1 | Adipor1 | Rn01483784_m1 |
| Agouti-regulated Protein | Agrp | Rn01431703_g1 |
| Brain Derived Neurotrophic Factor | Bdnf | Rn02531967_s1 |
| Cannabinoid Receptor 1 | Cnr1 | Rn02758689_s1 |
| CART prepropeptide | Cartpt | Rn01645174_m1 |
| Cholecystokinin Receptor 2 | Cck2r | Rn00565867_m1 |
| Estrogen Receptor α | Esr1 | Rn01640372_m1 |
| Estrogen Receptor β | Esr2 | Rn00562610_m1 |
| Forkhead box O1 | FoxO1 | Rn01494868_m1 |
| GABA-B Receptor 1 | Gabbr1 | Rn00578911_m1 |
| GABA-B Receptor 2 | Gabbr2 | Rn00582550_m1 |
| Galanin Receptor 3 | Galr3 | Rn02132531_s1 |
| Galanin-like Peptide | Galp | Rn00575275_m1 |
| Gastrin-releasing peptide receptor | Grpr | Rn01420745_m1 |
| Glucagon-like peptide 1 receptor | Glp1r | Rn00562406_m1 |
| Glucokinase | Gck | Rn00561265_m1 |
| Glutamate decarboxylase 1 | Gad1 | Rn00690300_m1 |
| Glutamate decarboxylase 2 | Gad2 | Rn00561244_m1 |
| Growth Hormone Secratogue Receptor | Ghsr | Rn00821417_m1 |
| Insulin Receptor Substrate 1 | Irs1 | Rn02132493_s1 |
| Insulin Receptor Substrate 2 | Irs2 | Rn01482270_s1 |
| Insulin-like Growth Factor 1 | Igf1 | Rn00710306_m1 |
| Insulin-like Growth Factor 1 Receptor | Igf1r | Rn00583837_m1 |
| Kisspeptin 1 | Kiss1 | Rn00710914_m1 |
| Leptin Receptor | Lepr | Rn01433205_m1 |
| Melanocortin 3 Receptor | Mc3r | Rn02585847_s1 |
| Metabotropic Glutamate Receptor 5 | Grm5 | Rn00566628_m1 |
| Muscarinc Receptor 3 | Chrm3 | Rn00560986_s1 |
| Neuropeptide Y | Npy | Rn01410145_m1 |
| NPY Y2 Receptor | Npy2r | Rn04219516_g1 |
| Opioid Receptor (μ) | Oprm1 | Rn01430371_m1 |
| Phosphatase and tensin homolog | Pten | Rn00477208_m1 |
| Prodynorphin | Pdyn | Rn00571351_m1 |
| Proopiomelanocortin | Pomc | Rn00595020_m1 |
| Serine/threonine-protein kinases (Akt3) | Akt3 | Rn00442194_m1 |
| Signal transducer and activator of transcription 3 | Stat3 | Rn00562562_m1 |
| Sulfonylurea Receptor 1 | Abcc8 | Rn00564778_m1 |
| Tyrosine Hydroxylase | Th | Rn00562500_m1 |
| Uncoupling Protein 2 | Ucp2 | Rn01754856_m1 |
For Experiment #2, samples were analyzed for relative gene expression only in those genes that had a statistically significant alteration in gene expression from the TLDA. Individual assays (FAM dye) for each gene (identical to those used in the array) were used to analyze the samples (see Table 2). Each gene assay was tested using BH cDNA while being multiplexed with a primer-limited endogenous control (Actb-VIC). Each gene was analyzed in one plate by multiplexing the gene assay with the endogenous control. The relative mRNA expression data were analyzed using the ΔΔCT method, where CT is cycle threshold [51]. The amplification protocol for all the genes was as follows: 95 °C for 10 min (initial denaturing) followed by 40 cycles of amplification at 94 °C for 15 sec (denaturing), 60 °C for 30 sec (annealing). Positive and negative BH cDNA controls were added to each amplification run including a water blank.
2.6. Data analysis
All data are expressed as mean ± SEM. We analyzed expression data from Experiment #1 using Data Assist® software (Life Technologies) to determine significant differences between EtOH:oil- and BPA-treated females. The false discovery rate was calculated (Benjamini-Hochberg method, P < 0.05) for Experiment #1. Secondary one-way ANOVA analysis was conducted on all significant data from the Data Assist analysis. All the data from the body weight measurements, pubertal age, and quantitative real-time PCR from Experiment #2 were analyzed using a one-way ANOVA followed by a post-hoc Bonferroni multiple comparison tests for each gene assay. All data were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA) or Statistica (Dell Software, Aliso Viejo, CA, USA). Because multiple genes were analyzed using the TLDA, we adjusted the alpha for Experiment #1 to p ≤ 0.01 for the ANOVA. For Experiment #2, the alpha was set at p ≤ 0.05 within each gene. A Grubb’s test was used to detect outliers in all experiments.
3. Results
3.1. Vaginal opening and body weight
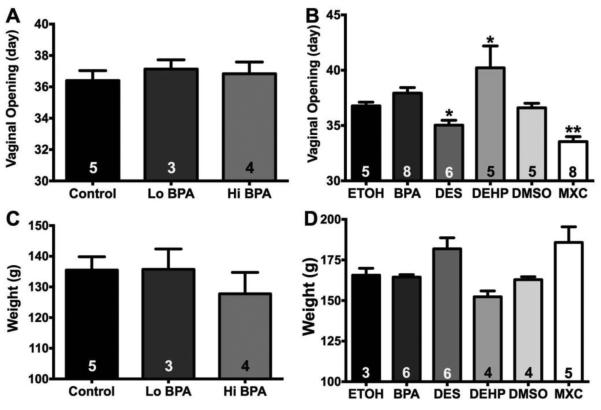
In Experiment #1, neither low dose (37.1 ± 0.6 d; n = 3) nor high dose (36.8 ± 0.8 d; n = 4) BPA altered the day of the vaginal opening as compared to control (36.4 ± 0.6 d; n = 5) (Figure 2A; F(2, 9) = 0.2853; not significant (ns)). In Experiment #2, there was an effect of EDC treatment on pubertal age (Figure 2B; F(5, 31) = 9.064; p < 0.0001). Pubertal age was advanced by both MXC (33.5 ± 0.5 d; n = 8; p < 0.01) and DES (35.0 ± 0.4 d; n = 6; p < 0.05) compared to their respective controls (DMSO:oil – 36.6 ± 0.4 d; n = 5, and EtOH:oil – 36.8 ± 0.4 d; n = 5). In contrast, DEHP delayed pubertal age (40.2 ± 2.0 d; n = 5; p < 0.05) vs. EtOH:oil control. As in Experiment, #1, BPA did not alter the pubertal age (37.9 ± 0.5; n = 8).
Figure 2.
Pubertal age (day of vaginal opening) and body weights (g) for female rats exposed to EDCs during gestation and neonatal development. A: Average day of vaginal opening in females from Experiment 1 (F(2, 9) = 0.2853, ns). B: Average day of vaginal opening in females from Experiment #2 (F(5, 31) = 9.064, p < 0.0001). C: Body weights from Experiment #1 (F(2, 9) = 0.6023, ns). D: Body weights from Experiment #2 (F(5, 22) = 4.624; p < 0.01). Data were analyzed by a one-way ANOVA with Bonferroni multiple comparison tests (* p < 0.05; ** p < 0.01). Numbers in column represent the sample size (n=litters) for each treatment.
In Experiment #1, female rats were weighed at the age of 50-60 days. There was no effect of treatment on age-matched body weights (Figure 2C; F(2, 9) = 0.6023; ns). Vehicle–treated females weighed an average of 135.5 ± 4.3 g (n = 5). Females exposed to the 50 µg/kg/day BPA dose weighed 135.7 ± 6.6 g (n = 3). Females exposed to 50 mg/kg/day BPA dose weighed 127.8 ± 7.0 g (n = 4). In Experiment #2, there was an effect of EDC treatment on body weights (Figure 2D; F(5, 22) = 4.624; p < 0.01). Control females were divided into those treated with EtOH:oil (1:9) (n = 3) and those treated with DMSO:oil (1:2) (n = 4). The average age-matched body weight for the two control groups did not differ (EtOH:oil – 165.6 ± 4.3 g; DMSO:oil - 162.9 ± 1.8 g). BPA (164.4 ± 1.5 g; n = 6), DES (181.8 ± 6.8 g; n = 6) and DEHP-treated (152.3 ± 3.6; n = 4) females were compared to EtOH:oil control and MXC-treated (185.8 ± 9.6 g; n = 5) was compared to DMSO:oil control. However, no individual EDC treatment altered body weights compared to controls.
3.2. Developmental exposures to BPA alters ARC gene expression
Because the TLDA was used initially as a screen for potential gene candidates and to reduce false discovery rate, we set the ANOVA at p≤0.01 as the cutoff. Of the 40 genes examined, BPA treatment altered the gene expression in two of these genes in a dose-dependent manner (ANOVA: p ≤ 0.01) (See Table 3). These two genes were Chrm3 and Adipor1. Chrm3, the muscarinic acetylcholine receptor 3 gene, was suppressed by the low (p < 0.05) and high (p < 0.01) doses of BPA (F(2, 8) = 15.07; p < 0.01). Chrm3 was also lower in the Hi-BPA than the Lo-BPA (p < 0.05). The adiponectin receptor 1 gene, Adipor1, was also suppressed by the low (p < 0.05) and high (p < 0.01) doses of BPA in the ARC (F(2, 8) = 9.868; p < 0.01).
Table 3.
Relative gene expression in Experiment #1.
| Gene Name | Vehicle | Lo-BPA | Hi-BPA | ANOVA p |
|---|---|---|---|---|
| Abcc8 | 1.47 ± 0.34 | 1.16 ± 0.14 | 1.14 ± 0.09 | 0.4391 |
| Adipor1 | 1.18 ± 0.06 | 0.95 ± 0.03 (a) | 0.82 ± 0.07 (a) | 0.0069 ** |
| Agrp | 0.93 ± 0.29 | 0.72 ± 0.06 | 1.19 ± 0.49 | 0.6985 |
| Akt3 | 1.42 ± 0.19 | 1.31 ± 0.12 | 1.37 ± 0.15 | 0.8784 |
| Bdnf | 1.12 ± 0.08 | 1.06 ± 0.12 | 0.60 ± 0.05 (a), (b) | 0.0158 * |
| Cnr1 | 0.95 ± 0.10 | 0.72 ± 0.10 | 0.55 ± 0.10 (a) | 0.0737 |
| Cartpt | 3.05 ± 0.13 | 3.88 ± 0.44 | 4.31 ± 0.84 | 0.4080 |
| Cck2r | 1.39 ± 0.16 | 0.91 ± 0.09 | 0.67 ± 0.16 (a) | 0.0190 * |
| Chrm3 | 0.92 ± 0.07 | 0.70 ± 0.08 (a) | 0.41 ± 0.03 (a), (b) | 0.0019 ** |
| Esr1 | 2.42 ± 0.51 | 2.53 ± 0.11 | 2.51 ± 0.22 | 0.9593 |
| Esr2 | 1.81 ± 0.10 | 1.81 ± 0.18 | 1.66 ± 0.13 | 0.7128 |
| FoxO1 | 1.60 ± 0.23 | 1.33 ± 0.11 | 1.46 ± 0.16 | 0.5508 |
| Gabbr1 | 1.19 ± 0.18 | 1.18 ± 0.05 | 0.93 ± 0.07 | 0.1482 |
| Gabbr2 | 1.22 ± 0.24 | 1.20 ± 0.11 | 1.21 ± 0.16 | 0.9951 |
| Galr3 | 0.45 ± 0.13 | 0.56 ± 0.11 | 0.33 ± 0.05 | 0.2596 |
| Galp | 6.05 ± 1.33 | 5.52 ± 0.43 | 7.08 ± 0.60 | 0.3627 |
| Grpr | 2.06 ± 0.16 | 1.65 ± 0.07 | 1.88 ± 0.16 | 0.1742 |
| Glp1r | 2.68 ± 0.28 | 2.36 ± 0.14 | 2.66 ± 0.39 | 0.7331 |
| Gck | 1.37 ± 0.20 | 1.16 ± 0.06 | 1.12 ± 0.10 | 0.3593 |
| Gad1 | 1.03 ± 0.25 | 0.97 ± 0.08 | 0.99 ± 0.12 | 0.9599 |
| Gad2 | 1.23 ± 0.14 | 0.99 ± 0.09 | 1.12 ± 0.12 | 0.3736 |
| Ghsr | 2.71 ± 0.25 | 2.32 ± 0.15 | 2.48 ± 0.15 | 0.3596 |
| Grm5 | 1.46 ± 0.15 | 1.00 ± 0.18 | 0.86 ± 0.11 (a) | 0.0641 |
| Htr2c | 0.75 ± 0.11 | 0.58 ± 0.04 | 0.40 ± 0.04 (a) | 0.0155 * |
| Irs1 | 0.71 ± 0.06 | 0.63 ± 0.04 | 0.57 ± 0.07 | 0.2858 |
| Irs2 | 1.62 ± 0.14 | 1.55 ± 0.23 | 1.49 ± 0.09 | 0.1504 |
| Igf1 | 1.25 ± 0.11 | 0.53 ± 0.05 (a) | 1.18 ± 0.28 | 0.0480 * |
| Igf1r | 1.54 ± 0.17 | 1.26 ± 0.10 | 1.52 ± 0.16 | 0.3345 |
| Kiss1 | 2.83 ± 0.58 | 4.93 ± 1.51 | 3.13 ± 0.76 | 0.3890 |
| Lepr | 1.42 ± 0.16 | 1.08 ± 0.03 | 1.12 ± 0.16 | 0.2242 |
| Mc3r | 1.34 ± 0.18 | 1.01 ± 0.03 | 1.19 ± 0.26 | 0.5012 |
| Npy | 0.76 ± 0.07 | 0.72 ± 0.10 | 0.89 ± 0.24 | 0.7240 |
| Npy2r | 1.50 ± 0.06 | 1.35 ± 0.07 | 1.44 ± 0.23 | 0.7979 |
| Oprm1 | 1.16 ± 0.15 | 1.09 ± 0.13 | 0.93 ± 0.07 | 0.4183 |
| Pten | 1.54 ± 0.23 | 1.24 ± 0.10 | 1.49 ± 0.13 | 0.3583 |
| Pdyn | 1.82 ± 0.11 | 1.49 ± 0.20 | 1.35 ± 0.14 | 0.2026 |
| Pomc | 5.32 ± 0.15 | 5.50 ± 1.55 | 4.38 ± 0.76 | 0.7398 |
| Stat3 | 1.76 ± 0.14 | 1.63 ± 0.10 | 1.80 ± 0.17 | 0.6721 |
| Th | 3.02 ± 0.34 | 2.82 ± 0.13 | 3.29 ± 0.54 | 0.6864 |
| Ucp2 | 1.78 ± 0.43 | 1.40 ± 0.14 | 1.56 ± 0.17 | 0.5638 |
Data were analyzed by a one-way ANOVA followed by Bonferroni multiple comparison test with litter as the unit. Samples size: vehicle = 3, Lo-BPA = 3, Hi-BPA = 4. ANOVA p values:
p < 0.05;
p < 0.01.
= p < 0.05 compared to vehicle;
= p < 0.05 compared to Lo-BPA.
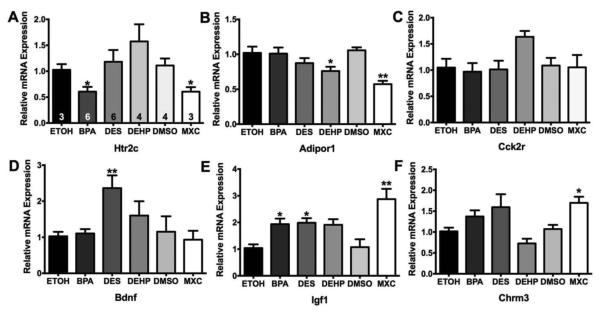
There were 4 other genes in the TLDA that were significantly altered by BPA treatment with ANOVA p values between 0.05 and 0.01. These genes included Bdnf, Cck2r, Htr2c, and Igf1 (See Table 2). Bdnf (brain-derived neurotrophic factor; F(2, 8) = 7.279; p < 0.05) was significantly suppressed by the high dose of BPA (p < 0.05). The cholecystokinin receptor 2 (Cck2r; (F(2, 8) = 6.770; p < 0.05) was significantly suppressed by the high BPA dose (p < 0.05). The serotonin receptor 5HT2c receptor (Htr2c; (F(2, 8) = 7.329; p < 0.05), was suppressed by the high dose of BPA (p < 0.05). Igf1 (insulin growth factor 1; F(2, 8) = 4.547; p < 0.05) was suppressed only by the low dose of BPA (p < 0.05). These six genes were examined again in the ARC samples generated from Experiment #2.
3.4. Endocrine disrupting compounds alter ARC gene expression
In Experiment #2, females were exposed during development to 4 EDCs - BPA, DES, DEHP, or MXC - and sacrificed at 70-90 days in age. Of the six genes, five were affected by EDC treatment (ANOVA: p ≤ 0.01) (See Figure 3). As in Experiment #1, BPA (p < 0.05) suppressed the expression of Htr2c in the ARC (F(5, 25) = 4.524; p < 0.01). MXC treatment also suppressed Htr2c expression (p < 0.05) (Figure 3A). Another gene that was suppressed by EDC treatment was Adipor1 (Figure 3B; F(5, 25) = 4.697; p < 0.01). This gene was suppressed by DEHP (p < 0.05) and MXC (p < 0.01) treatment in the ARC but not by BPA as it was in Experiment #1. Unlike in Experiment #1, there was not an effect of EDC treatment on Cck2r gene expression (Figure 3C; F(5, 25) = 2.067; p = 0.1036).
Figure 3.
Relative gene expression in ARC from young adult female rats exposed to EDC. A. Ht2cr, B. Adipor1, C. Cck2r, D. Bdnf, E. Igf1, and F. Chrm3. Data were analyzed using a one-way ANOVA followed by Bonferroni multiple comparison tests within each gene compared to the respective control samples (* p < 0.05; ** p < 0.01). Numbers in the columns of A are the sample sizes for each treatment (n = litters).
Only DES significantly affected Bdnf gene expression in the ARC (Figure 3D; F(5, 25) = 4.041; p < 0.01). EDC treatment also augmented Igf1 expression in the ARC (Figure 3E; F(5, 25) = 7.095; p < 0.001). Both BPA (p < 0.05) and DES (p < 0.05) significantly increased the expression of Igf1 by more than twofold and MXC treatment increased the expression of Igf1 compared to DMSO controls (p < 0.01). MXC also increased Chrm3 expression (Figure 3F; F(5, 25) = 2.868; p < 0.05) by about 50% in the ARC with no other compounds having a significant effect. Because of their involvement in reproduction (puberty) and energy homeostasis, we also examine gene expression of Kiss1, Igf-1r, Gabbr1, Esr1, and Esr2 in these samples. No effect of EDC treatment was found for any of these genes (data not shown).
4. Discussion
The goal of this study was to characterize the effects of developmental exposures to EDC (BPA, DES, DEHP, and MXC) on adult female ARC gene expression involved in reproduction, energy homeostasis, and hypothalamic neuronal functions. We selected a range of ARC genes including many GPCR, neuropeptides, signaling molecules, and transcription factors (reviewed in [6;30;31] and employed TLDA technology to screen for potential candidate genes using two doses of BPA. Of the 40 genes selected, six genes were affected by developmental BPA exposures. Four of these genes were receptors for neurotransmitters (serotonin, acetylcholine) and peripheral hormones (adiponectin) or brain-gut peptides (cholecystokinin). The remaining two genes were the neurotrophin BDNF and the paracrine hormone IGF-1. Five of these genes (Ht2cr, Adipor1, Bdnf, Igf1, and Chrm3) were significantly regulated in the second experiment, which examined the effects of 4 EDCs on ARC gene expression in adult female offspring. The inconsistency in Cck2r gene regulation between Experiment #1 and #2 by BPA may be due to age-dependent processes (> PD60) or may be ameliorated by adult influences on developmental programming.
Recent evidence has indicated that developmental exposures to EDC can affect hypothalamic functions, especially reproduction [38;39;52-54]. Many of these effects include changes in gene expression of relevant neuropeptides and hormone receptors. For example, developmental PCB exposures in rats suppressed POA expression of IGF-1, androgen receptor, and NDMA glutamate receptor subunit NR2b in females [54]. Developmental MXC exposures altered expression of ERα and other genes in the POA of female rats [38]. Neonatal and prepubertal exposures to dibutyl phthalates (DBP) increased ARC kisspeptin expression while suppressing GPR54/Kiss1r and ERα gene expression in female rats [21]. Finally, gestational exposures to BPA and 17α-ethinylestradiol increased neonatal expression of ERα in the ARC of male and female pups [39;52].
Few studies have examined the effects of EDC exposure on the expression of ARC genes involved in energy homeostasis and neuronal activity primarily focusing on the expression of the POMC and NPY neuropeptides. POMC neurons express the endogenous agonist for MC4 receptors, α-MSH, while NPY neurons express the endogenous MC4 antagonist, AgRP [55]. Recently, Mackay et al. (2013) reported that BPA and DES developmental exposures in CD-1 mice altered ARC expression of ERα in female offspring fed a control diet and augmented expression of NPY and AgRP in male offspring fed a high fat diet while suppressing the expression of POMC in females fed a high fat diet [10]. Additionally, the Mackay et al. (2013) study found alterations to hypothalamic melanocortin circuitry with a reduced POMC innervation of melanocortin receptor 4 (MC4) receptor-expressing PVH neurons.
Two of the genes regulated by BPA and EDCs in both experiments were receptors for neurotransmitters involved in reproduction and energy homeostasis. Serotoninergic neurons, originating from the dorsa raphe, are a major anorectic signal in the brain innervating both ARC POMC and NPY neurons [56]. The serotonin 5HT2c receptor is the primary receptor involved in serotonin’s anorectic actions in the ARC along with are the 5HT2a and 5HT1b receptors [57-59]. Agonists of the 5HT2c receptor reduce food intake and improve glucose homeostasis [60]. While the role of 5HT2c receptor in puberty is unknown, serotonin is involved in the initiation of puberty in rodents delaying puberty when injected systemically [26;61]. However, lesions of the dorsal raphe (containing serotonergic neurons) at PND 21 delayed vaginal opening and first vaginal estrus (ovulation) in rats while lesions after weaning (PND 24/27) did not [62], indicating that serotonin controls puberty onset. In our study, two compounds advanced puberty, DES and MXC, the latter also suppressed 5HT2c receptor expression. Conversely, in DEHP-treated females, there was a trend to elevated Htr2c expression (p = 0.154) and a delay in vaginal opening (p < 0.05).
There is no evidence that developmental or adult exposures to EDCs affect the expression of serotonin receptors and specifically the 5HT2c receptor in the hypothalamus. However, developmental exposures to estrogenic EDCs have been shown to alter serotoninergic innervation, release, and reuptake. Prenatal and lactational exposures to BPA increased the concentrations of serotonin and its metabolites in the hippocampus, thalamus, dorsal raphe, and substantia nigra [63;64]. MXC treatment in adulthood increased serotonin concentrations in the anterior hypothalamus while decreasing the concentrations in the posterior hypothalamus and the turnover in the anterior hypothalamus [65]. In our study, the reduction in 5HT2c receptor expression specifically in the ARC in BPA- and MXC-treated females could produce a decreased response to the anorectic serotoninergic signals originating from the dorsal raphe leading to hyperphagia and weight gain. Future studies should examine the expression and activity of 5HT2c receptors in ARC POMC neurons after developmental EDC exposure.
There are little data on the effects of EDC exposure on the activity and expression of Chrm3, an acetylcholine receptor, with this study being the first one to characterize a potential effect of developmental exposures on its expression in the hypothalamus. This receptor is thought to be involved in energy homeostasis due to genetic modification (Chrm3 knockout] in mice. The subsequent alterations in energy homeostasis suggests that Chrm3 has a role in glucose homeostasis, insulin sensitivity, food intake, and energy expenditure, which may be due to an increase in central sympathetic activity [66;67]. Although the role of M3 muscarinic receptor in ARC functions is unknown at this time, blocking muscarinic receptors with trihexyphenidyl or propantheline delays vaginal opening and first estrus in rats indicating cholinergic neurons are involved in the control of puberty [68]. The increase in Chrm3 by MXC in the ARC in Experiment #2 correlates with an advance in vaginal opening (puberty), suggesting that changes in muscarinic receptors in the hypothalamus due to EDC exposure may contribute to the dysregulation of puberty.
Receptors for two peptide hormones, adiponectin receptor 1 (Adipor1) and cholecystokinin receptor 2 (Cck2r), were significantly regulated in Experiment #1. However, in Experiment #2, there was no effect on Cck2r expression in the ARC, although there was a trend for elevated Cck2r expression (p = 0.07) in DEHP-exposed females. Conversely, the adiponectin receptor was consistently regulated in the ARC by EDC exposure. Adiponectin is an adipocyte peptide hormone and is highly expressed in lean animals to control insulin sensitivity and glucose homeostasis [69]. While controversial, adiponectin acts through its receptor in the hypothalamus to increase food intake and suppress energy expenditure by an AMPK-mediated pathway [70;71]. Adiponectin receptor 1 is expressed both in POMC and NPY neurons and the expression of this receptor is stimulated by fasting states [69]. While there are no data on the effects of EDC exposure on adiponectin receptor expression, both BPA and DEHP have been shown to suppress adiponectin expression in adipocytes in adult mice [72;73].
In our study, the reduction in Adipor1 expression specifically in the ARC in EDC-treated females may contribute to advance the onset of puberty found in the MXC-treated females. Adiponectin, in part, via actions through its receptor, inhibits the HPG axis and GnRH secretion, and controls puberty [74;75]. In GT1-7 cells, adiponectin inhibits production and secretion of GnRH [74]. Adiponectin also inhibits hypothalamic Kiss1 gene expression through AMPK-SP1 [76] and inhibits LH secretion from pituitary explants from rats by decreasing GnRH receptor expression in the pituitary [77]. In our study, MXC reduced Adipor1 expression in the ARC, thus potentially reducing the inhibition of the HPG axis and Kiss1 by adiponectin from peripheral adipose tissue to advance puberty and alter the estrous cycle.
Expression of two paracrine/autocrine neuropeptides was also differentially altered in our two experiments. Insulin-like Growth Factor 1 (Igf1) was suppressed by the low BPA dose in Experiment #1 while significantly increased by DES, MXC, and the high dose of BPA in Experiment #2. There are little to no data demonstrating developmental effects of EDC exposure on IGF-1 expression or activity in the brain. Recently, Huang et al. (2014) suggested that BPA exposure to human embryonic stem cells downregulated the expression of IGF-1 as well as other genes involved in neural development [78]. In the periphery, BPA exposure increased IGF-1 peptide expression in the liver of female rats [79] and MXC exposure altered proteins (e.g., Igf1r and Igfbp5) of the IGF-1 signaling in the ovary of rats [80].
IGF-1 signaling in the brain is an important regulator of neuroendocrine control of reproduction and energy balance [81;82]. While the direct effect of increase in Igf1 gene expression in the ARC on energy homeostasis is unknown, IGF-1 is a growth factor and an important hormone in the somatotropic axis [83]. Additionally, IGF-1 expression and activity is involved in the control of the HPG axis and the onset of puberty [81;82]. An increase in IGF-1 gene expression in the mediobasal hypothalamus is found in the neonate and in the adult (PND 60) [84]. Furthermore, peripheral administration of IGF-1 initiates the onset of puberty in prepubertal rats [85]. IGF-1 levels in ARC tanycytes is sexually dimorphic, is increased during the initiation of puberty, and is regulated by sex steroids including estradiol [86]. Previous studies have demonstrated that BPA and DES exposure advances puberty in female rodents [7;13;87]. However, in our study BPA did not advance vaginal opening while DES and MXC did. In the DES- and MXC-treated females, an increase in ARC IGF-1 expression may play a role in the effects on puberty and/or the estrous cycle.
The second paracrine/autocrine neuropeptide affected by EDC treatment was BDNF. In Experiments #1, Bdnf was suppressed by the high dose of BPA but not in Experiment #2 while being significantly increased by DES treatment in Experiment #2. In the hippocampus, developmental or neonatal exposures to DES increased Bdnf gene expression in male rats [66;88]. However, DES and BPA treatment suppressed BDNF protein expression in primary cultures of mouse cerebellar granule cells [89]. This suggests that EDC control BDNF expression through different mechanisms depending upon the species, the timing of exposure, and stage of development. During development, BDNF regulates neurogenesis and synaptic plasticity while during adulthood BDNF expression is associated with the control of food intake, body weight, and activity-dependent energy expenditure [53;90;91]. Deletion of BDNF in the VMH and DMH of adult mice increased food intake without affecting energy expenditure or locomotive behavior [92] although the involvement of ARC BDNF in energy homeostasis remains to be determined. As in the case of Igf1, the increase in Bdnf in the ARC with DES treatment is not associated with a higher weight gain in these females and therefore may be associated with the disruption of the HPG axis [93] or perhaps puberty, although the role of BDNF in puberty onset is unclear.
5. Conclusions
Our study indicates that solely examining the expression of anorectic and orexigenic neuropeptides or estrogen receptors and KNDy genes in the ARC is not sufficient to understanding the effects of developmental EDC exposures on homeostatic and reproductive functions. Many of the genes affected modulate energy homeostasis towards either a positive or negative balance (increase or decease food intake and/or energy expenditure) or are involved in the transmission of signals to initiate the onset of puberty. However, the effect of these changes in gene expression may offset each other and not disrupt reproduction or energy homeostasis, unless in an obesogenic environment [10]. The present study provides potential gene target for the developmental effects of EDCs on sexual maturity, estrous cycles, the HPG axis, and energy homeostasis that have been reported. Understanding these mechanisms of EDC toxicity is necessary in determining their respective effects on human and wild life health. For example, the consistent effect of BPA exposure on 5HT2c receptor has wide ranging potential to alter normal homeostatic functions increasing the propensity to obesity and Type II diabetes. Future experiments should examine these EDC effects in animal models that can target these receptors and neuropeptides at the level of specific neuronal cell types (POMC, NPY, KNDy, etc.). Due to small ARC tissue size, we did not measure protein levels, which are more functionally relevant. Because mRNA and protein levels may not correlate when comparing gene and protein expression [94], future studies will examine the expression and activity of the hormone and neurotransmitter receptors using electrophysiology and single cell gene expression assays in ARC neurons. Experiments such as those would determine if these effects on gene expression translate into neurological outcomes resulting in the dysregulation of reproduction and energy homeostasis.
Highlights.
Developmental exposure to BPA did not affect body weights or puberty.
Developmental exposure to DES & MXC advanced puberty and to DEHP delayed puberty.
Developmental exposure to EDC altered arcuate gene expression in early adulthood
Acknowledgements
This research is supported by National Institutes of Health Grant R00DK083457 (T.A.R.), and ES017847, ES017059 (M.U.), and NIEHS Center Grant ES005022 (T.A.R and M.U.). Dr. Elif Oruc, visiting scholar from Cukurova University, Turkey, supported in part by TUBITAK-BIDEB International, Postdoctoral Research Scholarship Program.
Abbreviations
- Adipor1
adiponectin receptor 1
- ARC
arcuate nucleus of the hypothalamus
- Bdnf
brain-derived neurotropic factor
- BPA
bisphenol A
- Cck2r
cholescystokinin 2 receptor
- Chrm3
muscarinic receptor 3
- DEHP
di(2-ethylhexyl)phthalate
- DES
diethylstilbestrol
- Htr2c
5-hydroxytryptamine receptor
- Igf1
insulin-like growth factor 1
- MXC
methoxychlor
- NPY
neuropeptide Y
- POMC
proopiomelanocortin
- TLDA
Taqman® Low-Density Array
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, bodyweight, and adiposity. J Ster Bioc Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [3].Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- [5].Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- [6].Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Human Reprod Update. 2014;20:153–174. doi: 10.1093/humupd/dmt033. [DOI] [PubMed] [Google Scholar]
- [7].Xi W, Lee CKF, Yeung WSB, et al. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31:409–417. doi: 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Arevalo M, Alonoso-Magdalena P, Dos Santos JR, Quesada I, Carnerio EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;6:1–13. doi: 10.1371/journal.pone.0100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Esterik JCJ, Dollé MET, van Leeuwen SPJ, Hamers T, Legler J, van der Ven LTM. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology. 2014;321:40–52. doi: 10.1016/j.tox.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [10].MacKay H, Patterson ZR, Khazall R, Patel S, Tsirlin D, Abizaid A. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology. 2013;154:1465–1475. doi: 10.1210/en.2012-2044. [DOI] [PubMed] [Google Scholar]
- [11].Bourguignon JP, Rasier G, Lebrethon MC, Gerard A, Naveau E, Parent AS. Neuroendocrine disruption of pubertal timing and interactions between homeostasis of reproduction and energy balance. Mol Cell Endocrinol. 2010;324:110–120. doi: 10.1016/j.mce.2010.02.033. [DOI] [PubMed] [Google Scholar]
- [12].Bourguignon JP, Franssen D, Gerard A, et al. Early neuroendocrine disruption in hypothalamus and hippocampus: developmental effects including female sexual maturation and implications for endocrine disrupting chemical screening. J Neuroendocrinol. 2013;25:1079–1087. doi: 10.1111/jne.12107. [DOI] [PubMed] [Google Scholar]
- [13].Franssen D, Ioannou YS, Alvarez-real A, et al. Pubertal timing after neonatal diethylstilbestrol exposure in female rats: neuroendocrine vs peripheral effects and additive role of prenatal food restriction. Reprod Toxicol. 2014;44:63–72. doi: 10.1016/j.reprotox.2013.10.006. [DOI] [PubMed] [Google Scholar]
- [14].Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol A alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–762. doi: 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- [16].Kwon S, Stedman DB, Elswick BA, Cattley RC, Welsch F. Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol Sci. 2000;55:399–406. doi: 10.1093/toxsci/55.2.399. [DOI] [PubMed] [Google Scholar]
- [17].Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems later in life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- [18].Gray LE, Jr., Ostby J, Sigmon R, et al. The development of a protocol to assess reproductive effects of toxicants in the rat. Reprod Toxicol. 1988;2:281–287. doi: 10.1016/0890-6238(88)90032-9. [DOI] [PubMed] [Google Scholar]
- [19].Lee KY, Shibutani M, Takagi H, et al. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology. 2004;203:221–238. doi: 10.1016/j.tox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- [20].Ma M, Kondo T, Ban S, et al. Exposure of prepubertal female rats to inhaled di(2-ethylhexyl)phthalate affects the onset of puberty and postpubertal reproductive functions. Toxicol Sci. 2006;93:164–171. doi: 10.1093/toxsci/kfl036. [DOI] [PubMed] [Google Scholar]
- [21].Hu J, Du G, Zhang W, Huang H, Wu CD, Wang X. Short-term neonatal/prepubertal exposure of dibutyl phthalate (DBP) advanced pubertal timing and afected hypothalamic kisspeptin/GR54 expression differently in female rats. Toxicology. 2013;314:65–75. doi: 10.1016/j.tox.2013.09.007. [DOI] [PubMed] [Google Scholar]
- [22].Moyer B, Hixon ML. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP) Reprod Toxicol. 2012;34:43–50. doi: 10.1016/j.reprotox.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC., Jr Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. Am J Physiol Endocrinol Metab. 2013;305:E1512–E1520. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ratra DV, Elias CF. Chemical identity of hypothalamic neurons engaged by leptin in reproductive control. J Chem Neuroanat. 2014;61-62:233–238. doi: 10.1016/j.jchemneu.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kasuya E, Nyberg CL, Mogi K, Terasawa E. A role of γ-aminobutyric acid (GABA) and glutamate in control of puberty in female rhesus monkeys: Effect of an antisense oligodeoxynucleotide for GAD67 messenger ribonucleic acid and MK801 on luteinizing hormone-releasing hormone release. Endocrinology. 1999;140:705–712. doi: 10.1210/endo.140.2.6574. [DOI] [PubMed] [Google Scholar]
- [26].Moran MJ, Ayala ME, Gallegos E, et al. Effects of systemic administration or intrabursal injection of serotonin on puberty, first ovulation and follicular development in rats. Reprod Fertil Dev. 2013;25:1105–1114. doi: 10.1071/RD12253. [DOI] [PubMed] [Google Scholar]
- [27].Parent AS, Matagne V, Bourguignon JP. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine. 2005;28:281–286. doi: 10.1385/ENDO:28:3:281. [DOI] [PubMed] [Google Scholar]
- [28].Giuliani FA, Escudero C, Casas S, et al. Allopregnanolone and puberty: modulatory effect on glutamate and GABA release and expression of 3alpha-hydroxysteroid oxidoreductase in the hypothalamus of female rats. Neuroscience. 2013;243:64–75. doi: 10.1016/j.neuroscience.2013.03.053. [DOI] [PubMed] [Google Scholar]
- [29].Divall SA, Williams TR, Carver SE, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu YL, Jackson VR, Civelli O. Orphan G protein-coupled receptors and obesity. Eur J Pharmacol. 2004;500:243–253. doi: 10.1016/j.ejphar.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [31].Schiöth HB. G protein-coupled receptors in regulation of body weight. CNS Neurol Disord Drug Targets. 2006;5:241–248. doi: 10.2174/187152706777452263. [DOI] [PubMed] [Google Scholar]
- [32].Lieberburg I, MacLusky N, McEwen BS. Androgen receptors in the perinatal rat brain. Brain Research. 196:125–138. doi: 10.1016/0006-8993(80)90721-0. 8-25-1980. [DOI] [PubMed] [Google Scholar]
- [33].Toyooka KR, Connolly PB, Handa RJ, Resko JA. Ontogeny of androgen receptors in fetal guinea pig brain. Biol Repro. 1989;41:204–212. doi: 10.1095/biolreprod41.2.204. [DOI] [PubMed] [Google Scholar]
- [34].Pérez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- [35].Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing Estrogen Receptor-α Immunoreactivity in the Preoptic Brain, the Diencephalon, and the Amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [36].Angelogianni P, Li HL, Gianoulakis C. Ontogenesis of proopiomelanocortin and its processing to beta-endorphin by the fetal and neonatal rat brain. Neuroendocrinology. 2000;72:231–241. doi: 10.1159/000054592. [DOI] [PubMed] [Google Scholar]
- [37].Coupe B, Bouret SG. Development of the hypothalamic melanocortin system. Front Endocrinol. 2013;4:38. doi: 10.3389/fendo.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to Endocrine-Disrupting Chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endo. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cao J, Reuli ME, Rogers J, et al. Prenatal bisphenol A exposure alters sex-specific estrogen recepotr expression in he neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133:157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Uzumcu M, Zama AM. Developmental Effects of Endocrine-Disrupting Chemicals in the Ovary and on Female Fertility. In: Rosenfeld CS, editor. The Epigenome and Developmental Origins of Health and Disease. Academic Press (Elsevier); Cambridge, MA, USA: 2016. pp. 143–170. [Google Scholar]
- [41].Rothschild TC, Calhoon RE, Boylan ES. Effects of diethylstilbestrol exposure in utero on the genital tracts of female ACI rats. Exp Mol Pathol. 1988;48:59–76. doi: 10.1016/0014-4800(88)90046-9. [DOI] [PubMed] [Google Scholar]
- [42].Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol Sci. 2006;91:247–254. doi: 10.1093/toxsci/kfj128. [DOI] [PubMed] [Google Scholar]
- [44].National Toxicology Report Di(2-ethylhexyl) phthalate. Report on Carcinogens. 2011;12:156–159. [PubMed] [Google Scholar]
- [45].Loff S, Kabs F, Witt K, et al. Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers. J Pediatr Surg. 2000;35:1775–1781. doi: 10.1053/jpsu.2000.19249. [DOI] [PubMed] [Google Scholar]
- [46].Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of Di-(2-ethylhexyl) Phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Repro. 2013;88:112. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol. 2006;191:549–558. doi: 10.1677/joe.1.06592. [DOI] [PubMed] [Google Scholar]
- [48].Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front Neuroendo. 2010;31:420–439. doi: 10.1016/j.yfrne.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alworth LC, Howdeshell KL, Ruhlen RL, et al. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- [50].Paxinos G, Charles W, Carrive P, Kirkcaldie M, Ashwell KWS. Chemoarchitectonic atlas of The Rat Brain second edition. Academic Press. 2008 [Google Scholar]
- [51].Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liao G-Y, Li Y, Xu B. Ablation of TrkB expression in RGS9-2 cells leads to hyperphagic obesity. Mol Metab. 2013;2:491–497. doi: 10.1016/j.molmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].IInytska O, Argyropoulos G. The role of the Agouti-Related Protein in energy balance regulation. Cell Mol Life Sci. 2008;65:2721–2731. doi: 10.1007/s00018-008-8104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bickerdike MJ. 5-HT2C receptor agonists as potential drugs for the treatment of obesity. Curr Top Med Chem. 2003;3:885–897. doi: 10.2174/1568026033452249. [DOI] [PubMed] [Google Scholar]
- [57].Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [58].Qiu J, Xue C, Bosch MA, et al. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharm. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- [59].Roepke TA, Smith AW, Ronnekleiv OK, Kelly MJ. Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am J Physiol Endocrinol Metab. 2012;302:1399–1406. doi: 10.1152/ajpendo.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moguilevsky JA, Faigon MR, Scacchi P, Szwarcfarb B. Effect of the serotoninergic system on luteinizing hormone secretion in prepubertal female rats. Neuroendocrinology. 1985;40:135–138. doi: 10.1159/000124064. [DOI] [PubMed] [Google Scholar]
- [62].Ayala ME, Monroy J, Morales L, Castro ME, Dominguez R. Effects of a lesion in the dorsal raphe nuclei performed during the juvenile period of the female rat, on puberty. Brain Res Bull. 1998;47:211–218. doi: 10.1016/s0361-9230(98)00074-4. [DOI] [PubMed] [Google Scholar]
- [63].Nakamura K, Itoh K, Yoshimoto K, Sugimoto T, Fushiki S. Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neurosci Lett. 2010;484:66–70. doi: 10.1016/j.neulet.2010.08.021. [DOI] [PubMed] [Google Scholar]
- [64].Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sajiki J, Shimizu E. Perinatal exposure to bisphenol A enhances contextual fear memory and affects the serotoninergic system in juvenile female mice. Horm Behav. 2013;63:709–716. doi: 10.1016/j.yhbeh.2013.03.016. [DOI] [PubMed] [Google Scholar]
- [65].Lafuente A, Cabaleiro T, Caride A, Esquifino AI. Toxic effects of methoxychlor administered subcutaneously on the hypothalmic-pituitary-testicular axis in adult rats. Food Chem Toxicol. 2007;46:1570–1575. doi: 10.1016/j.fct.2007.12.017. [DOI] [PubMed] [Google Scholar]
- [66].Gautam D, Gavrilova O, Jeon J, et al. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006;4:363–375. doi: 10.1016/j.cmet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- [67].Gautam D, Jeon J, Li JH, et al. Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review. J Recept Signal Transduct Res. 2008;28:93–108. doi: 10.1080/10799890801942002. [DOI] [PubMed] [Google Scholar]
- [68].Trkulja V, Lackovic Z. Involvement of muscarinic receptors in the control of female puberty in the rat. Eur J Pharmacol. 1996;297:93–96. doi: 10.1016/0014-2999(95)00821-7. [DOI] [PubMed] [Google Scholar]
- [69].Guillod-Maximin E, Roy AF, Vacher CM, et al. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200:93–105. doi: 10.1677/JOE-08-0348. [DOI] [PubMed] [Google Scholar]
- [70].Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [71].Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Brit J Pharmacol. 2012;165:313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander W. Bisphenol A at environmentally relevent doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2014;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adiopgenesis in C3H/N mice. Environ Health Perspect. 2012;120:1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cheng XB, Wen JP, Yang J, Yang Y, Ning G, Li XY. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011;39:6–12. doi: 10.1007/s12020-010-9375-8. [DOI] [PubMed] [Google Scholar]
- [75].Palin MF, Bordignon VV, Murphy BD. Adiponectin and the control of female reproductive functions. Vitam Horm. 2012;90:239–287. doi: 10.1016/B978-0-12-398313-8.00010-5. [DOI] [PubMed] [Google Scholar]
- [76].Wen JP, Liu C, Bi WK, et al. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J Endocrinol. 2012;214:177–189. doi: 10.1530/JOE-12-0054. [DOI] [PubMed] [Google Scholar]
- [77].Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- [78].Huang B, Jiang C, Luo J, Cui Y, Qin L, Liu J. Maternal exposure to bisphenol A may increase the risks of Parkinson's disease throught down-regulation of fetal IGF-1 expression. Med Hypotheses. 2013;82:245–249. doi: 10.1016/j.mehy.2013.10.023. [DOI] [PubMed] [Google Scholar]
- [79].Ramirez MC, Bourguignon NS, Bonaventura MM, Lux-Lantos V, Libertun C, Becú-Villalobos D. Neonatal xenoestrogen exposure alters growth hormone-dependent liver proteins and genes in adult female rats. Toxicol Lett. 2012;213:325–331. doi: 10.1016/j.toxlet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- [80].Zama AM, Uzumcu M. Targeted genome-wide methylation and gene expression analysis reveal signaling pathways involved in ovarian dysfunction after developmental EDC exposure in rats. Biol Repro. 2013;88:1–13. doi: 10.1095/biolreprod.112.104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wolfe A, Divall S, Wu S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1) Front Neuroendo. 2014;35:558–572. doi: 10.1016/j.yfrne.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med. 2005;230:292–306. doi: 10.1177/153537020523000503. [DOI] [PubMed] [Google Scholar]
- [83].Rogol AD. Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev. 2010;17:77–85. doi: 10.1159/000262530. [DOI] [PubMed] [Google Scholar]
- [84].Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- [85].Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3728. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- [86].Garcia-Segura LM, Duenas M, Fernandez-Galaz MC, et al. Interaction of the signalling pathways of insulin-like growth factor-I and sex steroids in the neuroendocrine hypothalamus. Horm Res. 1996;46:160–164. doi: 10.1159/000185016. [DOI] [PubMed] [Google Scholar]
- [87].Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2010;32:38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ramos JG, Varayoud J, Monje L, Moreno-Piovano G, Munoz-de-Toro M, Luque EH. Diethylstilbestrol alters the population dynamic of neural precursor cells in the neonatal male rat dentate gyrus. Brain Res Bull. 2007;71:619–627. doi: 10.1016/j.brainresbull.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [89].Imamura L, Kurashina K, Kawahira T, Omoteno M, Tsuda M. Additional repression of activity-dependent c-fos and BDNF mRNA expression by lipophilic compounds accompanying a decrease in Ca2+ influx into neurons. Neurotoxicology. 2005;26:17–25. doi: 10.1016/j.neuro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [90].Vanevski F, Xu B. Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci. 2013;7:1–10. doi: 10.3389/fnins.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fargali S, Sadahiro M, Jiang C, et al. Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis. J Mol Neurosci. 2012;48:654–659. doi: 10.1007/s12031-012-9790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nakamura T, Katsu Y, Watanabe H, Iguchi T. Estrogen receptor subtypes selectively mediate female mouse reproductive abnormalities induced by neonatal exposure to estrogenic chemicals. Toxicology. 2008;253:117–124. doi: 10.1016/j.tox.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [94].Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-Estradiol. J Comp Neurol. 2012;520:2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]